Abstract
Background
There has been a significant increase in the utilization of venoarterial extracorporeal membrane oxygenation (VA-ECMO) in recent years. Cardiothoracic surgery teams have historically led VA-ECMO care teams, with little data available on alternative care models.
Methods
We performed a retrospective review of a cardiovascular medicine inclusive VA-ECMO service, analyzing patients treated with peripheral VA-ECMO at a large quaternary care center from 2018 to 2022. The primary outcome was death while on VA-ECMO or within 24 hours of decannulation. Univariate and multivariate analyses were used to identify predictors of the primary outcome.
Results
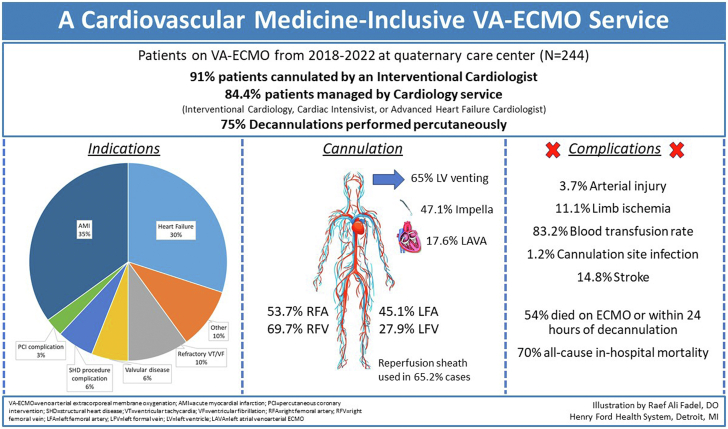
Two hundred forty-four patients were included in the analysis (median age 61 years; 28.7% female), of whom 91.8% were cannulated by interventional cardiologists, and 84.4% were managed by a cardiology service comprised of interventional cardiologists, cardiac intensivists or advanced heart failure cardiologists. Indications for VA-ECMO included acute myocardial infarction (34.8%), decompensated heart failure (30.3%), and refractory cardiac arrest (10.2%). VA-ECMO was utilized during cardiopulmonary resuscitation in 26.6% of cases, 48% of which were peri-procedural arrest. Of the patients, 46% survived to decannulation, the majority of whom were decannulated percutaneously in the cardiac catheterization laboratory. There was no difference in survival following cannulation by a cardiac surgeon vs interventional cardiologist (50% vs 45%; P = .90). Complications included arterial injury (3.7%), compartment syndrome (4.1%), cannulation site infection (1.2%), stroke (14.8%), acute kidney injury (52.5%), access site bleeding (16%) and need for blood transfusion (83.2%). Elevated baseline lactate (odds ratio [OR], 1.13 per unit increase) and sequential organ failure assessment score (OR, 1.27 per unit increase) were independently associated with the primary outcome. Conversely, an elevated baseline survival after VA ECMO score (OR, 0.92 per unit increase) and 8-hour serum lactate clearance (OR, 0.98 per % increase) were independently associated with survival.
Conclusions
The use of a cardiovascular medicine inclusive ECMO service is feasible and may be practical in select centers as indications for VA-ECMO expand.
Keywords: cardiogenic shock, extracorporeal membrane oxygenation, mechanical circulatory support
Introduction
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides temporary cardiopulmonary support to patients with circulatory collapse and is utilized both as a bridge to definitive therapy and recovery. There has been a significant increase in the utilization of ECMO over the past decade.1 Sauer et al2 reported a 422% increase in the use of ECMO from 2006 to 2011 in the United States whereas Becher et al3 reported a similar 30-fold increase from 2007 to 2015 in Germany.
The clinical utility of ECMO has expanded well beyond the operating room. ECMO is commonly utilized in the management of patients with cardiogenic shock, including those with acute myocardial infarction (AMI), cardiac arrest (CA), decompensated heart failure, and pulmonary embolism.4, 5, 6, 7 With the growing indications and use of ECMO, health care systems may benefit by diversifying the clinicians providing ECMO care including granting privileges to cardiovascular-medicine physicians to implant, manage, and explant ECMO.
Our report highlights the experience of a cardiovascular medicine inclusive VA-ECMO service within a larger comprehensive acute mechanical circulatory support (MCS) program. Since the inclusion of cardiologists in our ECMO program in 2017, our program has seen steady growth which can serve as a blueprint for select centers.
Methods
Study design and population
We performed a retrospective review of patients admitted to a quaternary care center from January 2018 through September 2022. All patients admitted to the cardiac intensive care unit on an ECMO circuit were reviewed for inclusion in this analysis. Patients were included if they were ≥18 years old and required peripheral VA-ECMO. Patients were excluded if ECMO cannulation occurred at an outside hospital, if ECMO cannulation occurred via central access, if patients died before being admitted to the cardiac intensive care unit, or if there were missing data regarding the indication for ECMO, procedural details, or outcomes.
Data collection and missing data
All data were gathered through chart review and manual abstraction from the electronic health record. Baseline demographics, comorbidities, severity scores, and outcomes were evaluated by the research team and adjudicated by study investigators. Information on ECMO cannulation, including indication, procedural details, and complications, was manually abstracted from procedural documentation and follow-up.
Multiple imputation was used for specific missing variables and values in order to calculate baseline survival after VA ECMO (SAVE) and sequential organ failure assessment (SOFA) scores, as well as to calculate 8-hour lactate clearance. The following laboratory values required imputation for missing data points: baseline creatinine (2/244 patients), baseline bicarbonate (2/244 patients), baseline bilirubin (21/244 patients), baseline platelet count (4/244 patients), baseline serum lactate (33/244 patients), post-ECMO cannulation 0-hour (12/244 patients) and 8-hour (28/244 patients) lactate levels.
Study definitions and calculations
The primary end point of “ECMO death” was defined as death while on ECMO or within 24 hours of decannulation. This end point allowed better characterization of ECMO management characteristics including ECMO decannulation. Secondary end points included inpatient death, defined as any death occurring during the index hospitalization. Death postdischarge was defined as death from any cause after discharge from the index hospitalization. Acute kidney injury (AKI), defined by the Kidney Disease Improving Global Outcomes 2012 guidelines, based on creatinine elevation and a reduction in urine output, was recorded as mL/kg/h. Thrombocytopenia was defined as a drop in platelets by ≥50% of baseline or to a nadir of <100,000/μL. ECMO days were calculated from the day of cannulation to decannulation or death. Hospital length of stay was calculated as time from index admission to discharge or death.
Statistical analysis
Continuous variables were described by medians (with interquartile ranges) and categorical variables by frequency rates and percentages. The Mann-Whitney U test or t test was used for continuous variables, whereas χ2 or Fisher exact tests were used for categorical variables. A univariate analysis was performed to determine the association of underlying risk factors with the primary outcome of ECMO death. A multivariate logistic regression analysis was then performed with ECMO death as the dependent variable. The risk factors chosen for the model were based on association with the dependent variable by univariate analysis with a P < .05 on 2-sided alpha testing. The adjusted odds ratio (OR) and CI were reported for each risk factor. Kaplan-Meier analysis was used to analyze 30-day mortality depending on the indication for VA-ECMO. SPSS Statistics software (IBM) was used for imputation of missing variables and all statistical analysis.
