Abstract
Background and Aims
Eosinophilic gastritis and eosinophilic duodenitis (EoG/EoD) are often misdiagnosed as functional gastrointestinal (GI) disorders. Consequently, patients with GI symptoms of EoG/EoD may not undergo the necessary steps for diagnosis. We studied gastroenterologists’ evaluations of patients with chronic, unexplained, moderate-to-severe GI symptoms that were unresponsive to over-the-counter medications.
Methods
We performed a cross-sectional online survey of 202 board-certified gastroenterologists at office-based practices, community hospitals, or academic institutions. Respondents had been in active clinical practice for 3–35 years post-residency training, spent most of their time on direct patient care, managed ≥1 patient with irritable bowel syndrome and/or functional dyspepsia, and performed ≥1 endoscopy per month. Responses were analyzed to identify barriers to EoG/EoD diagnosis and management.
Results
Respondents managed a mean of 1880 patients per year; the most common diagnoses were functional dyspepsia (36%) and gastroesophageal reflux disease (19%). Mean proportions of patients who underwent upper endoscopy ranged from 42% to 84%. Biopsies were collected from >90% of patients with visible endoscopic mucosal abnormalities vs 42%–72% of patients with normal-appearing mucosae. Approximately 20% of respondents collected only 1–2 biopsies from each site of the GI tract. Only 30% routinely requested pathologists to count eosinophils, and nearly 40% had no histologic threshold for EoG/EoD diagnosis.
Conclusion
Gastroenterologists vary in their evaluation of patients with chronic, unexplained moderate-to-severe GI symptoms. Limited gastric and duodenal biopsy collection, particularly from normal-appearing mucosae, and failure to request tissue eosinophil counts might contribute to underdiagnosis of EoG/EoD. Availability and awareness of EoG/EoD diagnostic guidelines should improve detection in clinical practice.
Keywords: Gastrointestinal, Eosinophil, Misdiagnosed, Symptoms, Pathology
Introduction
Eosinophilic gastritis and/or eosinophilic duodenitis (EoG/EoD) are chronic inflammatory diseases characterized by nonspecific gastrointestinal (GI) symptoms and accumulation of activated eosinophils and mast cells in the stomach or duodenum.1,2 EoG and EoD were thought to be rare, but studies suggest that they are common and often misdiagnosed among patients with chronic, unexplained, moderate-to-severe GI symptoms.3,4 EoG prevalence has been estimated at 5.1 to 6.3 per 100,000 in the United States, while US prevalence of EoG with proven EoD was estimated to be 8.4 per 100,000.5, 6, 7 However, these estimates are likely low, as EoG/EoD is often underdiagnosed because of substantial delays and barriers to diagnosis, including delayed gastroenterologist referral, misdiagnoses, lack of biopsy and/or histopathologic evaluation, and delayed esophagogastroduodenoscopy (EGD).8,9 Furthermore, in a prospective study of 122 patients with refractory upper GI symptoms, 5.7% had a missed diagnosis for EoG.4 Indeed, patients with EoG/EoD often suffer for years before receiving an accurate diagnosis—many are misdiagnosed with functional GI disorders (FGIDs), such as irritable bowel syndrome (IBS), functional dyspepsia (FD), gastroesophageal reflux disease (GERD), or chronic gastritis.8,10 Reasons for missed or delayed diagnosis of EoG/EoD might include their nonspecific clinical presentation, perceived rarity, and lack of standardized methods for detection (including analysis of multiple biopsies).11
Publication of diagnostic guidelines for eosinophilic esophagitis (EoE)12 has increased its detection over the past 2 decades. Although there are no diagnostic guidelines for EoG/EoD, researchers have offered suggestions for increasing detection.13, 14, 15, 16, 17 Many patients with EoG/EoD have normal-appearing mucosae during endoscopy, and biopsies must be collected systematically to detect eosinophilia.3,17,18 Experts have aligned on counts of ≥30 eosinophils/high-power field (eos/hpf) in 5 hpfs in the stomach and 3 hpfs in the duodenum for diagnosis of EoG and EoD, respectively, based on findings from studies that compared patients with controls.19 Eosinophils can be easily missed during histopathologic analyses. Therefore, for clinical trials, pathologists have developed a systematic approach to counting eosinophils in multiple hpfs in each gastric and duodenal biopsy.17,20 In a recent trial of patients with chronic, moderate-to-severe, treatment-refractory GI symptoms undergoing EGD, these systematic biopsy and histopathologic approaches detected high proportions of patients with EoG/EoD.21
Millions of patients in the United States have chronic, unexplained moderate-to-severe GI symptoms or an FGID diagnosis, and these recently published data suggest that many have undetected EoG/EoD. We surveyed a representative group of practicing gastroenterologists in the United States to investigate their evaluations of adults with chronic, unexplained GI symptoms or FGID diagnoses. We aimed to identify factors that contribute to missed diagnoses of EoG/EoD, barriers to diagnosis, and gastroenterologists’ impressions of available therapy options.
