Abstract
Background
Approximately 30% to 50% of patients who are referred for diagnostic coronary angiography are found to have no obstructive coronary artery disease (CAD). Ischemia and nonobstructive coronary arteries (INOCA) is increasingly recognized and encompasses coronary microvascular dysfunction, vasospastic angina, symptomatic myocardial bridging, and other vasomotor disorders. However, the prevalence of these disorders and whether underlying atherosclerotic plaque burden and morphology affect the long-term outcomes of each physiologic phenotype is unknown.
Methods
The DISCOVER INOCA registry is ongoing at 8 centers in the United States and plans to enroll 500 patients with ischemic heart disease referred for angiography undergoing coronary function testing (CFT). All participants will complete patient-reported outcome measures and undergo protocol-guided angiography, acetylcholine provocation, coronary thermodilution, and intravascular imaging. Follow-up assessments occur at 30 days, 6 months, 1 year, and annually for 5 years. The primary short-term end point is the prevalence of INOCA phenotypes based on physiology and the degree of atherosclerosis based on intravascular ultrasound or optical coherence tomography (intravascular imaging). The primary long-term end point is the incidence of major adverse cardiovascular events, defined as a composite of cardiovascular death, myocardial infarction, hospitalization for cardiovascular causes, or coronary revascularization at a follow-up of 5 years. At the time of this publication, 100 participants have been enrolled.
Conclusions
DISCOVER INOCA is the first prospective study of INOCA patients to integrate anatomic and physiologic measures of disease and correlate them with long-term outcomes. DISCOVER INOCA will report on the prevalence of INOCA phenotypes, the safety of comprehensive invasive CFT, and the impact of testing on diagnoses and medical therapy. Symptoms and cardiovascular adverse events at long-term follow-up will be determined in patients with no obstructive CAD undergoing angiography.
Keywords: coronary, intravascular imaging, physiology, stable ischemic heart disease
Introduction
Among patients referred for invasive coronary angiography (ICA) with or without ischemia on noninvasive stress testing, nearly 50% are found to have no obstructive coronary artery disease (CAD).1, 2, 3 Myocardial ischemia and nonobstructive coronary arteries (INOCA) is an umbrella term encompassing specific syndromes including coronary microvascular dysfunction (CMD) due to structural abnormalities or abnormally elevated resting flow, vasospastic angina (VSA), diffuse physiologically significant epicardial CAD, endothelial dysfunction, and symptomatic myocardial bridging. Previous studies suggest that there are 3 to 4 million people in the United States who suffer from INOCA.4,5 Compared with patients with obstructive CAD, these patients are more likely to suffer from anxiety, depression, and recurrent symptoms.6 Furthermore, compared with healthy controls, these patients are at increased risk for major adverse cardiovascular events (MACE) and cardiovascular mortality.7,8