Results
Baseline demographics, indications, medical history
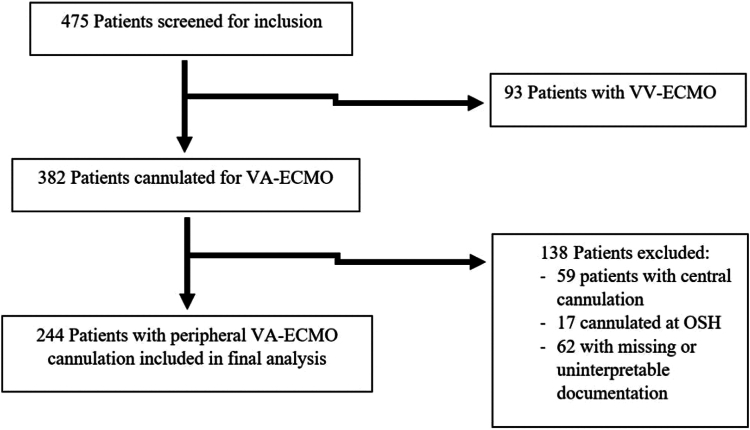
A total of 475 patients were screened for inclusion and 244 patients were included in the final analysis, 132 (54%) patients died while on ECMO, and 112 (46%) survived 24 hours from decannulation. The primary reasons for exclusion were central ECMO cannulation and missing or incomplete data. Figure 1 includes the information on patient screening, inclusion, and exclusion.
Figure 1.
Patient screening and inclusion. OSH, outside hospital; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VV-ECMO, veno-venous extracorporeal membrane oxygenation.
Our patient cohort comprised 71.3% males and 58.2% White patients, with a median age of 61 years (IQR, 49.8-69.0) and a BMI of 26.7 kg/m2 (IQR, 23.1-31.4). Indications for ECMO included AMI (34.8%), heart failure (HF) (30.3%), refractory ventricular tachycardia (VT)/ventricular fibrillation (VF) (10.2%), valvular disease (7.0%), structural heart disease complication (5.7%), and complication from a heart catheterization (4.9%). Patients frequently experienced CA (52%), with a median cardiopulmonary resuscitation time of 20 minutes (IQR, 10-30). ECMO was utilized for cardiopulmonary resuscitation in 26.6% of patients, including 9.4% for primary CA, and 12.7% for periprocedural arrest. A total of 224 (91.8%) patients were cannulated by an interventional cardiologist and 206 (84.4%) patients were managed by a primary cardiology service.
The most prevalent comorbidities included hypertension (70.1%), diabetes mellitus (35.7%), chronic kidney disease (25.8%), and prior myocardial infarction (25%), with 20.9% having received prior percutaneous coronary intervention and 13.1% having received prior CABG. Additionally, 25.4% of patients had a history of arrhythmia (7.0% VT/VF, 18.4% atrial fibrillation, 2.9% sick sinus syndrome/complete heart block), and 13.1% had an implantable cardioverter defibrillator implanted prior to the index hospitalization. Patients were frequently transferred from another health care facility (53.7%) and 15.6% had some form of MCS prior to ECMO cannulation (3.7% intraaortic balloon pump, 9.8% Impella, 0.4% veno-venous extracorporeal membrane oxygenation, 1.6% durable left ventricular assist device). Table 1 includes baseline demographics, indications for ECMO, and past medical history of the cohort. Supplemental Tables S1 and S2 expand on the indications for ECMO and the use of ECMO in the peri-arrest setting, respectively.
Table 1.
Baseline demographics, ECMO indication, and medical history.
| Variable | Overall (N = 244) | ECMO death (n = 132) | Survival to decannulation (n = 112) | P value |
|---|---|---|---|---|
| Baseline demographics | ||||
| Age, y | 61.0 (49.8-69.0) | 62.0 (53.5-69.3) | 58.0 (47.8-68.0) | .139 |
| Body mass index, kg/m2 | 26.7 (23.1-31.4) | 27.1 (23.3-31.7) | 26.5 (22.9-30.8) | .661 |
| Male sex | 174.0 (71.3) | 97.0 (73.5) | 77.0 (68.8) | .417 |
| Ethnicity | .088 | |||
| White | 142.0 (58.2) | 72.0 (54.5) | 70.0 (62.5) | |
| Black | 71.0 (29.1) | 39.0 (29.5) | 32.0 (28.6) | |
| Latino | 3.0 (1.2) | 2.0 (1.5) | 1.0 (0.9) | |
| Other | 28.0 (11.5) | 19.0 (14.4) | 9.0 (8.0) | |
| Index hospitalization | ||||
| Outside hospital transfer | 131.0 (53.7) | 73.0 (55.3) | 58.0 (51.8) | .585 |
| ECMO indicationb | .356 | |||
| Acute myocardial infarction | 85 (35.0) | 48 (36.4) | 37 (33.0) | |
| Heart failure | 74 (30.3) | 42 (31.8) | 32 (28.6) | |
| Refractory VT/VF | 25 (10.2) | 12 (9.09) | 13 (11.6) | |
| Acute valvular disease | 15 (6.1) | 4 (3.0) | 11 (9.8) | |
| SHD procedure complication | 15 (6.1) | 8 (6.1) | 7 (6.3) | |
| PCI/LHC complication | 6 (2.5) | 3 (2.3) | 3 (2.7) | |
| ECMO implanter | .910 | |||
| Interventional cardiologist | 222.0 (91.0) | 121 (91.7) | 101 (90.2) | |
| Cardiac surgeon | 22.0 (9.0) | 11 (8.3) | 11 (9.8) | |
| Primary management team | .638 | |||
| Interventional cardiology | 166.0 (68.0) | 94.0 (71.2) | 72.0 (64.3) | |
| Heart failure | 40.0 (16.4) | 16.0 (12.1) | 24.0 (21.4) | |
| Cardiac surgery/anesthesia | 38.0 (15.6) | 22.0 (16.7) | 16.0 (14.3) | |
| Cardiac arrest | 127.0 (52.0) | 82.0 (62.1) | 45.0 (40.2) | .001d |
| Arrest site | .002d | |||
| Out-of-hospital | 16.0 (6.6) | 11.0 (8.3) | 5.0 (4.5) | |
| Emergency department | 4.0 (1.6) | 3.0 (2.3) | 1.0 (0.9) | |
| In-hospital | 107.0 (43.9) | 68.0 (51.5) | 39.0 (34.8) | |
| Arrest rhythm | <.001d | |||
| VF | 35.0 (14.3) | 21.0 (15.9) | 14.0 (12.5) | |
| VT | 13.0 (5.3) | 7.0 (5.3) | 6.0 (5.4) | |
| Pulseless electrical activity | 67.0 (27.5) | 48.0 (36.4) | 19.0 (17.0) | |
| Asystole | 9.0 (3.7) | 5.0 (3.8) | 4.0 (3.6) | |
| Total CPR time, min | 20.0 (10.0-30.0) | 20.0 (10.0-40.0) | 15.0 (10.0-25.0) | .043d |
| ECPRc | 65.0 (26.6) | 44.0 (33.3) | 21.0 (18.8) | .010d |