Methods
Study Participants
From February through March of 2021, IQVIA Inc administered an anonymous online survey (Supplement 1) to a cross-sectional sample of board-certified gastroenterologists from their proprietary database, including those at office-based private or group practices, nonteaching community hospitals, or academic institutions in the United States. Participants were required to have practiced for 3–35 years, spend ≥80% of their time (≥50% for academics) in direct patient care, personally treat ≥1 patient with IBS or FD, perform ≥1 endoscopic procedure per month, and have no affiliation with pharmaceutical or diagnostic testing companies beyond clinical trials. Respondents who failed to meet all screening criteria in the survey (Supplement 2) were excluded. Respondents were compensated for their time with a fair-market-value honorarium.
Survey Development
Open-ended survey questions were created based on pilot interviews with gastroenterologists and hypotheses generated from studies on the prevalence and underdiagnosis of EoG/EoD.9,17 Respondents were asked questions related to diagnosis and care of patients with chronic, unexplained GI symptoms, including those diagnosed with FGIDs (including IBS and FD), GERD, or chronic gastritis; those with inflammatory bowel diseases (IBDs) were included for comparison purposes. Respondents were additionally asked about perceptions of available treatments and potential barriers to diagnosing EoG/EoD. Details on survey development can be found in the Supplementary Methods.
Statistical Analysis
Respondent demographics and survey responses were analyzed with descriptive statistics. The χ2 and Student’s t tests were used to detect differences (in means and distribution) in subgroup analysis (details in Supplementary Methods). Statistical significance was defined as a P value of .05.
Results
Respondent Demographics
Two hundred eighty-four of 10,416 surveyed gastroenterologists answered the screening questions (3% response rate). Of those, 202 participants completed the survey (Table 1). Respondents were distributed throughout the United States, had a mean of 17.7 ± 8.8 years in gastroenterology practice, and spent 95% of their time in direct patient care. Most worked in a community group or solo practice (68%), and an average of 35% practiced in an integrated delivery network or managed care organization.
Table 1.
Respondent Demographics
| Demographic | Survey respondents (n = 202) |
|---|---|
| Years in gastroenterology practice, mean ± SD | 17.7 ± 8.8 |
| Percent of time spent in patient care, mean ± SD | 94.8 ± 9.1 |
| Practice setting, n (%) | |
| Academic medical center | 40 (20) |
| Community hospital | 25 (12) |
| Private (solo or group) practice | 137 (68) |
| Practice location, n (%) | |
| Urban | 87 (43) |
| Suburban | 97 (48) |
| Rural | 18 (9) |
| Number of gastroenterologists within practice, excluding self, mean ± SD | 4.3 ± 7.6 |
| Affiliation with integrated delivery network or managed care organization, n (%) | 71 (35) |
| US census region, n (%) | |
| Northeast | 60 (30) |
| Midwest | 35 (17) |
| South | 76 (38) |
| West | 31 (15) |
| Pathology laboratory use | |
| Academic laboratory | 45 (23) |
| Commercial laboratory | 38 (19) |
| Other laboratory (at a private or group practice or community hospital) | 118 (58) |
SD, standard deviation.
Patient Characteristics
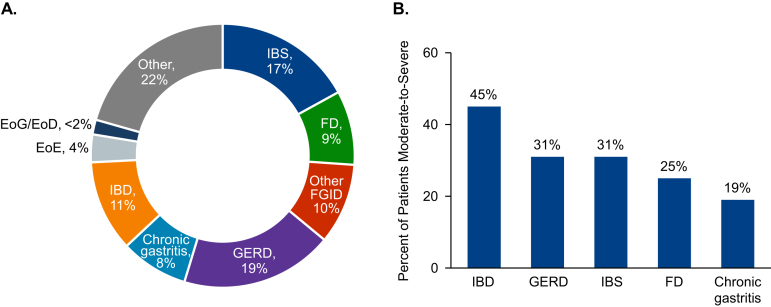
Respondents reported that they personally treated a mean 1880 ± 1230 unique patients in a typical year. The reported average proportions of patients diagnosed with EoE (4%) and EoG/EoD (<2%) were low compared with those of other diagnoses (which ranged between 8% and 19%; Figure 1A). Respondents estimated that 30% of their patients had moderate-to-severe symptoms (abdominal pain, nausea, vomiting, bloating, diarrhea, and/or early satiety; Figure 1B).
Figure 1.