Invasive anatomic and physiologic assessment of patients with INOCA has revealed epicardial endothelial dysfunction, CMD, myocardial bridging, or diffuse epicardial atherosclerosis in >75% of patients.2 Recent studies have shown that angina and quality of life can be improved when coronary physiology assessment is incorporated into clinical care.9,10 However, comprehensive assessments of coronary physiology (including the epicardial vessels and microvascular bed) with coronary function testing (CFT) are currently not performed routinely in most medical centers despite being recommended by professional society guidelines.6,11,12 The rationale for performing intravascular imaging and comprehensive coronary physiology assessment in patients with INOCA is that ICA alone cannot diagnose or exclude CMD, diffuse atherosclerosis, physiologically significant myocardial bridging, endothelial dysfunction, or dynamic processes such as coronary vasospasm.9 Additionally, determining the specific INOCA physiologic phenotype can help guide medical therapy and long-term prognosis.6,13
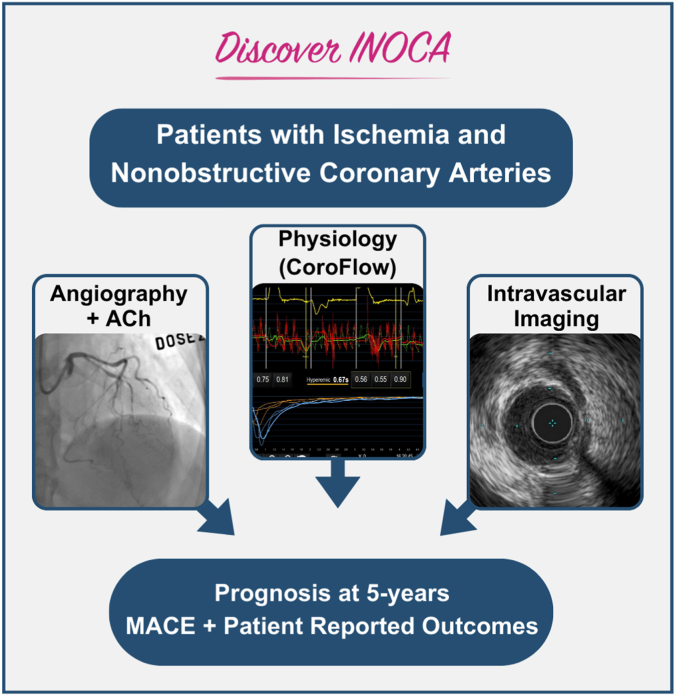
This prospective, multicenter registry will enroll up to 500 patients presenting with symptomatic ischemic heart disease and referred for ICA based on clinical indications. The overall objective of the Determining the Cause of Coronary Vasomotor Disorders in Patients With Ischemia and Nonobstructive Coronary Arteries (DISCOVER INOCA) Registry is to characterize phenotypes of INOCA and long-term outcomes based on both an anatomic evaluation (ICA and intravascular imaging) and physiologic assessment with the CoroFlow Cardiovascular System (Abbott Vascular) (Central Illustration). In this study, we aim to: (1) describe the prevalence of the INOCA phenotypes: CMD, VSA, mixed CMD/VSA, myocardial bridging, and other disorders of coronary physiology; (2) characterize the burden of epicardial coronary artery atherosclerosis and myocardial bridging; and (3) characterize the natural history and outcomes of patients with INOCA and determine variables associated with MACE.
Central Illustration.
The DISCOVER INOCA multicenter registry aims to identify specific phenotypes of ischemia and nonobstructive coronary arteries through combined anatomic and physiological assessments (CoroFlow Cardiovascular System) and determine long-term outcomes. MACE, major adverse cardiovascular events (composite of cardiovascular death, myocardial infarction, hospitalization for cardiovascular causes, or coronary revascularization).
Methods
Study design
DISCOVER INOCA is a prospective, multicenter registry of patients with INOCA evaluated by ICA, intravascular imaging, and physiologic assessment with the CoroFlow Cardiovascular System (Central Illustration). The study is registered at ClinicalTrials.gov (NCT05288361). Participants will be enrolled at up to 10 investigational sites in the United States with prior experience performing CFT. A current list of investigational sites is provided in Supplemental Table S1. Clinical end points will be adjudicated by independent physician review by a provider who is not part of the study team. The relationship of these events to the procedure and physiology assessment protocol will also be adjudicated. Index procedure angiographic data, physiologic assessments, and intracoronary imaging will be analyzed offline by an angiographic core laboratory and intravascular imaging core laboratory (Yale Cardiovascular Research Group).