| Primary cardiac arrest | 23.0 (9.4) | 18.0 (13.6) | 5.0 (4.5) | |
| Procedural complication | 31.0 (12.7) | 16.0 (12.1) | 15.0 (13.4) | |
| Medical history | ||||
| Hypertension | 171.0 (70.1) | 90.0 (68.2) | 81.0 (72.3) | .484 |
| Diabetes mellitus | 87.0 (35.7) | 46.0 (34.8) | 41.0 (36.6) | .776 |
| COPD | 23.0 (9.4) | 12.0 (9.1) | 11.0 (9.8) | .846 |
| On home oxygen | 9.0 (3.7) | 8.0 (6.1) | 1.0 (0.9) | .033d |
| Cirrhosis | 7.0 (2.9) | 4.0 (3.0) | 3.0 (2.7) | .870 |
| Chronic kidney disease | 63.0 (25.8) | 32.0 (24.2) | 31.0 (27.7) | .543 |
| On dialysis | 9.0 (3.7) | 3.0 (2.3) | 6.0 (5.4) | .204 |
| Venous thromboembolism | 26.0 (10.7) | 13.0 (9.8) | 13.0 (11.6) | .659 |
| Tobacco use | 85.0 (34.8) | 46.0 (34.8) | 39.0 (34.8) | .996 |
| Coronary artery disease | 109.0 (44.7) | 58.0 (43.9) | 51.0 (45.5) | .804 |
| Prior MI | 61.0 (25.0) | 34.0 (25.8) | 27.0 (24.1) | .768 |
| Prior PCI | 51.0 (20.9) | 26.0 (19.7) | 25.0 (22.3) | .617 |
| Prior CABG | 32.0 (13.1) | 17.0 (12.9) | 15.0 (13.4) | .906 |
| Arrhythmia | 62.0 (25.4) | 29.0 (22.0) | 33.0 (29.5) | .182 |
| VT/VF | 17.0 (7.0) | |||
| Atrial fibrillation | 45.0 (18.4) | |||
| SSS/CHB | 7.0 (2.9) | |||
| ICD implanted | 32.0 (13.1) | 17.0 (12.9) | 15.0 (13.4) | 0.906 |
| MCS prior to index eventa | 38.0 (15.6) | 24.0 (18.2) | 14.0 (12.5) | 0.224 |
| Intraaortic balloon pump | 9.0 (3.7) | |||
| Impella | 24.0 (9.8) | |||
| VV-ECMO | 1.0 (0.4) | |||
| Left ventricular assist device | 4.0 (1.6) |
Values are median (IQR) or n (%).
BP, blood pressure; CABG, coronary artery bypass graft; CHB, complete heart block; CICU, cardiac intensive care unit; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; DM, diabetes mellitus; ECPR, extracorporeal membrane oxygenation (ECMO) cardiopulmonary resuscitation; HF, heart failure; ICD, implantable cardioverter defibrillator; MCS, mechanical support; MI, myocardial infarction; PCI, percutaneous coronary intervention; SHD, structural heart disease; SICU, surgical intensive care unit; SSS, sick sinus syndrome; VF, ventricular fibrillation; VT, ventricular tachycardia; VV-ECMO, veno-venous extracorporeal membrane oxygenation.
Highest recorded value prior to VA ECMO cannulation.
See Supplemental Table S1 for the full list of indications.
See Supplemental Table S2 for the full list of ECPR indications.
Significant P values.
Pre-ECMO laboratory values, hemodynamics, and severity scores
Prior to ECMO cannulation, baseline laboratory markers and hemodynamic parameters were evaluated. Patients frequently presented with AKI prior to ECMO (58.2%), with a median creatinine of 1.7 mg/dL (IQR, 1.2-2.5). Baseline lactate was elevated at 4.1 mmol/L (IQR, 1.9-9.7). Baseline echocardiogram prior to ECMO cannulation was available in 70.5% of patients with a baseline LVEF of 43% (IQR, 20-60).
Patients were frequently intubated (85.7%), 80.3% required vasopressors, with 46.3% of patients requiring 2 or more vasopressors, and 29.9% required inotropes.
The median SAVE score prior to VA-ECMO was 0.0 (IQR, –4.0 to 3.0), which correlates to predicted in-hospital mortality of 58%. Similarly, median baseline SOFA score was 13.0 (IQR, 10.0-16.0) which correlates to a predicted in-hospital mortality of >95%. Table 2 highlights the pre-VA-ECMO laboratory values, hemodynamic parameters, and severity scores.
Table 2.
Pre-ECMO laboratory and hemodynamic assessment, and severity scores.
| Variable | Overall (N = 244) | ECMO death (n = 132) | Survival to decannulation (n = 112) | P value |
|---|---|---|---|---|
| Pre-ECMO labs | ||||
| Creatinine, mg/dLa,b | 1.7 (1.2-2.5) | 1.8 (1.2-2.5) | 1.6 (1.1-2.5) | .933 |
| AKI prior to VA ECMO | 142.0 (58.2) | 79.0 (59.8) | 63.0 (56.3) | .572 |
| Bicarbonate, mEq/La | 20.0 (16.0-24.0) | 19.0 (14.5-24.0) | 21.0 (17.0-25.0) | .221 |
| Bilirubin, mg/dL a,b | 1.2 (0.6-2.3) | 1.2 (0.7-2.4) | 1.0 (0.6-2.2) | .109 |
| Lactate, mmol/L a,b | 4.1 (1.9-9.7) | 6.6 (2.8-12.0) | 2.5 (1.6-6.0) | <.001e |
| Platelet count, K/μL a,b | 183.5 (130.0-253.3) | 169.5 (118.5-247.0) | 197.0 (150.0-258.3) | .010e |
| PaO2, mm Hg a,b | 113.0 (78.8-246.0) | 106.5 (69.0-198.0) | 128.0 (84.9-284.0) | .096 |
| Pre-ECMO imaging and hemodynamics | ||||
| Echo prior to ECMO | ||||
| Baseline LVEF, % | 43.0 (20.0-60.0) | 45.0 (22.0-60.0) | 40.5 (20.0-60.0) | .509 |
| MR (mod-severe) | 68.0 (27.9) | 23.0 (17.4) | 45.0 (40.2) | .269 |
| MS (mod-severe) | 11.0 (4.5) | 5.0 (3.8) | 6.0 (5.4) | .727 |
| TR (mod-severe) | 51.0 (20.9) | 26.0 (19.7) | 25.0 (22.3) | .946 |
| AI (mod-severe) | 14.0 (5.7) | 4.0 (3.0) | 10.0 (8.9) | .087 |
| AS (mod-severe) | 24.0 (9.8) | 12.0 (9.1) | 12.0 (10.7) | .951 |
| LA dilation | 69.0 (28.3) | 33.0 (25.0) | 36.0 (32.1) | .557 |
| RV dysfunction | 86.0 (35.2) | 46.0 (34.8) | 40.0 (35.7) | .449 |
| LVED diameter, cm | 5.1 (4.5-6.1) | 4.8 (4.1-6.0) | 5.3 (4.8-6.2) | .101 |
