Abstract
Chronic hepatitis B virus (HBV) infection affects about 262 million people worldwide, leading to over 820,000 deaths each year primarily due to cirrhosis and hepatocellular carcinoma. The World Health Organization has pledged to eliminate HBV as a health threat by 2030, but currently, no countries are on track to achieve this goal. One of the barriers to HBV elimination is stigma, causing shame, denial, self-isolation, self-rejection, and depression leading to those with chronic HBV less likely to get tested or seek treatment and more likely to conceal their infection. Other barriers include limited access to care and complicated and restrictive clinical practice guidelines. Increasing public and political efforts are necessary to raise awareness, increase access to care, and change screening and treatment guidelines. The current guidance of the American Association for the Study of Liver Diseases (AASLD) recommends testing only if patients are considered at risk, but this has proven to be ineffective. We propose a simplified “test all and treat all” approach with a 5-line guideline for HBV infection. Universal screening and treatment of adults is cost-effective and can prevent transmission by effectively managing chronic HBV. All patients who are hepatitis B surface antigen (HBsAg) positive with detectable HBV-DNA should receive treatment until HBsAg is undetectable for 12 months, as HBV-DNA transmission via blood transfusion can occur even at low viral loads of 16 copies/mL, and mother-to-child transmission is still a risk even with passive-active immunoprophylaxis. Furthermore, clinical outcomes after HBsAg clearance are significantly better than the clinical outcomes of those who remain HBsAg positive.
Keywords: Hepatitis B Virus, Chronic Hepatitis B, Five-line guideline for HBV, Test all and treat all
Introduction
Chronic hepatitis B virus (HBV) infection is a significant global health issue affecting an estimated 262 million people worldwide with 1.5 million new infections each year.1,2 It is associated with more than 820,000 deaths per year, and patients face substantial risk of complications, as 15%–40% of patients with chronic HBV (CHB) may develop cirrhosis, liver failure, and hepatocellular carcinoma (HCC).1,3, 4, 5 Prevalence of HBV is highest in African, Western Pacific, East Asia, and Southeast Asia regions, with the Western Pacific accounting for approximately 50% of all CHB infections worldwide.1,4 Unfortunately, the prevalence is underestimated partly due to limited surveillance systems within different countries, reactivation risk of hepatitis B surface antigen (HBsAg)-negative patients, and occult HBV infection.6
In 2021, the World Health Organization (WHO) estimated that approximately 15%–25% of people with CHB will require treatment1. Based on all the global guidelines, patients who have HBV-DNA ≥2000 IU/mL and elevated alanine aminotransferase (ALT) should begin treatment.7, 8, 9, 10, 11 The goal of the current treatment regimen has 2 targeted end points: suppression of HBV-DNA to undetectable levels and the loss of HBsAg or HBsAg seroconversion (ie, functional cure). Studies have shown that these proposed goals for treatment have been associated with improved long-term outcomes.12,13 Although HBV-DNA suppression occurs in more than 70% of patients, the loss of HBsAg occurs only in a small subset of patients. The loss of HBsAg has been difficult to achieve since the first-line therapies have very little effect on the HBsAg production and do not modify the immune response.9,14 Because the mortality rate for HBV has not changed in the last 20 years, major research and investments have been made in the development of new medications.