Screening process and eligibility criteria
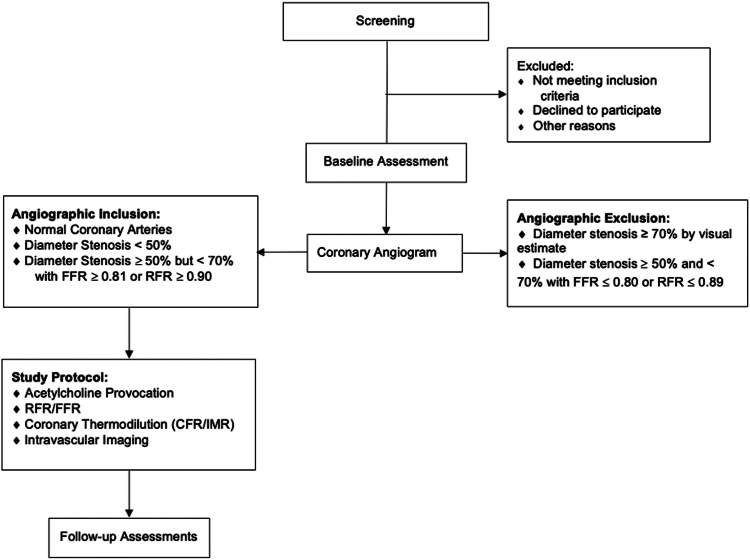
Patients with suspected ischemic heart disease that are scheduled for clinically indicated cardiac catheterization at the recommendation of their physician will be screened for inclusion in the registry. Participants must speak English or Spanish as the primary language. Up to 500 eligible patients who meet all inclusion criteria (Table 1) and no exclusion criteria (Table 2) will be enrolled in the study. The point of enrollment is investigator certification at the completion of the index procedure cardiac catheterization that all eligibility criteria are met. Participants will remain enrolled in the study regardless of the findings of the study procedure, including those with normal coronary physiology. The study flow diagram is presented in Figure 1. The following study measures must be assessed prior to the index procedure: Canadian Cardiovascular Society Anginal Classification, Seattle Angina Questionnaire, EuroQol 5 Dimensions – 5 Levels, Patient Health Questionnaire-8, and Generalized Anxiety Disorder-7, as well as preprocedure medications.
Table 1.
DISCOVER INOCA registry inclusion criteria.
| Potential subjects must meet all of the following criteria to be eligible for inclusion in the study: |
General inclusion criteria:
|
CAD, coronary artery disease; FFR, fractional flow reserve; RFR, resting full cycle ratio.
Table 2.
DISCOVER INOCA registry exclusion criteria.
| Potential subjects will be excluded if any of the following conditions apply: |
General Exclusion Criteria
|
Angiographic Exclusion Criteria
|
eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RFR, resting full cycle ratio.
Figure 1.
DISCOVER INOCA registry patient flow diagram. Patients who meet all inclusion and no exclusion criteria will be eligible for enrollment. CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; RFR, resting wave-free ratio.
Index procedure
Invasive CFT is intended to be performed as part of the standard of care evaluation for patients who present to the cardiac catheterization laboratory. After excluding the presence of obstructive CAD, the purpose of the procedure is to evaluate coronary vasoreactivity with acetylcholine provocation, physiology with thermodilution, identify myocardial bridging, and describe the burden of atherosclerosis by intravascular imaging (the DISCOVER INOCA index procedure protocol is included as a Supplemental Appendix).
The target vessel for physiologic analysis and intracoronary imaging will be the vessel in which physiologic assessment is clinically indicated or the left anterior descending artery. Coronary angiography will be performed using a standardized acquisition protocol. Angiograms will be acquired in at least 2 projections for each major artery or branch, and the treating physician will record an estimation of stenosis location and severity. The sequence of procedural elements including acetylcholine testing, wire-based testing, and intravascular imaging are at the discretion of the operator and will be recorded.
Coronary vasoreactivity testing will be performed with bolus injection or infusion of escalating doses of intracoronary acetylcholine via a guide catheter. Recommended doses for acetylcholine testing are listed in the Supplemental Appendix. Angiography will be performed after each dose with documentation of electrocardiogram changes and/or symptoms to evaluate for endothelial dysfunction, epicardial coronary vasospasm, and microvascular spasm (Figure 2).
Figure 2.