| Estimated PAP, mm Hg | 42.0 (29.0-55.0) | 42.5 (32.8-57.3) | 41.0 (26.0-53.0) | .050e |
| TAPSE, cm | 1.7 (1.3-2.2) | 1.6 (1.2-2.1) | 1.7 (1.4-2.3) | .298 |
| RHC hemodynamics peri-ECMOc | 215.0 (88.1) | — | — | — |
| RA mean, mm Hg | 15.0 (10.0-20.0) | 14.0 (9.0-18.0) | 17.0 (11.3-22.0) | .115 |
| PA systolic, mm Hg | 51.5 (40.3-64.3) | 54.0 (42.0-65.0) | 51.0 (39.0-62.0) | .959 |
| PA diastolic, mm Hg | 25.5 (20.0-30.8) | 25.0 (20.0-32.0) | 26.0 (20.0-30.0) | .785 |
| PA mean, mm Hg | 35.0 (28.3-44.0) | 36.0 (28.0-45.0) | 34.0 (29.0-42.0) | .765 |
| PCWP mean, mm Hg | 24.5 (16.0-30.8) | 24.0 (16.0-30.0) | 25.0 (17.0-31.0) | .801 |
| Mixed venous O2, % | 54.0 (44.0-60.5) | 52.0 (42.0-61.0) | 55.5 (45.8-60.0) | .605 |
| Cardiac output, L/min | 4.0 (3.0-5.1) | 3.8 (3.1-5.5) | 4.4 (2.9-5.1) | .349 |
| Cardiac index, L/min/m2 | 1.8 (1.5-2.3) | 1.7 (1.5-2.5) | 2.0 (1.4-2.3) | .682 |
| Intubated prior to VA ECMO | 209.0 (85.7) | 124.0 (93.9) | 85.0 (75.9) | <.001e |
| PIP prior to VA ECMO, cm H2O | 24.0 (20.0-30.0) | 25.0 (21.0-31.0) | 23.0 (18.3-27.0) | .014e |
| Systolic BP, mm Hga | 90.5 (79.0-107.0) | 87.5 (76.0-105.0) | 93.5 (83.8-109.0) | .050e |
| Diastolic BP, mm Hga | 59.0 (49.0-68.0) | 57.5 (49.0-65.0) | 63.0 (50.8-69.3) | .152 |
| Requiring vasopressor | 196.0 (80.3) | 115.0 (87.1) | 81.0 (72.3) | .004e |
| Number of vasopressors required | <.001e | |||
| 0 | 48.0 (19.7) | 17.0 (12.9) | 31.0 (27.7) | |
| 1 | 78.0 (32.0) | 38.0 (28.8) | 40.0 (35.7) | |
| 2 | 59.0 (24.2) | 38.0 (28.8) | 21.0 (18.8) | |
| 3 | 54.0 (22.1) | 34.0 (25.8) | 20.0 (17.9) | |
| 4 | 5.0 (2.0) | 5.0 (3.8) | 0 (0) | |
| Requiring inotropic support | 73.0 (29.9) | 36.0 (27.3) | 37.0 (33.0) | .329 |
| Dobutamine | 47.0 (19.3) | 26.0 (19.7) | 21.0 (18.8) | |
| Milrinone | 26.0 (10.7) | 10.0 (7.6) | 16.0 (14.3) | |
| Severity scores | ||||
| SAVE scorea,d | 0.0 (–4.0 to 3.0) | –2.0 (–6.0 to 2.0) | 0.5 (–4.0 to 4.3) | <.001e |
| SOFA scorea,d | 13.0 (10.0-16.0) | 15.0 (12.0-17.0) | 12.0 (8.0-14.0) | <.001e |
Values are median (IQR) or n (%).
AI, aortic insufficiency; AKI, acute kidney injury; AS, aortic stenosis; LA, left atrium; LVED, left ventricular end diastolic; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MS, mitral stenosis; PA, pulmonary artery; PaO2, partial pressure of arterial oxygen; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PI, pulmonary insufficiency; RA, right atrium; RHC, right heart catheterization; RV, right ventricle; SaO2, oxygen saturation; SAVE, survival after VA ECMO; SOFA, sequential organ failure assessment; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TS, tricuspid stenosis; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Highest or worst recorded value prior to VA ECMO cannulation.
Data available for baseline creatinine (2/244 patients), baseline bicarbonate (2/244 patients), baseline bilirubin (21/244 patients), baseline platelet count (4/244 patients), baseline serum lactate (33/244 patients), post-ECMO cannulation 0-h (12/244 patients) and 8-h (28/244 patients).
Values obtained prior to, during, or immediately after ECMO cannulation, to guide clinical decision making for device selection lactate levels. Multiple imputation was used for missing variables as highlighted in the methods section.
SAVE and SOFA scores calculated with values that required imputation for missing variables as highlighted in the methods section.
Significant P values.
Procedural characteristics
Patients were frequently treated using a venting strategy (64.7%), 17.6% via left-atrial VA-ECMO cannulation, and 47.1% via additional insertion of an Impella. The majority of patients received femoral vein (97.6%) and femoral artery cannulation (98.8%). Table 3 highlights the technical and procedural characteristics of ECMO cannulation.
Table 3.
VA-ECMO cannulation procedural information.
| Variable | Overall (N = 244) | ECMO death (n = 132) | Survival to decannulation (n = 112) | P value |
|---|---|---|---|---|
| LV venting | 158.0 (64.7) | 79 (59.9) | 79 (70.5) | 0.210 |
| LAVA | 43.0 (17.6) | 15.0 (11.4) | 28.0 (25.0) | 0.005a |
| LV Impella | 115.0 (47.1) | 64.0 (48.5) | 51.0 (45.5) | 0.647 |
| Venous cannula location | 0.592 | |||
| RFV | 170.0 (69.7) | 94.0 (71.2) | 76.0 (67.9) | |
| LFV | 68.0 (27.9) | 35.0 (26.5) | 33.0 (29.5) | |
| RIJV | 6.0 (2.5) | 3.0 (2.3) | 3.0 (2.7) | |
| Arterial cannula location | 0.266 | |||
| RFA | 131.0 (53.7) | 70.0 (53.0) | 61.0 (54.5) | |
| LFA | 110.0 (45.1) | 59.0 (44.7) | 51.0 (45.5) | |
| Axillary | 3.0 (1.2) | 3.0 (2.3) | 0 (0) |
Values are n (%).
LAVA, left-atrial venoarterial; LFA, left femoral artery; LFV, left femoral vein; LV, left ventricle; RFA, right femoral artery; RFV, right femoral vein; RIJV, right internal jugular vein; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Significant P values.