Patient characteristics. (A) Diagnoses of respondents’ typical patients. (B) Mean percentage of patients diagnosed with each disorder perceived to be moderate-to-severe. EoE, eosinophilic esophagitis; EoG/EoD, eosinophilic gastritis and/or eosinophilic duodenitis; FD, functional dyspepsia; FGID, functional gastrointestinal disorder; GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
Patient Evaluations
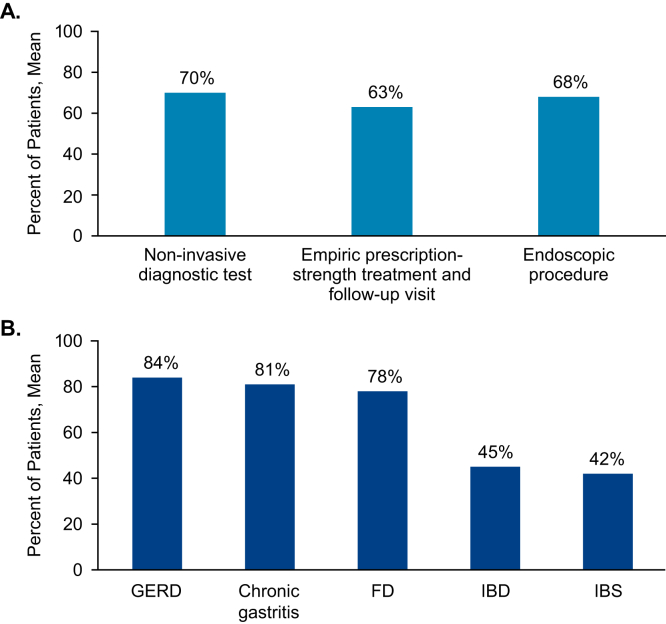
In response to questions regarding initial management and treatment (first 6 months) of younger patients (<50 years) with chronic moderate-to-severe GI symptoms (unresponsive to over-the-counter medications), respondents indicated that 70% of patients would undergo a noninvasive diagnostic workup (imaging, blood, or stool tests); 63% would begin empiric, prescription-strength drug therapy with follow-up; and 68% would undergo an endoscopic procedure (EGD or colonoscopy; Figure 2A). Respondents estimated that 78%–84% of patients diagnosed with moderate-to-severe GERD, chronic gastritis, or FD underwent EGD at some point during their care, whereas 45% of patients with moderate-to-severe IBD and 42% of those with moderate-to-severe IBS underwent EGD (Figure 2B).
Figure 2.
Diagnostic approach. (A) Mean percentage of new patients with chronic, unexplained, moderate-to-severe symptoms unresponsive to over-the-counter medications who undergo these procedures within the first 6 months of their initial appointment. (B) Mean percentage of patients diagnosed with each condition who undergo EGD at any point during their care. EGD, esophagogastroduodenoscopy; FD, functional dyspepsia; GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
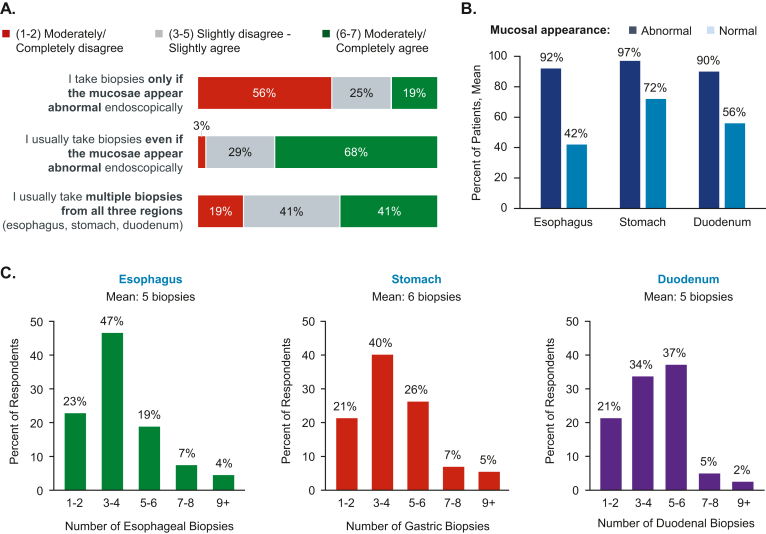
Most respondents agreed (score 6–7 of 7) that they would perform a biopsy during EGD even if mucosae appeared normal (68%), and 41% agreed that they would biopsy 3 regions (esophagus, stomach, and duodenum; Figure 3A). Respondents indicated that they would biopsy most of the time (on average, 92%, 97%, and 90% in esophagus, stomach, and duodenum, respectively) when mucosal abnormalities were identified, but less so (42%, 72%, and 56%) in the absence of mucosal abnormalities (Figure 3B). The mean numbers of biopsies taken from esophagus, stomach, and duodenum were 5, 6, and 5, respectively (Figure 3C).
Figure 3.
Biopsy collection. (A) Percentage of respondents reporting levels of agreement with statements related to collection of biopsies during EGD, rated on a scale of 1 (completely disagree) to 7 (completely agree). (B) Mean percentage of patients from whom respondents collect esophageal, stomach, and duodenum biopsies based on mucosal appearance. (C) Percentage of respondents who report collecting each number of biopsies from the esophagus, stomach, and duodenum; the survey question required respondents to enter numerical, open-ended responses, which were grouped post hoc into the ranges shown. EGD, esophagogastroduodenoscopy.