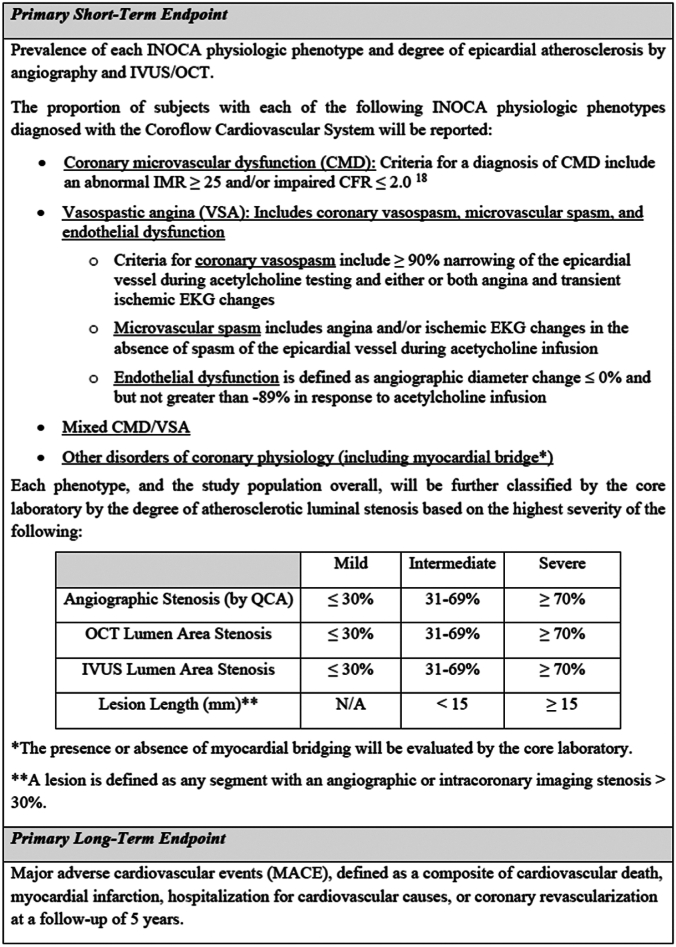
Primary end points. Primary short-term and primary long-term end points. CFR, coronary flow reserve, CMD, coronary microvascular dysfunction; EKG, electrocardiogram; IMR, index of microcirculatory resistance; INOCA, ischemia and nonobstructive coronary arteries; IVUS, intravascular ultrasound; N/A, not applicable; OCT, optical coherence tomography; QCA, quantitative coronary angiography; VSA, vasospastic angina.
Physiologic assessment will be performed with the CoroFlow Cardiovascular System and PressureWire X guidewire (Abbott Vascular) per the instructions for use. A PressureWire X guidewire will be advanced at least 6 cm or two-thirds of the way down the vessel. Any contrast will be cleared from the catheter, and the temperature will be zeroed. Resting Pd/Pa and resting full cycle ratio will be recorded. Baseline saline thermodilution will be performed and repeated 3 times to determine the baseline mean transit time (Tmn). Adenosine will be administered intravenously at 140 μg/kg/min. During hyperemia, thermodilution will be repeated 3 times to determine the hyperemic mean transit time (Tmn-hyp). Coronary flow reserve (CFR), index of microcirculatory resistance (IMR), and fractional flow reserve (FFR) will be recorded (Figure 2).
Intracoronary imaging will be performed with optical coherence tomography (OCT) or intravascular ultrasound (IVUS) according to the acquisition guidelines. Imaging should be performed on the vessel that underwent guidewire assessment using an automated pullback for a minimum of 54 mm.
Core laboratory analysis
Offline postprocedure analyses will be made by the imaging and physiology core laboratories and include quantitative coronary angiography (QCA) evaluation of all epicardial vessels, quantitative interpretation of IVUS or OCT imaging, resting Pd/Pa, resting full cycle ratio, or other nonhyperemic indices, IMR, CFR, and FFR, and quantitative flow ratio (Medis Medical Imaging).
Follow-up
All subjects will have clinical follow-up prior to discharge from the index procedure. Changes in medical therapy are at the discretion of the treating provider and will be recorded postprocedure and at all follow-up time points. Screening, procedural, and primary follow-up data collection will occur through the electronic medical record based on the local standard of care, augmented by study-specific telehealth contacts from a centralized call center at 30 days, 6 months, 12 months, and annually through 5 years (Supplemental Table S2). Symptom assessment and patient-reported outcome measures will be completed by the centralized call center at each time point.
End points
The primary short-term end point is to characterize the prevalence of each INOCA physiologic phenotype and degree of epicardial atherosclerosis (none, mild, moderate) and presence/absence of a myocardial bridge by angiography and IVUS/OCT (Figure 2). The primary long-term end point will be the incidence of MACE, defined as a composite of cardiovascular death, myocardial infarction, hospitalization for cardiovascular causes, or coronary revascularization at a follow-up of 5 years (Figure 2).
Secondary end points
Secondary end points are patient-related end points and procedural end points. Secondary patient-related end points include MACE and its components, stroke, progression to obstructive CAD, major bleeding, major vascular complications, angina severity, and patient-reported outcomes (Table 3). Procedural end points include reclassification from preprocedure diagnosis, completion of CFT without device-related serious adverse events or major angiographic complications, and frequency/nature of major angiographic complications (Table 3).