Successful decannulation from ECMO occurred in 115 (47.1%) patients. Of the 115 decannulated, 75% were performed percutaneously with Perclose (55) or Manta (31), whereas 15 (13%) were performed surgically, and 14 (5.7%) cases lacked documentation on closure technique. Pre-Perclose was used in 29 patients. “Dry” closure technique using balloon tamponade from another access occurred in 43 patients. Table 4 highlights details of decannulation.
Table 4.
Decannulation.
| Variable | Overall (N = 244) | ECMO death (n = 132) | Survival to decannulation (n = 112) | P value |
|---|---|---|---|---|
| Decannulated | 115.0 (47.1) | 13.0 (9.8) | 102.0 (91.1) | <.001a |
| Decannulation closure used | 115.0 (47.1) | 10 (7.6) | 78 (69.6) | <.001a |
| Perclose | 55.0 (22.5) | 6.0 (4.5) | 42.0 (37.5) | |
| MANTA | 31.0 (12.7) | 3.0 (2.3) | 22.0 (19.6) | |
| Surgical | 15.0 (6.1) | 1.0 (0.8) | 14.0 (12.5) | |
| Undocumented | 14.0 (5.7) | |||
| Dry closure technique used | 43.0 (17.6) | 8.0 (6.1) | 35.0 (31.3) | |
| Pre-Perclose used | 29.0 (11.9) | 14.0 (10.6) | 15.0 (13.4) | .505 |
Values are n (%).
Significant P values.
Complications
Complication rates postcannulation were similar between patients who died while on ECMO and those surviving decannulation, and included 3.7% arterial injury, 11.1% limb ischemia, 4.1% compartment syndrome, and 1.2% cannulation site infection. Bleeding from the access site occurred in 16% of patients and overall 83.2% of patients required blood transfusion. Thrombocytopenia occurred in 84.8% of patients and 26.2% of patients receiving platelet transfusion. A total of 36 (14.8%) patients developed stroke while on ECMO including 6.6% hemorrhagic stroke, and 8.2% ischemic stroke. The incidence of AKI post-ECMO was 52.5%, with 22.5% of patients requiring renal replacement therapy for renal failure. Complications during the hospitalization while on ECMO are highlighted in Table 5.
Table 5.
Complications.
| Complications | Overall (N = 244) | ECMO death (n = 132) | Survival to decannulation (n = 112) | P value |
|---|---|---|---|---|
| Arterial injury | 9.0 (3.7) | 5.0 (3.8) | 4.0 (3.6) | .929 |
| Dissection | 6.0 (2.5) | 4.0 (3.0) | 2.0 (1.8) | – |
| Pseudoaneurysm | 2.0 (0.8) | 0.0 (0.0) | 2.0 (1.8) | – |
| Retroperitoneal bleed | 1.0 (0.4) | 1.0 (0.8) | 0 (0) | – |
| Venous injury | 16.0 (6.6) | 11.0 (8.3) | 5.0 (4.5) | .225 |
| Dissection | 1.0 (0.4) | 0.0 (0.0) | 1.0 (0.9) | – |
| Hematoma | 15.0 (6.1) | 11.0 (8.3) | 4.0 (3.6) | – |
| Limb ischemia | 27.0 (11.1) | 19.0 (14.4) | 8.0 (7.1) | .073 |
| Compartment syndrome | 10.0 (4.1) | 7.0 (5.3) | 3.0 (2.7) | .305 |
| Return to cath lab | 33.0 (13.5) | 18.0 (13.6) | 15.0 (13.4) | .956 |
| Bleeding from access | 39.0 (16.0) | 24.0 (18.2) | 15.0 (13.4) | .311 |
| Thrombocytopenia | 207.0 (84.8) | 109.0 (82.6) | 98.0 (87.5) | .287 |
| Required platelet transfusion | 64.0 (26.2) | 29.0 (22.0) | 35.0 (31.3) | .101 |
| Required pRBC transfusion | 203.0 (83.2) | 103.0 (78.0) | 100.0 (89.3) | .019a |
| Infected cannulation site | 3.0 (1.2) | 0 (0) | 3.0 (2.7) | .059 |
| Bacteremia | 14.0 (5.7) | 6.0 (4.5) | 8.0 (7.1) | .387 |
| Need for wound vac placement | 10.0 (4.1) | 5.0 (3.8) | 5.0 (4.5) | .792 |
| Stroke | 36.0 (14.8) | 17.0 (12.9) | 19.0 (17.0) | .372 |
| Hemorrhagic | 16 (6.6) | –– | –– | – |
| Ischemic | 20.0 (8.2) | –– | –– | – |
| AKI post VA ECMO | 128.0 (52.5) | 80.0 (60.6) | 48.0 (42.9) | .006a |
| Required inpatient RRT | 55.0 (22.5) | 28.0 (21.2) | 27.0 (24.1) | .591 |
Values are n (%).
AKI, acute kidney injury; pRBC, packed red blood cells; RRT, renal replacement therapy; SAH, subarachnoid hemorrhage; SDH, subdural hematoma; vac, vacuum; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Significant P values.
Outcomes
The median post-ECMO 0-hour lactate level was 7.0 mmol/L (IQR, 2.8-12.4), with a post-ECMO 8-hour lactate level of 4.1 mmol/L (IQR, 1.9-9.3), and 8-hour lactate clearance of 22.3% (1.1-42.4).
The median days on VA-ECMO was 4.0 days (IQR, 2.0-8.0). The median hospital length of stay was 10.5 days (IQR, 3.0-23.8). The 30-day mortality was 66.8%, and 90-day mortality was 70.9%. Overall, in-hospital mortality was 69.7%. Table 6 includes post-ECMO outcomes.
Table 6.
Outcomes.
| Outcomes | Overall (N = 244) | ECMO death (n = 132) | Survival to decannulation (n = 112) | P value |
|---|---|---|---|---|
| 0-h lactate post VA ECMO | 7.0 (2.8-12.4) | 10.3 (5.2-14.8) | 4.1 (2.0-8.5) | <.001b |
| 8-h lactate post VA ECMO | 4.1 (1.9-9.3) | 8.4 (3.3-14.9) | 2.7 (1.4-4.9) | <.001b |
| 8-h lactate clearance, % | 22.3 (1.1-42.4) | 13.5 (–7.5 to 36.2) | 33.3 (7.8-49.3) | .002b |
| 24 h lactate post VA ECMO | 1.9 (1.2-4.2) | 3.9 (2.0-8.8) | 1.3 (1.0-2.2) | <.001b |
| 48 h lactate post VA ECMO | 1.4 (1.0-2.2) | 2.2 (1.4-3.1) | 1.2 (0.9-1.6) | <.001b |
| 72 h lactate post VA ECMO | 1.2 (0.8-1.8) | 1.7 (1.2-3.2) | 1.0 (0.8-1.4) | <.001b |
| Total VA-ECMO d | 4.0 (2.0-8.0) | 2.0 (2.0-5.3) | 6.0 (4.0-11.0) | <.001b |
| Hospital LOS, d | 10.5 (3.0-23.8) | |||
| Inpatient deatha | 170.0 (70.0) | |||
| Death post discharge | 4.0 (1.6) | |||
| 30-d survivala | 81.0 (33.2) | |||
| 90-d survivala | 71.0 (29.1) | |||
| 6-mo survivala | 62.0 (25.4) | |||
| 1-y survivala | 54.0 (22.1) |
Values are median (IQR) or n (%).