The “test all and treat all” approach within the 5-line guideline of HBV discussed herein will likely increase HBV diagnosis, reduce transmission, extrahepatic manifestations of HBV, stigma, and rates of hepatitis Delta virus (HDV) coinfection, improve quality of life, and be cost-effective overall.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 This will increase the number of patients with HBsAg loss since a far greater number of patients will be receiving treatment especially as advanced treatments become available in clinics. Lower costs for low- and middle-income countries (LMICs) can also help improve access to treatment, and emerging therapies for the treatment of CHB under investigation may increase the number of patients with HBsAg loss (ie, functional cure).12,13
Transmission of Hepatitis B
Blood Exposures, Relationship to Viral Load, and Infectivity
The risk of infection from blood exposures, such as blood transfusions, is dependent on the circulating infectious agent, antibodies bound to virions, stage of infection of the donor, blood volume received, degree of immunosuppression in the recipient, and type of blood product received.26,27 Hepatitis B core antibody (anti-HBc) negativity or low levels of anti-HBc have been related to higher infectivity rates. There was 50% transmission in those without anti-HBc and HBV <100 copies/mL vs 3% transmission in those with anti-HBc and HBV <100 copies/mL.26,27 In a study involving human hepatocytes transplanted into chimeric mice, low anti-HBc titers were related to ∼3% infectivity, whereas those without anti-HBc had 37%–50% infectivity. Therefore, transmission may be higher during the window period and in the acute phase than in the chronic phase.28
Patients with occult HBV infection are also HBV-DNA positive, anti-HBc positive, and HBsAg negative. While most patients are found to have HBV-DNA levels around 20,000–90,000 copies/mL (approximately 4000–22,000 IU/mL), much lower titers of HBV-DNA levels can also be observed.26,29,30 However, there are no data on whether blood products from these patients are infectious.26 There may be a small relationship between viral load in infectious blood and noninfectious blood in instances where infection was reported with viral copies as low as 2–5 copies/mL and no infection with 200 copies/mL.27 Some studies have proposed that the minimum infectious dose of HBV may be as low as 16 copies/mL.31 Therefore, it is important to treat all those who are HBV-DNA positive as even low levels have the potential to spread HBV infection.32
HBV Mother-to-Child Transmission
Mother-to-child transmission (MTCT) of HBV during the perinatal period continues to be a global health issue especially in high-endemic areas.33, 34, 35 Even in low-endemic areas, MTCT accounts for more than one-third of CHB infections.36, 37, 38 If newborns are born to hepatitis B e antigen (HBeAg)-positive mothers and do not receive hepatitis B immunoglobulin (HBIG) and hepatitis B vaccine soon after birth, 70%–90% will develop HBV infection. If the newborns are born to HBeAg-negative mothers and do not receive HBIG and hepatitis B vaccine, 10%–20% will develop HBV infection.39,40 Also, despite treatment with HBIG and hepatitis B vaccine in neonates, about 9% of newborns will acquire HBV infection if their mother has a CHB infection. This is especially true if the mother is HBeAg positive or she has high HBV-DNA levels.41 For example, there was 9% transmission rate when mother’s viral load was >8 log10 copies/ml (7.3 log10 IU/ml) even after administration of passive-active immunoprophylaxis.42 Recent studies propose lower levels of HBV-DNA <6 log10 copies/ml (5.3 log10 IU/ml) to reduce MTCT rates.43,44
Additionally, newborns have a 90% chance of developing a CHB infection compared to a 50% chance for children younger than 3 year old and only a 5% chance for adults after being infected with HBV.41 In high-endemic areas such as China, the percentage of women of childbearing age with CHB infection is up to 17.8%.45 Therefore, we reiterate the importance and the need to “test all and treat all” and vaccinate all who are susceptible. This also recognizes that there are areas of the world where HBV-DNA testing is twice the price of 1 year of tenofovir and other areas where nucleic acid testing is not available for pregnant women.
Sexual Transmission
One of the main modes of transmission for HBV is through sexual contact. In low-prevalent areas, intravenous drug users (IVDUs) and sexual exposure account for most cases of HBV exposure. Historically, men who have sex with men (MSM) are at the highest risk for infection. This has been associated with anal intercourse, increased number of sexual partners, and the number of years of sexual activity.46 About 70% of MSM were infected after 5 years of activity.47 However, it is not only limited to MSM. Women and heterosexual men also have an increased risk of HBV infection with an increased number of sexual partners, years of sexual activity, and a history of sexually transmitted diseases.48
Hepatocellular Carcinoma
HCC is the leading cause of death in patients with cirrhosis.25 It was ranked the third-highest cause of mortality due to cancer in 2018,49 and 90% of primary liver cancers are due to HCC. Unfortunately, HBV is still a major risk factor for HCC development in East Asian countries.50 The risk for HCC development is increased for those who have coinfection with hepatitis C virus (HCV), HDV, and or HIV.50,51 Although there is still a risk for HCC with HBsAg clearance even in the absence of cirrhosis, the risk was significantly lower compared to those who were HBsAg positive.52 The continued risk in those who are HBsAg negative is likely due to integrated HBV-DNA in the hepatocyte genome.52
Although there have been improvements in therapeutic options for HCC, the 5-year survival rate for HCC is lower than 20%.25 An important factor for HCC prognosis is the tumor stage. The 5-year survival rate can be greater than 60% for those in earlier stages compared to those in advanced stages (median survival of 1–2 years).25 Surveillance with ultrasound is recommended every 6 months with or without alpha-fetoprotein (AFP) serum testing to reduce mortality due to HCC. Table 1 summarizes HCC surveillance recommendations from different guidelines. Unfortunately, a recent study found that less than 40% of patients underwent annual HCC surveillance.55
Table 1.