Table 3.
Secondary end points.
| Patient-Related End Points |
The following end points will be reported at all time points, unless otherwise specified:
|
| Procedural End Points |
The following end points will be reported in-hospital (or within 48 h of the index procedure):
|
BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass grafting; CAD, coronary artery disease; EQ-5D-5L, EuroQol 5 Dimensions – 5 Levels; GAD, Generalized Anxiety Disorder; MACCE, major adverse cardiovascular and cerebrovascular events; MACE, major adverse cardiovascular events (composite of cardiovascular death, myocardial infarction, hospitalization for cardiovascular causes, or coronary revascularization); NeuroARC, Neurologic Academic Research Consortium; PHQ, Patient Health Questionnaire; PCI, percutaneous coronary intervention.
Statistical analysis
Primary short-term end point
The prevalence of the various phenotypes will be estimated using the Exact (Clopper-Pearson) formula. Assuming the prevalence ranges between 10% and 50% in each phenotype, with a sample size of 500, the width of the CI will range from 5.5% to 8.9% (with a maximum interval width of 8.9%) allowing for adequate estimation of the prevalence within each group.
Primary end point
The study sample size was calculated to provide adequate power to identify variables associated with primary long-term end point events on the basis of a range of assumptions about the frequency of high-risk characteristics, their predictive accuracy, the overall rate of such events, and the hazard ratio (HR) for the risk factor. For example, if 32% of patients had a high-risk variable, 500 patients would be needed to provide 70% and 90% power to detect a HR of 4.0 for MACE of 3% and 5%, respectively, with a 1-sided alpha of 0.025. With 500 subjects, the minimum HR that will be able to be detected with event rates of 3% and 5% is 3.0 and 2.3, respectively (assuming 32% prevalence of the high-risk factor as noted above).
Baseline variables that are considered clinically relevant or that show a univariate relationship with outcome will be entered into multivariate Cox proportional hazards regression models. Variables for inclusion will be chosen, given the number of events available, to ensure parsimony of the final models. Lesion-level multivariable models will be adjusted for patient effects by means of the marginal Cox model, and nonsignificant variables dropped by backward selection. Statistical analyses will be performed using SAS software version 9.4 (SAS Institute).
Secondary end points will be reported in the overall population and by phenotype using appropriate descriptive statistics. In general, statistics for continuous variables will include mean, median, quartiles, SD, minimum, maximum, and sample size. Binary variables will be summarized using frequencies, percentages, and sample size. No formal hypothesis testing will be performed, and there is no plan to adjust alpha to account for multiple testing of exploratory secondary end points. Subgroup analyses will be performed for all primary and secondary end points for the following subgroups: presentation, categorization of plaque burden, diabetes status, sex, and plaque characterization.
Data collection and monitoring
All centers are qualified before participation. During enrollment and follow-up, monitors will review consistency on the electronic data capture system and source documents. Monitoring ensures that the trial complies with current Good Clinical Practice, relevant laws and regulations, and trial protocol, and finally, obtains secure and accurate data.
Discussion
DISCOVER INOCA is the first prospective, multicenter registry of patients with INOCA undergoing comprehensive CFT with intravascular imaging in the United States. The study is designed to integrate anatomic and physiologic measures of disease and correlate them with long-term outcomes. The standardized CFT protocol was designed by the study executive committee and is shared in its entirety in the Supplemental Appendix. The design of DISCOVER INOCA is unique due to required protocol elements of ICA, acetylcholine provocation, thermodilution assessment with the CoroFlow Cardiovascular System, and intravascular imaging. This is one of the only studies of patients with INOCA that includes intravascular imaging with IVUS and OCT to assess for myocardial bridging, quantify atherosclerotic plaque burden, and characterize plaque morphology to determine whether cardiovascular events are related to the physiology of an underlying vasomotor disorder or the natural history of atherosclerotic cardiovascular disease.