Counting Eosinophils in Gastric and Duodenal Biopsies
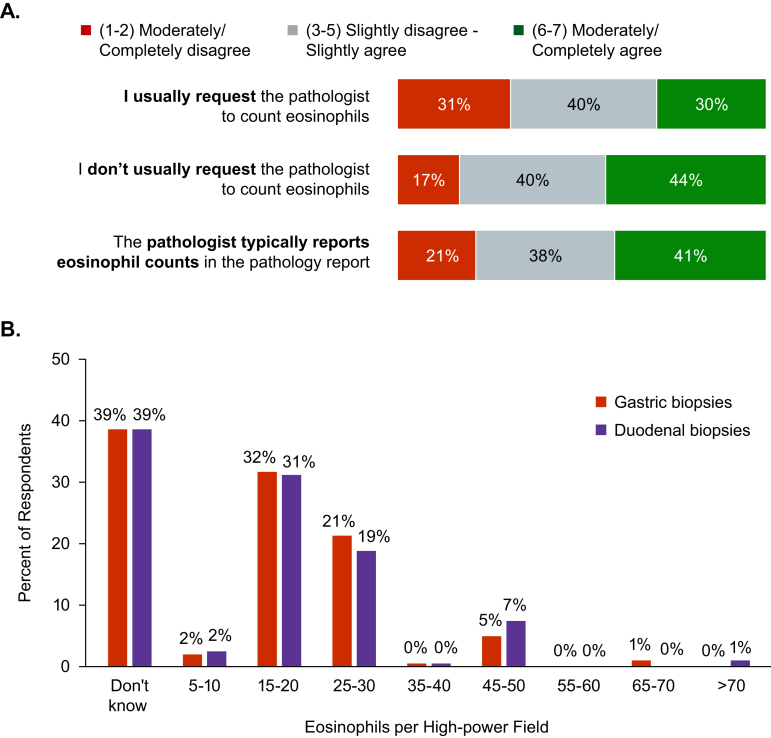
Only 30% of respondents agreed (score 6–7 of 7) that they typically ask pathologists to count eosinophils in gastric and duodenal biopsies, whereas 44% agreed that they do not typically request eosinophil counts. Only 41% of respondents agreed that their pathologist typically reports gastric and duodenal eosinophil counts (Figure 4A), and 39% did not know the threshold number of gastric or duodenal eosinophil counts that they would use as the histologic threshold for diagnosis of EoG/EoD (Figure 4B). Sixteen percent of respondents reported using the published threshold of 30 eos/hpf in the stomach and 13% used that threshold in the duodenum to diagnose EoG/EoD19; other responses ranged from 15 to 20 eos/hpf (Figure 4B).
Figure 4.
Counting eosinophils in gastric and duodenal biopsies. (A) Percentage of respondents reporting levels of agreement with statements related to pathologists’ counting of eosinophils in gastric and duodenal biopsy specimens, rated on a scale of 1 (completely disagree) to 7 (completely agree). (B) Percentages of respondents reporting number of eosinophils per high-power field in biopsies that they use as thresholds for diagnosis of EoG/EoD; the question required respondents to enter numerical, open-ended responses, which were grouped post hoc into the ranges shown. EoG/EoD, eosinophilic gastritis and/or eosinophilic duodenitis.
Respondents reported using pathology laboratories at academic medical centers (23%), private or group practices or community hospitals (58%), or commercial pathology laboratories (19%) (Table 1). Responses to questions related to eosinophil counting in gastric and duodenal biopsies were similar, regardless of whether respondents used academic, commercial, or other types of pathology laboratories (Supplements 3 and 4). The exception was that a higher proportion of respondents who used academic pathology laboratories agreed that they usually ask pathologists to count eosinophils in gastric and duodenal biopsy specimens (45.6% vs 25.0% of respondents who use nonacademic pathology laboratories; P = .020; Supplement 3).