AASLD, EASL, and APASL Guidelines for HCC Surveillance
| Guidelines | Patients without cirrhosis | Patients with cirrhosis |
|---|---|---|
American Association for the Study of Liver Diseases (AASLD)11,53
|
HBsAg seroclearance:
|
|
| ||
European Association for the Study of the Liver (EASL)54
|
HBsAg positive:
|
|
| ||
Asian Pacific Association for the Study of the Liver (APASL)51
|
HBsAg seroclearance:
|
|
|
HDV, hepatitis D virus; US, ultrasound.
PAGE-B score: 5-y HCC, risk predictor for Caucasian patients with chronic HBV, receiving nucleos(t)ide analogs.
Ultrasound has a higher sensitivity, specificity, and diagnostic accuracy compared to AFP serum testing, but AFP can be considered for those without ultrasound access.11,56 However, the accuracy of AFP in detecting HCC may also vary depending on the cutoff value, prevalence of the disease, and patients’ virological status. AFP is Food and Drug Administration-cleared as a risk test for HCC and not for diagnosis.
A recent cohort study of 534 patients showed that none of the biomarkers (AFP, AFP-L3, des-gamma-carboxy prothrombin [DCP]) individually or in combination had promising results in risk prediction for HCC.57 Furthermore, these biomarkers’ detection accuracy is also dependent on their cutoff values. Overall, the combination of these serum markers alone or together has shown only marginal improvement in HCC surveillance or diagnosis.
There have been several scoring systems created to help predict the risk of developing HCC in patients with CHB. The PAGE-B model was developed previously to predict 5-year HCC development in Caucasians with CHB receiving nucleos(t)ide analogs. In 2018, a modified PAGE-B model was released, and it showed improved prediction for HCC risk in Asians with CHB infection receiving nucleos(t)ide analogs.58 Unfortunately, because many of these predictive scoring models are dependent on many factors such as ethnicity, stages of liver disease, and antiviral treatment, their use is limited.
The GALAD scoring system was developed to improve early-stage HCC detection in patients with HBV and HCV. The GALAD scoring system looks at gender, age, AFP level, AFP-L3 level, and DCP level. It has been shown to detect early-stage HCC with 86% sensitivity and 96% sensitivity in a British cohort.59 In a European cohort study in 2021, the GALAD system was superior at detection of early-stage Barcelona Clinic Liver Cancer stage 0 or A compared to biomarkers AFP, AFP-L3, or DCP alone.60 However, a recent study found that although GALAD score had significantly higher sensitivity, it also had an increase in false-positive rates.57
Treatment of HBV-Associated HCC
The treatment of HCC is dependent on the stage of the tumor, disease burden, patient functional status, and underlying hepatic function. If HCC is found earlier, initial curative treatment entails resection and or ablation or liver transplantation. The 5-year recurrence rate is 4%–18% for those treated with liver transplantation vs 50%–75% for those treated with resection or local ablation. In patients with liver transplantation for HBV-associated HCC, the 5-year survival rate is approximately 80%. For those with advanced disease, palliative treatments include local-regional therapies such as transarterial chemoembolization or transarterial radioembolization followed by systemic therapy.56
The 5-year recurrence risk for HCC after curative resection is between 60% and 80%.61 About 70% of recurrences are within the first 2 years (early recurrence).62 The recurrence of HBV-associated HCC is independently related to high HBV viral load.63,64 Therefore, HBV antivirals are used to prevent recurrence for those who received a curative resection. Unfortunately, even with the antivirals, the recurrence rate is between 40% and 60%.62,65 A 2022 systematic review and meta-analysis showed that HCC recurrence and mortality were lower with tenofovir disoproxil fumarate (TDF) than with entecavir (ETV). This was mostly in the prevention of late recurrence (after 2 years).66
Other Consequences of HBV
Extrahepatic Manifestations of CHB
About 20% of patients with HBV infection develop extrahepatic manifestations67 as shown in Table 2. Like HCV, many of the extrahepatic manifestations are thought to be due to the deposition of immune complexes (ICs) with an inflammatory response.77 These ICs consist of HBsAg, again highlighting the importance of treating all who are HBV-DNA positive until HBsAg loss is achieved.
Table 2.