Prior studies have demonstrated a wide range for the incidence of adverse cardiovascular events in patients with INOCA7; however, the hazard of so-called “hard events” has generally been reported to be lower than that of patients with obstructive CAD.8,14 This contrasts with the high burden of symptoms and repeat cardiovascular testing seen in this population.4 In DISCOVER INOCA, short-term and long-term outcomes will be assessed for change in response from baseline for patient-reported outcome measures including Seattle Angina Questionnaire, EuroQol 5 Dimensions – 5 Levels, Patient Health Questionnaire-8, and Generalized Anxiety Disorder-7, as well as for procedural complications, progression of CAD, changes in medication over time, and MACE. The data elements included in this study will help characterize the natural history and prognosis of patients with INOCA with patient-reported outcomes such as angina burden and quality of life, as well as with adverse cardiovascular events (including re-presentation for chest pain or invasive angiography). A centralized call center was developed to ensure that patient follow-up includes symptom assessment and serial assessment of patient-reported outcome measures and to capture hospitalizations or cardiac procedures regardless of the location of care.
DISCOVER INOCA is complimentary to other active registry studies of patients with INOCA. The Inclusive Invasive Physiological Assessment in Angina Syndromes Registry (ILIAS, NCT04485234) is a global registry with enrolling sites in Korea, the Netherlands, Japan, Spain, Denmark, Italy, and the United States.15,16 Participants in ILIAS underwent CFT according to the local protocol of the enrolling institution, data was entered into a shared electronic data capture system, and patient follow-up was performed by the enrolling site. More than 2000 patients with stable ischemic heart disease or acute coronary syndromes (nonculprit vessels only) who underwent assessment of coronary pressure and flow in at least 1 vessel have been enrolled. In contrast to ILIAS, DISCOVER INOCA will exclude patients with myocardial infarction and has a prespecified diagnostic protocol. The Ischemia in Patients With Nonobstructive Disease (INOCA) in Italy INOCA IT Multicenter Registry (INOCAIT, NCT05164640) is a prospective study of 200 consecutive patients with INOCA at 3 centers in Italy.17 All subjects in INOCAIT will undergo assessment of CFR and IMR followed by provocative testing with acetylcholine. The Coronary Microvascular Disease Registry (NCT05960474) is a retrospective/prospective descriptive study of patients with stable ischemic heart disease and acute coronary syndromes who undergo invasive physiologic assessment of the microvasculature.18
These efforts demonstrate the intensive global efforts to characterize INOCA, which is a prevalent and increasingly recognized syndrome that has historically been poorly defined and challenging to treat. To date, 100 participants have been enrolled in DISCOVER INOCA, and the dissemination of results is anticipated in early 2025. The completion of this study will add to the growing body of evidence defining the patient population, physiologic phenotypes, and long-term prognosis of INOCA.
Acknowledgments
The authors extend their heartfelt gratitude to all study participants, hospitals, and researchers contributing to the DISCOVER INOCA registry. We would like to acknowledge Regina A. Deible, RN, BSN, for her insight and support as well as Lydia Tran, PharmD, with the Yale New Haven Hospital pharmacy.
Peer review statement
Editor in Chief Alexandra J. Lansky had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Deputy Editor Suzanne J. Baron.
Declaration of competing interest
Samit M. Shah reports research support from Abbott Vascular. Jennifer A. Tremmel reports consulting and advisory boards with Abbott Vascular; consulting, advisory boards, and research support with Boston Scientific; consulting and research support with Shockwave; and consulting with Avinger. Nathaniel R. Smilowitz is supported, in part, by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL150315 and reports consulting for Abbott Vascular. Yuhei Kobayashi is a consultant for Abbott Vascular. Bruce A. Samuels reports speaker/advisory board for Abbott Vascular and Philips. Jeffrey W. Moses reports equity with Xenter and Orchestra Biomed. Alexandra J. Lansky reports institutional support from Abbott Vascular. Habib Samady reports research grants from Abbott Vascular, Philips, and Medtronic, and receives consulting fees from Abbott Vascular and Philips. Timothy D. Henry, Megha Prasad, Glen Henry, Amir Lerman, Cody Pietras, Zhiyuan Zhang, Daniela Tirziu, Helen Parise, Ecaterina Cristea, Daniel Chamié, Daniel Grubman, Kyna Henrici, Elzar Matmusaeva, Nida Latif, and Natasha Cigarroa reported no financial interests.