Perceptions of EoG/EoD
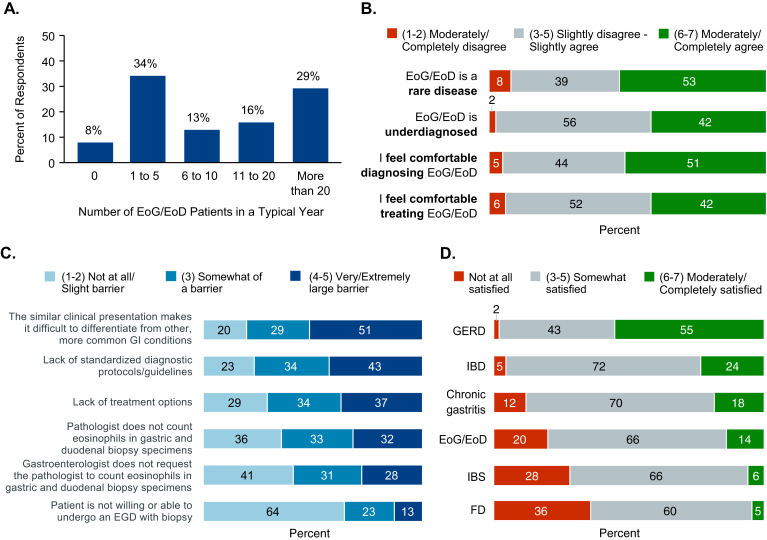
The number of patients with EoG/EoD treated annually by respondents varied, but 55% reported treating 10 or fewer (Figure 5A). Respondents were shown a series of statements about EoG/EoD and asked to rate their level of agreement with each. Although 53% agreed (score 6–7 of 7) with the statement that EoG/EoD is a rare disease, 42% had the same level of agreement that EoG/EoD is underdiagnosed (Figure 5B); 51% agreed that they feel comfortable diagnosing EoG/EoD, and 42% agreed that they feel comfortable treating it (Figure 5B). Response concordance was high, as 73% of respondents had similar levels of comfort for diagnosing and treating EoG/EoD (Supplement 5).
Figure 5.
Diagnosing and managing patients with EoG/EoD. (A) Percentages of respondents reporting the number of patients with EoG/EoD that they typically treat in a 12-month period. (B) Percentages of respondents reporting levels of agreement with statements about diagnosis and treatment of EoG/EoD on a scale of 1 (completely disagree) to 7 (completely agree). (C) Percentages of respondents ranking their perception of potential barriers related to the diagnosis of EoG/EoD on a scale of 1 (not at all a barrier) to 5 (extremely large barrier). (D) Percentage of respondents reporting their level of satisfaction with treatment options for gastrointestinal disorders on a scale of 1 (not at all satisfied) to 7 (extremely satisfied). EoG/EoD, eosinophilic gastritis and/or eosinophilic duodenitis; FD, functional dyspepsia; GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
The highest perceived barrier to diagnosing EoG/EoD was that the nonspecific presentation makes it difficult to differentiate from other more common GI disorders; 51% of respondents rated this as a very large or extremely large barrier (score 4–5 of 5; Figure 5C). Lack of standardized diagnostic guidelines and lack of treatment options were rated as barriers by 43% and 37% of respondents, respectively. Statements related to pathologists not counting eosinophils in biopsies and gastroenterologists not requesting eosinophil counts were rated as large or extremely larger barriers by 32% and 28% of respondents, respectively. Patient unwillingness or inability to undergo EGD with biopsy was perceived to be the lowest barrier—64% of respondents stated that it is not at all or only a slight barrier (score 1–2 of 5). Overall satisfaction with treatment options was lower for EoG/EoD, FD, and IBS (36%, 28%, and 20% of respondents indicated low/no satisfaction) than for other diagnoses, such as GERD (55% were extremely satisfied; Figure 5D).
Subgroup Comparisons
We compared differences in diagnosis and treatment patterns between respondents who practice in academic and nonacademic settings (such as community hospitals or private/group practices). Academic respondents were more likely to practice in an urban setting (75.0% vs 35.6% of nonacademic respondents; P < .001), practice in the Northeast region of the United States (47.5% vs 25.3% of nonacademic respondents; P = .041), and use an academic pathology laboratory (95.0% vs 4.9% of nonacademic respondents; P < .001). Other demographic characteristics, including volumes of EoG/EoD patients, were similar between academic and nonacademic respondents (Supplement 6). Respondents who practice in academic settings were more likely to agree (score 6–7 of 7) that they usually ask pathologists to count eosinophils in gastric and duodenal biopsies (45.0% vs 25.9% of nonacademic respondents; P = .048) and were more likely to agree that EoG/EoD is underdiagnosed (47.5% vs 40.1% of nonacademic respondents; P = .037; Supplement 7).
We compared patterns between respondents who treat high volumes of patients with EoG/EoD (>20 patients/y) and those whose patient volumes are low (0–20 patients/y). Although the groups had no demographic differences (Supplement 8), they did differ in their reported behaviors and attitudes. A significantly higher percentage of respondents with a high volume of EoG/EoD patients agreed that they collect biopsies from all 3 areas of the GI tract during EGD (52.5% vs 35.7% with typical patient volumes; P = .039) and collect significantly more duodenal biopsies (mean 7.0 biopsies vs 4.7 biopsies by respondents with lower volumes; P = .035; Supplement 9). A significantly larger percentage of respondents with a high EoG/EoD patient volume agreed that they usually ask pathologists to count eosinophils in gastric and duodenal biopsies (45.8% vs 23.1% with typical volumes; P = .002) and that their pathologists typically report eosinophil counts (57.6% vs 34.3% with lower volumes; P = .008; Supplement 9). Additionally, significantly higher percentages of respondents with high volumes of EoG/EoD patients agreed that EoG/EoD is underdiagnosed (54.2% vs 36.4% with typical volumes; P = .011), that they feel comfortable diagnosing EoG/EoD (72.9% vs 42.0% with typical volumes; P = .001), and that they feel comfortable treating EoG/EoD (59.3% vs 34.3% with typical volumes; P = .003; Supplement 9).