Extrahepatic Manifestations of HBV
| Types of manifestations | Symptoms |
|---|---|
| Periarteritis or polyarthritis nodosa (PAN) |
|
| Mixed cryoglobulinemia (MC) vasculitis |
|
| Rheumatological manifestations |
|
| Skin manifestations | |
| Renal manifestations |
|
| Hematologic malignancies |
ANCAs, antineutrophil cytoplasmic antibodies; anti-PLAR, antiphospholipase A2 receptor antibodies; CTA, computed tomography angiography; DLCL, diffuse large B-cell lymphoma; Ig, immunoglobulin; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephropathy; MRA, magnetic resonance angiography; NHL, non-Hodgkin lymphoma.
Coinfection With HDV Systemic Manifestations
HDV is the smallest human RNA virus and is considered a defective subvirus that can only propagate with the existence of HBV. It envelopes within the HBV surface proteins and uses HBV as a helper for hepatocyte entry, intrahepatic spread, and host dissemination.83 Modes of transmission occur via simultaneous coinfection with HBV or superinfection with a CHB carrier.84 A simultaneous infection occurring with HBV can cause severe to fulminant hepatitis with a high mortality rate; however, individuals who are able to achieve recovery typically have clearance of both viruses.85 Superinfection with CHB usually causes HDV persistence, which can cause rapid progression to cirrhosis and increased risk of HCC, making it one of the most severe forms of CHB.83,86 Approximately 50%–70% of patients with HBV-HDV coinfection develop cirrhosis within 5–10 years of diagnosis, a 3-fold increase compared to HBV monoinfection.86
A systematic review and meta-analysis from 2020 estimated prevalence among HBsAg carriers as 4.5%, with a global HDV prevalence of 0.16%, equaling approximately 12 million people,85 but exact global HDV infection prevalence is unknown due to lack of universal testing and reporting and limited laboratory test access, making epidemiological data scant or not available for many countries.
Chronic viral infection with HDV has been suspected to cause primary Sjögren syndrome. In a viral microarray analysis of salivary glands of patients with and without Sjögren syndrome, HDV was found 50% more often in those with primary Sjögren syndrome compared to healthy controls. However, these patients were negative for HBsAg, anti-HBc antibodies, and HDV antigens in the serum.87 Additionally, patients with chronic HDV tend to have higher frequency of autoantibodies compared to those with HBV alone. These autoantibodies are antiliver kidney microsomes, antibasal cell layer antibodies, and antithymic antibodies (stellate epithelial cell antibodies, thymic reticular cell antibodies, and perithymocytic cell antibodies).88 Therefore, the “test all and treat all who are HBV-DNA positive” approach may reduce the risk of contaminant HDV infections and associated systemic manifestations.
Quality of Life
Clinicians should be aware of the reduced quality of life for patients with HBV, which is due to the stigmatization, chronicity of HBV infection, and the extrahepatic manifestations of HBV. Current evidence suggests that there is a gap between the clinician’s and the patient’s perspective on the impact of the disease on patients’ daily lives.20,22 This can be bridged with patient-reported outcomes (PROs) questionnaires. PROs come directly from the patient without any interpretation by a health-care professional.22 Recently, Younossi et al developed and validated a new PROs instrument known as the HBV-specific version of Chronic Liver Disease Questionnaire (CLDQ-HBV).21 This questionnaire can be used to monitor disease progression and identify factors that can strengthen patients’ adherence to treatment.22,23 Younossi et al found that patients with depression, type 2 diabetes, and obesity had more impaired health-related quality of life.21 In a recent cohort study, depression was independently and positively correlated with liver-related mortality and liver cancer mortality.24 Therefore, we should treat all who are HBV-DNA positive.
Stigma
In the United States, HBV is highly prevalent in Asian immigrants.89,90 Unfortunately, many of these immigrants neither have access to nor seek testing, vaccination, or care for HBV, increasing the risk of transmission and disease progression.89 A study performed on Vietnamese Americans in Chicago showed that up to 55% of the population is unaware that HBV can be spread by sexual intercourse, instead believing that it can be transmitted by sharing utensils.90 In the same study, more than 60% believed that HBV carriers put others at risk and should avoid close contact with others.89 This social stigma is compounded by self-stigma, in which people living with CHB blame themselves for their infection. The resulting shame, denial, self-isolation, self-rejection, self-hatred, and depression result in a withdrawal from public activities, family, and friends, which can increase risk of depression and suicidal ideation, increasing the mortality risk for these patients. These misconceptions and stigma affect all aspects of the HBV pandemic from primary prevention to prevention of progression and spread. At-risk populations are less likely to get vaccinated, tested, or seek treatment and are more likely to conceal their infection, further increasing the risk of spread.19,89 Therefore, by adopting the “test all and treat all who are HBV-DNA positive” approach, these authors emphasize the ability to reduce spread and the stigma associated with HBV infection.