Funding sources
The DISCOVER INOCA registry is sponsored by the Yale Cardiovascular Research Group (PIs Samit M. Shah, MD, and Alexandra J. Lansky, MD) through an investigator-initiated research grant from Abbott Vascular. The funding source was not involved in the design or implementation of the study.
Ethics statement and patient consent
This study will be conducted in compliance with the protocol, the sponsor’s standard operating procedures and/or guidelines, FDA regulations, ICH GCP guidelines, and EN/ISO 14155:2020. The investigator will assure that an appropriately constituted institutional review board (IRB) complies with the requirements of the International Conference on Harmonization Guideline. Prior to initiation of the study, the investigator will forward copies of the protocol to the IRB for its review and approval. Relevant study information will be summarized on a patient information and consent form (“informed consent form”) that has been approved by applicable regulatory authorities. This document, or a modification based on local IRB recommendations, must be approved by the applicable IRB and signed by each subject or his/her legal representative prior to the performance of any study-specific procedures or assessments.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.102046.
Supplementary material
References
- 1.Patel M.R., Peterson E.D., Dai D., et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272. Published correction appears in N Engl J Med. 2010;363(5):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee B.K., Lim H.S., Fearon W.F., et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131(12):1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMaria A.N., Lee G., Amsterdam E.A., Low R., Mason D.T. The anginal syndrome with normal coronary arteries. Etiologic and prognostic considerations. JAMA. 1980;244(8):826–828. [PubMed] [Google Scholar]
- 4.Bairey Merz C.N., Pepine C.J., Walsh M.N., Fleg J.L. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw L.J., Shaw R.E., Merz C.N., et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117(14):1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 6.Samuels B.A., Shah S.M., Widmer R.J., et al. Comprehensive management of ANOCA, part 1-definition, patient population, and diagnosis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2023;82(12):1245–1263. doi: 10.1016/j.jacc.2023.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Gdowski M.A., Murthy V.L., Doering M., Monroy-Gonzalez A.G., Slart R., Brown D.L. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc. 2020;9(9) doi: 10.1161/JAHA.119.014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jespersen L., Hvelplund A., Abildstrøm S.Z., et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 9.Ford T.J., Stanley B., Good R., et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Ford T.J., Stanley B., Sidik N., et al. 1-Year outcomes of angina management guided by invasive coronary function testing (CorMicA) J Am Coll Cardiol Intv. 2020;13(1):33–45. doi: 10.1016/j.jcin.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knuuti J., Wijns W., Saraste A., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. Published correction appears in Eur Heart J. 2020;41(44):4242. [DOI] [PubMed] [Google Scholar]
- 12.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029. Published correction appears in Circulation. 2023;148(24):e281. [DOI] [PubMed] [Google Scholar]
- 13.Ford T.J., Corcoran D., Berry C. Coronary artery disease: physiology and prognosis. Eur Heart J. 2017;38(25):1990–1992. doi: 10.1093/eurheartj/ehx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radico F., Zimarino M., Fulgenzi F., et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2018;39(23):2135–2146. doi: 10.1093/eurheartj/ehy185. [DOI] [PubMed] [Google Scholar]
- 15.van de Hoef T.P., Lee J.M., Boerhout C.K.M., et al. Combined assessment of FFR and CFR for decision making in coronary revascularization: from the multicenter international ILIAS registry. J Am Coll Cardiol Intv. 2022;15(10):1047–1056. doi: 10.1016/j.jcin.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.H., Shin D., Lee J.M., et al. Clinical relevance of ischemia with nonobstructive coronary arteries according to coronary microvascular dysfunction. J Am Heart Assoc. 2022;11(9) doi: 10.1161/JAHA.121.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghizzoni G., Leone A.M., di Serafino L., et al. “The INOCA-IT: rationale and design of a multicenter registry investigating ischemia in patients with non-obstructive coronary artery (INOCA) disease in Italy”. Int J Cardiol. 2024 doi: 10.1016/j.ijcard.2024.131859. [DOI] [PubMed] [Google Scholar]
- 18.Case B.C., Merdler I., Medranda G.A., et al. Understanding patient characteristics and coronary microvasculature: early insights from the coronary microvascular disease registry. Am J Cardiol. 2023;205:97–103. doi: 10.1016/j.amjcard.2023.07.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.