LOS, length of stay; VA ECMO, venoarterial extracorporeal membrane oxygenation.
1 patient still admitted at the time of data collection and analysis.
Significant P values.
Predictors of outcomes
Factors associated with ECMO death on univariate analysis included the following: occurrence of CA prior to cannulation (P = .001); past medical history of chronic respiratory failure on home oxygen (P = .033); baseline serum lactate level (P < .001) and platelet count (P = .010); intubation prior to cannulation (P < .001); vasopressor use (P = .004); SAVE score (P < .001) and SOFA score (P < .001); major bleeding requiring blood transfusion (P = .019); AKI postcannulation (P = .006); and postcannulation 8-hour serum lactate clearance (P = .002).
Elevated baseline lactate (OR, 1.13 per mmol/L increase; 95% CI, 1.04-1.23; P = .003) and elevated baseline SOFA scores (OR, 1.27 per unit increase; 95% CI, 1.15-1.40; P < .001) were independently associated with the primary end point on multivariate logistic regression analysis. Conversely, elevated baseline SAVE score (OR, 0.923 per unit increase; 95% CI, 0.859-0.993; P = .031) and 8-hour serum lactate clearance (OR, 0.987 per % increase, 95% CI, 0.979-0.994, P = .001) were independently associated with survival. Table 7 demonstrates all factors included in the multivariate regression analysis.
Table 7.
Multivariate logistic regression analysis of risk factors for ECMO death.
| Variable | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| On home oxygen prior to admit | 10.47 (0.8768-125.0555) | .063 |
| Initial serum lactate (mmol/L) | 1.13 (1.0416-1.2287) | .003a |
| Initial platelet count (1000) | 1.00 (0.99-1.00) | .067 |
| Cardiac arrest | 1.56 (0.13-19.20) | .728 |
| Arrest site | 1.04 (0.47-2.32) | .920 |
| Arrest rhythm | 0.84 (0.51-1.38) | .488 |
| Total CPR time (min) | 1.02 (0.99-1.05) | .181 |
| Intubated prior to VA ECMO | 2.18 (0.57-8.31) | .252 |
| Initial SBP prior to VA ECMO | 1.00 (0.99-1.02) | .629 |
| Required vasopressors prior to VA ECMO | 1.12 (0.38-3.32) | .843 |
| Required inotropes prior to VA ECMO | 0.94 (0.45-1.96) | .871 |
| SAVE score prior to VA ECMO (unit) | 0.92 (0.86-0.99) | .031a |
| SOFA score prior to VA ECMO (unit) | 1.27 (1.15-1.40) | <.001a |
| LAVA ECMO | 0.43 (0.16-1.20) | .109 |
| Required blood transfusion post VA ECMO | 0.39 (0.15-1.03) | .057 |
| 8-h serum lactate clearance (%) | 0.99 (0.98-0.99) | .001a |
| AKI post VA ECMO | 1.16 (0.57-2.37) | .689 |
Model inclusive of risk factors associated with ECMO death with a P value of <.05 on univariate analysis (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6). Dependent variable = ECMO death defined as inpatient mortality while on ECMO circuit, or within 24 h of decannulation.
AKI, acute kidney injury; CPR, cardiopulmonary resuscitation; LAVA, left-atrial venoarterial; SAVE, survival after VA ECMO; SOFA, sequential organ failure assessment; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Significant P values.
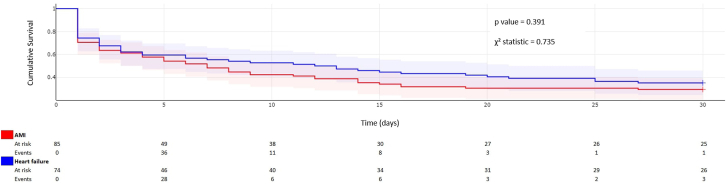
Kaplan Meier analysis (Figure 2) demonstrates no significant difference in 30-day survival based on indication for VA-ECMO when comparing the major subgroups of AMI and HF-related cardiogenic shock.
Figure 2.
Kaplan Meier analysis of 30-day mortality by indication for venoarterial extracorporeal membrane oxygenation. AMI, acute myocardial infarction.
Discussion
Our analysis highlights several important findings: (1) the majority of patients treated with peripheral ECMO were cannulated and decannulated by an interventional cardiologist demonstrating the feasibility of such an approach; (2) the majority of patients were managed by a primary cardiology service comprising interventional, HF or critical care cardiologists, highlighting the potential role of a cardiology inclusive ECMO service; (3) there was no difference in survival between patients cannulated on ECMO by an interventional cardiologist compared to a cardiac surgeon, suggesting comparable safety with such an approach; (4) preoperative lactate and SOFA scores were independently associated with VA-ECMO death; (5) 8-hour lactate clearance and higher SAVE scores were independently associated with survival (Central Illustration).
Central Illustration.
Important details and findings of our study.
The most important finding from our analysis is that use of a cardiovascular medicine inclusive ECMO service is feasible. Previous groups have highlighted the use of an intensivist inclusive service. Kouch et al8 described their experience transitioning from a surgical-based model to an intensivist-based model in patients requiring veno-venous ECMO. Similarly, Kraai et al9 have described their experience of an intensivist-led ECMO program encompassing both veno-venous and VA-ECMO. Our experience similarly supports the concept that cardiologists who are knowledgeable about ECMO and critical care management can serve as primary providers for this critically ill patient population. In our program, we utilized numerous pathways to define competency for implantation and explantation as well as management of ECMO. We utilized commonly available resources provided by Extracorporeal Life Support Organization (ELSO) for training in the management of ECMO. Additional hands-on experience was obtained by coscrubbing into early cases.
Although there is no doubt to the importance of cardiac surgeons in a successful ECMO program, there are distinct advantages of being inclusive of cardiologists in ECMO programs. Cardiologists are overall well suited for ECMO care, as they are knowledgeable of hemodynamic derangements, which are frequent in those with cardiogenic shock, allowing for assistance in MCS device selection and interpretation of invasive hemodynamics and cardiovascular imaging. Garan et al10 for example demonstrated that in >1400 patients with cardiogenic shock, there was an improvement to in-hospital mortality when care was guided by a pulmonary artery catheter (PAC) monitoring. Similarly, Osman et al11 demonstrated improved outcomes with the use of PAC monitoring which in their analysis was more frequently associated with delivery of advanced HF therapies. In our analysis, for 88% of the patients, a PAC was used to guide decision making for MCS device selection.
Cardiologists also implant and manage other forms of MCS, such as intraaortic balloon pump, Impella, and Tandem Heart, and thus can carefully evaluate the risks and benefits of select devices. In our cohort, 64.8% of patients had some form of left ventricle venting when using ECMO. Use of LV venting has been associated with improved outcomes in patients treated with VA-ECMO.12 In particular, there was growing use in patients receiving left-atrial VA-ECMO cannulation and these patients had increased likelihood of survival.