Discussion
We used a cross-sectional survey of US gastroenterologists to study evaluation patterns of adults with chronic, unexplained, moderate-to-severe GI symptoms and identify factors that might contribute to underdiagnosis of EoG/EoD. Patients with EoG/EoD made up <2% of respondents’ patient volume, whereas IBS, FD, other FGIDs, GERD, and chronic gastritis accounted for 63%—some of these patients might have undetected EoG/EoD.3,4 We found that the largest perceived barrier to diagnosis of EoG/EoD was its nonspecific clinical presentation. Symptoms of EoG/EoD (abdominal pain, diarrhea, and early satiety) overlap with those of other GI disorders, and many patients do not have signs such as peripheral eosinophilia.3,22,23
Survey respondents took different approaches to treating patients who present with chronic, unexplained moderate-to-severe GI symptoms and are not responsive to over-the-counter medications. Within the first 6 months, most patients received empiric prescription-strength medications and underwent noninvasive diagnostic tests or endoscopic procedures. Gastroenterologists, therefore, spend considerable time and resources trying to identify causes of patients’ symptoms and relieve them. EGD was used for 78% of patients with moderate-to-severe FD and 85% of patients with GERD but only 42% of patients with moderate-to-severe IBS. Presumably, this discrepancy is because FD and GERD are classified as gastroduodenal and esophageal disorders, whereas IBS is classified as a bowel disorder,24 despite having overlapping symptoms.25 In patients with abdominal pain and diarrhea or constipation, or with suspected IBS, thorough assessment of upper GI symptoms, with EGD and biopsies when appropriate, can optimize evaluations.
Although respondents reported collecting biopsies from stomach and duodenum in ≥90% of patients with abnormal-appearing mucosae, biopsies were collected from only 42% to 72% of patients with normal-appearing mucosae. Unlike patients with IBD, many patients with EoG/EoD have normal-appearing mucosae or only minor abnormalities observed during EGD3,17,26; tissue eosinophilia can be accurately detected only by high-power magnification of tissue specimens.4,27,28 Eosinophils are patchy in gastric and duodenal tissues, and eosinophil densities vary among biopsies and within the same specimens among hpfs. Detection of EoG/EoD requires the examination of eosinophils in hpfs of at least 8 biopsies from the stomach and 4 from the duodenum.17 It is common for guidelines to require multiple biopsies to detect disease; for example, guidelines recommend collection of 5 gastric biopsies for detection of Helicobacter pylori infection29 and 5–6 duodenal biopsies for celiac disease.30 We found high variation in reported biopsy collection—on average, respondents collected 6 gastric and 5 duodenal biopsies, but approximately 20% reported collecting only 1–2 biopsies from each site of the GI tract. Systematic collection of multiple biopsies from the stomach and duodenum, even from normal-appearing mucosae, could improve detection rates of EoG/EoD.19
Seventeen percent of gastroenterologists reported that they do not routinely ask pathologists to count eosinophils in gastric and duodenal biopsies and assume that pathologists will report eosinophil counts if they are abnormal, despite a lack of standard for normal levels. Approximately 36% of gastroenterologists stated that pathologists not counting eosinophils were not a barrier to diagnosis of EoG/EoD. Nearly 40% of respondents stated that they did not know what tissue eosinophil counts are considered abnormal and are required for diagnosis of EoG/EoD. Most gastroenterologists, therefore, defer to and trust their pathologist to evaluate eosinophils and report eosinophilia. Analysis of the gastroenterologist subgroup with a high volume of EoG/EoD patients (>20 patients/year) offered insights into ways to increase detection and diagnosis of EoG/EoD in practice. These gastroenterologists were significantly more likely than others to collect biopsies from all 3 regions (esophagus, stomach, and duodenum) and to collect significantly more duodenal biopsies during EGD. Additionally, they were more likely to ask pathologists to count gastric and duodenal eosinophils and to receive counts in pathology reports. Recent studies of patients with EoG/EoD indicated that systematic collection of multiple gastric and duodenal biopsies followed by systematic counting and reporting of eosinophils in biopsy specimens increases diagnostic yield of EoG/EoD.17,19,20,31 Findings from our study appear to support this concept.
Although steps were taken to minimize bias in this study such as validating survey questions with 3 independent gastroenterologists and varying question formats, limitations common to all survey research should be taken into consideration. This study had a low response rate of 3% (284 screening questions answered out of 10,416 gastroenterologists surveyed), resulting in a moderate sample size (n = 202), suggesting possible response bias. Additionally, a few survey questions asked for self-reported approximations of patient volumes and percentages, which may be misrepresented owing to improper recall and social desirability.