The cardiac catheterization laboratory is also well equipped for ECMO cannulation and decannulation. Cath labs are operational 24 hours a day, with systems in place for rapid activation, as is necessary for ST-segment elevation myocardial infarction (STEMI) care. Although investigators have previously described the use of VA-ECMO in the cath lab,13, 14, 15 these cohorts have predominantly comprised patients with in-hospital CA admitted to a tertiary academic medical center14; in our cohort, we describe a comprehensive cohort of patients who were safely treated in the cath lab.
Interventional cardiologists are also well equipped to manage large bore MCS, including use of modern-day vascular access best practices, such as the use of ultrasound, fluoroscopy, micropuncture needles, peripheral angiography, the routine use of reperfusion sheaths and vascular access closure. This skill set is important not just for successful cannulation but more importantly to mitigate vascular access complications, all of which significantly impact survival.16 In our study, 3.7% of patients suffered from arterial complications, 11.1% had limb ischemia, and 4.1% suffered compartment syndrome while on support. These complications are lower than prior reports.17 Insertion of distal perfusion sheaths has also been shown to reduce the incidence of limb ischemia18 and is routinely used in the majority of ECMO patients. Furthermore, only 13.5% of patients in the cohort required transport back to the catheterization lab due to complications, and though 16% of the cohort suffered bleeding at the site of cannulation it is still lower than in previous cohorts.19 Lastly, 1.2% of patients in our study experienced infection at the site of cannulation, and 5.7% were found to have bacteremia during the entire time on ECMO. Prior reports have suggested rates of groin cannulation infection ranging from 10% to 20%, with obesity and malnourishment being 2 primary risk factors.18,20 We saw far fewer infections and suspect this may be due to better sterile cannulation techniques used in the catheterization laboratory compared to the intensive care unit.
Another advantage of cardiology involvement in ECMO care is the ability to perform percutaneous decannulation. Kraai et al9 have previously described their experience of intensivists implanting ECMO. In their cohort, 50% of the patients were cannulated with the use of transesophageal echocardiography at the bedside and the other 50% were cannulated using a portable fluoroscopy unit. Although the investigators reported safety of using such an approach, the majority of patients were decannulated in the operating room. In our cohort, 115 patients were decannulated, the vast majority of whom were decannulated percutaneously using suture-based closure, collagen-based closure, or “dry” closure using balloon tamponade and manual pressure. Previous reports have demonstrated that percutaneous closure, which interventional cardiologists are apt to perform, is associated with 80% less likelihood of limb complications and bleeding and therefore may be the preferred strategy when feasible.21
An inclusive ECMO program also allows surgeons to focus and have more time for the placement of durable left ventricular assist devices, cardiac transplants, and centrally placed temporary MCS devices including ECMO. This allows cardiologists to provide more complete care to patients they are already caring for. For example, in our cohort, 32% of patients required ECMO for AMI complicated by cardiogenic shock, 30% for decompensated heart failure, 10% for refractory arrhythmias, and 7% for severe valvular disease. Cardiologists are heavily involved in the care of these patients and the ability to use more robust MCS devices such as ECMO can often be lifesaving. However, caution is necessary when selecting appropriate ECMO candidates, as ECMO is a resource-heavy therapy. In our cohort, 10% of the patients required ECMO for intraprocedural complications, 26.6% were placed on ECMO in a peri-arrest setting, and of these patients, 67% died.
It is also important to emphasize that in our cohort, approximately 10% of patients were cannulated at a satellite hospital with no cardiothoracic surgery backup. Having ECMO cannulation capabilities at such facilities can be vitally important to patient care.22 In fact, this highlights the need to expand the accessibility of ECMO, particularly as part of the treatment of patients with cardiogenic shock diagnosed at institutions without surgical teams.
Although recent clinical trials including ECLS-SHOCK and ECMO-CS have suggested no significant benefit in routine use of VA-ECMO in AMI-CS,23,24 it is important to highlight that only a small portion of our current, real-world cohort would have met either trial inclusion criteria if applied at the time of cannulation (Supplemental Figure S1). Additionally, although we demonstrated no significant difference in overall 30-day survival on Kaplan Meier analysis between the major subgroups of AMI and HF-CS, the various other indications for ECMO show the heterogeneity of cardiogenic shock and highlight the utility of VA-ECMO beyond AMI-CS, particularly in cases of VT/VF, procedural complications, and HF shock as a bridge to durable support or transplant.
Lastly, our overall in-hospital mortality was 69.7%. This is comparable to the estimated mortality of 58% to 95% based on the patients' SAVE and SOFA scores, as well as the high rate of cannulation in the peri-arrest setting.25, 26, 27 The difference between the rate of all-cause in-hospital mortality and survival to decannulation from ECMO was due to various reasons, but most notable was the fact that patients who were decannulated went on to undergo surgical procedures or developed infections/complications at which point they were no longer candidates for advanced therapies. This is an area of interest, which should be the focus of future studies.
Limitations
There are several limitations of our study. Our study was a retrospective single-center study, prone to selection and treatment bias which limits the generalizability of our results. Additionally, the determination for use of MCS was decided by the treatment team. However, the purpose of our study was to describe the outcomes of a cardiology inclusive ECMO service; so the selection of a support device does not alter the interpretation of results. In addition, data on the use of reperfusion catheters were omitted due to lack of confidence in the accuracy of the data. Regarding statistical analysis, it is worth noting that imputation was used for missing variables to calculate severity scores, and this should be considered when drawing conclusions. Although this is a validated and widely used method for missing data, it introduces a margin of error that must be considered. Furthermore, none of the patients who expired while on ECMO underwent autopsy, and thus may have had missing diagnoses for the cause of decompensation such as bleeding, infection, or thrombosis that was not previously identified, potentially impacting our results.
Looking ahead
As the accessibility of ECMO increases across health systems, there remains a need for continued studies. Clinical trials to identify patient cohorts who may benefit or be harmed by ECMO, as well as continued study of the processes of care that affect outcomes are necessary.
Acknowledgments
Declaration of competing interest
Mohammad Alqarqaz received research funding from Abiomed. Jennifer Cowger is a consultant for Abbott, Medtronic, CH Biomedical, Procyrion, BioVentrix, and CorWave. Khaldoon Alaswad is a consultant for Arrow, Cardiovascular Systems Inc, Teleflex, Abbott, and Boston Scientific. William O’Neill is a consultant for Abbott, Abiomed, Boston Scientific, Edwards Lifesciences, and Medtronic. Pedro Villablanca is a consultant for Edwards Lifesciences, Medtronic, Medicure Pharma, and Angiodynamics. Brian O’Neill is a consultant for Edwards Lifesciences, Abbott, Medtronic, Inari, Medicure Pharma, and Angiodynamics. Herb Aronow is a consultant for Philips. Hassan Nemeh is a consultant for Edwards Lifesciences and Abbott. Tiberio Frisoli is a consultant for Edwards Lifesciences, Abbott, Boston Scientific, and Angiodynamics. Celeste Williams is a consultant for Zoll, Abbott, Medtronic, and St. Jude. Mir Babar Basir is a consultant Abiomed, Boston Scientific, Chiesi, Saranas, and Zoll. All other authors report no conflicts of interest.
Funding sources
This work was not supported by funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
The research was carried out in accordance with appropriate ethical guidelines. The Henry Ford Health System Institutional Review Board approved the study and waived the need for informed consent due to the retrospective nature of the study.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.101359
Supplementary material
Application of clinical trial inclusion/exclusion criteria.
References
- 1.Extracorporeal Life Support Organization . 2020. ECLS Registry Report United States Regional Summary.https://www.elso.org/registry/internationalsummaryandreports.aspx Accessed June 20, 2023. [Google Scholar]
- 2.Sauer C.M., Yuh D.D., Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61(1):31–36. doi: 10.1097/MAT.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 3.Becher P.M., Schrage B., Sinning C.R., et al. Venoarterial extracorporeal membrane oxygenation for cardiopulmonary support. Circulation. 2018;138(20):2298–2300. doi: 10.1161/CIRCULATIONAHA.118.036691. [DOI] [PubMed] [Google Scholar]
- 4.Yandrapalli S., Sanaani A., Harikrishnan P., et al. Cardiogenic shock during heart failure hospitalizations: age-, sex-, and race-stratified trends in incidence and outcomes. Am Heart J. 2019;213:18–29. doi: 10.1016/j.ahj.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Shah M., Patnaik S., Patel B., et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287–303. doi: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 6.Buda K.G., Sedhom R., Elbadawi A., et al. Trends and outcomes of veno-arterial extracorporeal membrane oxygenation for acute myocardial infarction-cardiogenic shock. Eur Heart J. 2022;43(Suppl 2) ehac544.1325. Accessed July 13, 2023. https://doi.org/10.1093/eurheartj/ehac544.1325. [Google Scholar]
- 7.O'Neill W., Basir M., Dixon S., Patel K., Schreiber T., Almany S. Feasibility of early mechanical support during mechanical reperfusion of acute myocardial infarct cardiogenic shock. JACC Cardiovasc Interv. 2017;10(6):624–625. doi: 10.1016/j.jcin.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Kouch M., Green A., Damuth E., et al. Rapid development and deployment of an intensivist-led venovenous extracorporeal membrane oxygenation cannulation program. Crit Care Med. 2022;50(2):e154–e161. doi: 10.1097/CCM.0000000000005282. [DOI] [PubMed] [Google Scholar]
- 9.Kraai E., Teixeira J.P., Patel I.A., et al. An intensivist-led extracorporeal membrane oxygenation program: design, implementation, and outcomes of the first five years. ASAIO J. 2023;69(5):451–459. doi: 10.1097/MAT.0000000000001870. [DOI] [PubMed] [Google Scholar]
- 10.Garan A.R., Kanwar M., Thayer K.L., et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail. 2020;8(11):903–913. doi: 10.1016/j.jchf.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Osman M., Syed M., Patel B., et al. Invasive hemodynamic monitoring in cardiogenic shock is associated with lower in-hospital mortality. J Am Heart Assoc. 2021;10(18) doi: 10.1161/JAHA.121.021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrage B., Becher P.M., Bernhardt A., et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142(22):2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastoris I., Tonna J.E., Hu J., et al. Use of extracorporeal membrane oxygenation as bridge to replacement therapies in cardiogenic shock: insights from the Extracorporeal Life Support Organization. Circ Heart Fail. 2022;15(1) doi: 10.1161/CIRCHEARTFAILURE.121.008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim Y., Kim M.C., Jeong I.S., et al. Outcomes of extracorporeal cardiopulmonary resuscitation for in-hospital cardiac arrest according to cannulation sites: cath lab vs non-cath lab. J Cardiovasc Interv. 2022;1(1):40–48. doi: 10.54912/jci.2021.0001. [DOI] [Google Scholar]
- 15.Parr C.J., Sharma R., Arora R.C., Singal R., Hiebert B., Minhas K. Outcomes of extracorporeal membrane oxygenation support in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2020;96(3):547–555. doi: 10.1002/ccd.28492. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka D., Hirose H., Cavarocchi N., Entwistle J.W. The impact of vascular complications on survival of patients on venoarterial extracorporeal membrane oxygenation. Ann Thorac Surg. 2016;101(5):1729–1734. doi: 10.1016/j.athoracsur.2015.10.095. [DOI] [PubMed] [Google Scholar]
- 17.Cheng R., Hachamovitch R., Kittleson M., et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Lamb K.M., DiMuzio P.J., Johnson A., et al. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. J Vasc Surg. 2017;65(4):1074–1079. doi: 10.1016/j.jvs.2016.10.059. [DOI] [PubMed] [Google Scholar]
- 19.Saeed D., Stosik H., Islamovic M., et al. Femoro-femoral versus atrio-aortic extracorporeal membrane oxygenation: selecting the ideal cannulation technique. Artif Organs. 2014;38(7):549–555. doi: 10.1111/aor.12245. [DOI] [PubMed] [Google Scholar]
- 20.Bisdas T., Beutel G., Warnecke G., et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. 2011;92(2):626–631. doi: 10.1016/j.athoracsur.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Chandel A., Desai M., Ryan L.P., Clevenger L., Speir A.M., Singh R. Preclosure technique versus arterial cutdown after percutaneous cannulation for venoarterial extracorporeal membrane oxygenation. JTCVS Tech. 2021;10:322–330. doi: 10.1016/j.xjtc.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ya'Qoub L., Alqarqaz M., Cowger J., et al. Cardiogenic shock in a young woman with SCAD: The importance of early access to VA-ECMO in the community. Cardiovasc Revasc Med. 2024;59:81–83. doi: 10.1016/j.carrev.2023.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Thiele H., Freund A., Gimenez M.R., et al. Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock - Design and rationale of the ECLS-SHOCK trial. Am Heart J. 2021;234:1–11. doi: 10.1016/j.ahj.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Ostadal P., Rokyta R., Karasek J., et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO-CS randomized clinical trial. Circulation. 2023;147(6):454–464. doi: 10.1161/CIRCULATIONAHA.122.062949. [DOI] [PubMed] [Google Scholar]
- 25.Chen W.C., Huang K.Y., Yao C.W., et al. The modified SAVE score: predicting survival using urgent veno-arterial extracorporeal membrane oxygenation within 24 hours of arrival at the emergency department. Crit Care. 2016;20(1):336. doi: 10.1186/s13054-016-1520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czobor P., Venturini J.M., Parikh K.S., et al. Sequential organ failure assessment score at presentation predicts survival in patients treated with percutaneous veno-arterial extracorporeal membrane oxygenation. J Invasive Cardiol. 2016;28(4):133–138. [PubMed] [Google Scholar]
- 27.Laimoud M., Alanazi M. The validity of SOFA score to predict mortality in adult Patients with cardiogenic shock on venoarterial extracorporeal membrane oxygenation. Crit Care Res Pract. 2020;2020 doi: 10.1155/2020/3129864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Application of clinical trial inclusion/exclusion criteria.