Many respondents perceived EoG/EoD to be rare, but many also believed that these disorders are underdiagnosed. Although these beliefs appear contradictory, they make sense in the context of gastroenterologists’ experience in diagnosis of EoE over the last 2 decades. Although EoE is still considered a rare or orphan disease, diagnoses of EoE have increased substantially with heightened awareness and publication of diagnostic guidelines.12 Our respondents might anticipate a similar pattern for EoG/EoD—lack of diagnostic guidelines was perceived to be one of the top barriers to diagnosis, along with lack of treatment options (respondents reported a low level of satisfaction with available treatments for EoG/EoD). Although respondents expressed relatively high satisfaction for IBD and GERD treatments, satisfaction was lowest for IBS and FD treatments. Detecting eosinophil-mediated inflammation and providing patients with proper diagnoses are first steps toward effective treatment.
Conclusion
Diagnosis of EoG/EoD should be relatively straightforward, involving common procedures: EGD with biopsies and histopathologic evaluation of biopsy specimens. However, the specific methods for optimizing detection of EoG/EoD have been described only recently and are not widely implemented in standard practice. Development of diagnostic guidelines with recommendations for systematic biopsy and histopathology protocols is expected to greatly improve detection of EoG/EoD and reduce the long diagnostic delays endured by patients. It will also open the door to potential treatment options, such as therapies that target eosinophils and mast cells, important mediators of pathogenesis.
Acknowledgments:
Medical writing and editorial support were funded by Allakos and provided under the direction of the authors by Shina Satoh, MD, and Claire Levine, MS, ELS, of MedThink SciCom.
Authors' Contributions:
Mirna Chehade and Lauren T. Gehman were involved in conceptualizing the project. Jingwen Tan and Lauren T. Gehman oversaw project administration. All authors were involved in the formal analysis. Jingwen Tan wrote the first draft, and all authors reviewed, edited, and approved the final draft for submission.
Footnotes
Conflicts of Interest: These authors disclose the following: MC received consultant fees from Regeneron, Allakos, Adare/Ellodi, Shire/Takeda, AstraZeneca, Sanofi, Bristol Myers Squibb, and Phathom and research funding from Regeneron, Allakos, Shire/Takeda, AstraZeneca, Adare/Ellodi, and Danone. JT is a former employee of Allakos who was employed by Allakos at the time this study was conducted, the data were analyzed, and the manuscript was drafted and reviewed. LTG is an employee of Allakos and owns stocks and stock options.
Funding: The study was funded by Allakos (the sponsor), San Carlos, CA. The sponsor participated in the data analysis and interpretation, preparation, and review of the manuscript.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Data, analytic methods, and study materials will be made available to other researchers via contacting the corresponding author.
Reporting Guidelines: SRQR.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.11.010.
Supplementary Materials
References
- 1.Gonsalves N. Eosinophilic gastrointestinal disorders. Clin Rev Allergy Immunol. 2019;57:272–285. doi: 10.1007/s12016-019-08732-1. [DOI] [PubMed] [Google Scholar]
- 2.Dellon E.S., Gonsalves N., Abonia J.P., et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20:2474–2484.e3. doi: 10.1016/j.cgh.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhmoud T., Hanson J.A., Parasher G. Eosinophilic gastroenteritis: an underdiagnosed condition. Dig Dis Sci. 2016;61:2585–2592. doi: 10.1007/s10620-016-4203-5. [DOI] [PubMed] [Google Scholar]
- 4.Abassa K.K., Lin X.Y., Xuan J.Y., et al. Diagnosis of eosinophilic gastroenteritis is easily missed. World J Gastroenterol. 2017;23:3556–3564. doi: 10.3748/wjg.v23.i19.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker M.M., Potter M., Talley N.J. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol Hepatol. 2018;3:271–280. doi: 10.1016/S2468-1253(18)30005-0. [DOI] [PubMed] [Google Scholar]
- 6.Mansoor E., Saleh M.A., Cooper G.S. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15:1733–1741. doi: 10.1016/j.cgh.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Jensen E.T., Martin C.F., Kappelman M.D., et al. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J Pediatr Gastroenterol Nutr. 2016;62:36–42. doi: 10.1097/MPG.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chehade M., Kamboj A.P., Atkins D., et al. Diagnostic delay in patients with eosinophilic gastritis and/or duodenitis: a population-based study. J Allergy Clin Immunol Pract. 2021;9:2050–2059.e20. doi: 10.1016/j.jaip.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Chehade M., Gehman L., Kamboj A., et al. A longitudinal, population-based study of the difficult journey to diagnosis endured by patients with eosinophilic gastritis and eosinophilic gastroenteritis. J Allergy Clin Immunol. 2020;145:AB40. [Google Scholar]
- 10.Bedell A., Taft T., Craven M.R., et al. Impact on health-related quality of life in adults with eosinophilic gastritis and gastroenteritis: a qualitative assessment. Dig Dis Sci. 2018;63:1148–1157. doi: 10.1007/s10620-018-4978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiremath G., Kodroff E., Strobel M.J., et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol. 2018;42:483–493. doi: 10.1016/j.clinre.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellon E.S., Liacouras C.A., Molina-Infante J., et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022–1033.e10. doi: 10.1053/j.gastro.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoda T., Wen T., Caldwell J.M., et al. Molecular, endoscopic, histologic, and circulating biomarker-based diagnosis of eosinophilic gastritis: multi-site study. J Allergy Clin Immunol. 2020;145:255–269. doi: 10.1016/j.jaci.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genta R.M., Sonnenberg A., Turner K. Quantification of the duodenal eosinophil content in adults: a necessary step for an evidence-based diagnosis of duodenal eosinophilia. Aliment Pharmacol Ther. 2018;47:1143–1150. doi: 10.1111/apt.14558. [DOI] [PubMed] [Google Scholar]
- 15.Joo J.Y., Cho J.M., Yoo I.H., et al. Eosinophilic gastroenteritis as a cause of non-Helicobacter pylori, non-gastrotoxic drug ulcers in children. BMC Gastroenterol. 2020;20:280. doi: 10.1186/s12876-020-01416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egritas Gurkan O., Ozturk H., Karagol H.I.E., et al. Primary eosinophilic gastrointestinal diseases beyond eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr. 2021;72:294–299. doi: 10.1097/MPG.0000000000002925. [DOI] [PubMed] [Google Scholar]
- 17.Dellon E.S., Gonsalves N., Rothenberg M.E., et al. Determination of biopsy yield that optimally detects eosinophilic gastritis and/or duodenitis in a randomized trial of lirentelimab. Clin Gastroenterol Hepatol. 2022;20:535–545.e15. doi: 10.1016/j.cgh.2021.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed C., Woosley J.T., Dellon E.S. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis. 2015;47(3):197–201. doi: 10.1016/j.dld.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed C.C., Genta R.M., Youngblood B.A., et al. Mast cell and eosinophil counts in gastric and duodenal biopsy specimens from patients with and without eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2021;19:2102–2111. doi: 10.1016/j.cgh.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dellon E.S., Peterson K.A., Murray J.A., et al. Histologic and symptomatic improvement across multiple forms of eosinophilic gastrointestinal diseases in ENIGMA, a randomized, double-blind, placebo-controlled trial of antolimab (AK002) [AGA abstract 932] Gastroenterology. 2020;158:S186. [Google Scholar]
- 21.Dellon E.S., Peterson K.A., Murray J.A., et al. Anti–Siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020;383:1624–1634. doi: 10.1056/NEJMoa2012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldwell J.M., Collins M.H., Stucke E.M., et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol. 2014;134:1114–1124. doi: 10.1016/j.jaci.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko H.M., Morotti R.A., Yershov O., et al. Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol. 2014;109:1277–1285. doi: 10.1038/ajg.2014.166. [DOI] [PubMed] [Google Scholar]
- 24.Drossman D.A. Functional gastrointestinal disorders: What's new for Rome IV? Lancet Gastroenterol Hepatol. 2016;1:6–8. doi: 10.1016/S2468-1253(16)30022-X. [DOI] [PubMed] [Google Scholar]
- 25.Talley N.J., Dennis E.H., Schettler-Duncan V.A., et al. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454–2459. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 26.Grandinetti T., Biedermann L., Bussmann C., et al. Eosinophilic gastroenteritis: clinical manifestation, natural course, and evaluation of treatment with corticosteroids and vedolizumab. Dig Dis Sci. 2019;64:2231–2241. doi: 10.1007/s10620-019-05617-3. [DOI] [PubMed] [Google Scholar]
- 27.Ashitani K., Tsuzuki Y., Yamaoka M., et al. Endoscopic features and diagnostic procedures of eosinophilic gastroenteritis. Intern Med. 2019;58:2167–2171. doi: 10.2169/internalmedicine.2298-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X.X., Guan D.X., Zhou J., et al. Clinical analysis of eosinophilic gastroenteritis in 71 children. Zhounghua Er Ke Za Zhi. 2018;56:500–504. doi: 10.3760/cma.j.issn.0578-1310.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y.X., Brill J., Krishnan P., et al. American Gastroenterological Association Institute guideline on the role of upper gastrointestinal biopsy to evaluate dyspepsia in the adult patient in the absence of visible mucosal lesions. Gastroenterology. 2015;149:1082–1087. doi: 10.1053/j.gastro.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Rubio-Tapia A., Talley N.J., Gurudu S.R., et al. Gluten-free diet and steroid treatment are effective therapy for most patients with collagenous sprue. Clin Gastroenterol Hepatol. 2010;8:344–349.e3. doi: 10.1016/j.cgh.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lwin T., Melton S.D., Genta R.M. Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol. 2011;24:556–563. doi: 10.1038/modpathol.2010.221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.