Current Therapies
The treatment goals of managing HBV are directed toward managing stigma, preventing transmission, and preventing liver cirrhosis, decompensated cirrhosis, HCC, liver transplantation, and death. This can be achieved through normalizing liver enzyme levels and halting necroinflammatory activity by stopping HBV replication. There are currently 9 approved drugs for CHB, though only 3 are in active use globally. There are 2 interferon (INF) formulations: conventional and pegylated interferon (pegINF). There are 7 nucleos(t)ide analogs: lamivudine, telbivudine, adefovir, ETV, TDF, tenofovir alafenamide fumarate (TAF), and clevudine and besifovir dipivoxil (only in Korea) (Table 3).
Table 3.
Summary of Current HBV Therapies
| Types | Notes |
|---|---|
| Interferon formulations | |
| Conventional INF pegINF | |
| TDF | |
| Lamivudine Telbivudine Adefovir ETV TDF TAF Clevudine Besifovir dipivoxila |
|
CrCl, creatinine clearance; NA, nucleos(t)ides analog.
Only available in Korea.
A virological response with INF treatment is defined as HBV-DNA <2000 IU/mL after 6 months of therapy. For treatment with nucleos(t)ide analogs, virological response is defined as HBV-DNA <10–20 IU/mL. A biochemical response is defined as normalization of ALT.93 For CHB treatments, cutoff values of 35 U/L for males and 25 U/L for females for ALT are used to help guide treatment decisions.11 Although our overall goal is to functionally cure and resolve HBV infection (HBsAg loss or detection of HBsAg lower than 0.05 IU/mL), it is extremely rare to achieve (<1% of patients per year).11,93 Given the high rates of relapse after cessation of nucleos(t)ide analogs, the American Association for the Study of Liver Diseases (AASLD) recommends stopping treatment only after HBsAg loss with or without seroconversion. Emerging data on hepatitis B core-related antigen (HBcrAg), quantitative HBV-RNA (qHBV-RNA), and other biomarkers will help with the viral clearance prediction. Discontinuation is not recommended for those with cirrhosis due to hepatitis flares after virological response and the risk of liver failure and death.92
Standard of Care
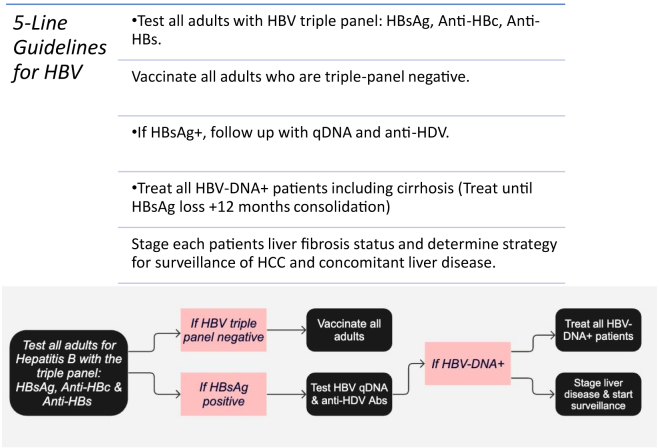
Five-Line Guidelines for HBV
We agree with the recent Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention on HBV testing and vaccinations. They advise testing all adults who are 18 years or older with a triple panel consisting of HBsAg, anti-HBc, and hepatitis B surface antibody (anti-HBs) at least once in their lifetime.16 If the person is at increased risk for HBV, periodic testing should take place regardless of age, as long as the risk for exposure persists.16 A recent article by the Infectious Disease Society of America found that screening everyone with HBsAg once is more cost-effective compared to the current practice guided by the current HBV guidelines.15 The study found that it would prevent an additional 23,000 deaths from liver disease and liver cancer, resulting in an estimated savings of $596,000,000.15 Universal screening of adults is cost-effective, and it may prevent transmission with the management of CHB, identify those who are at risk of HBV reactivation, and identify those who would benefit from HBV vaccination.16 A 2022 Morbidity and Mortality Weekly Report recommends HBV vaccination for all, as it has proven to be safe, efficacious, and well tolerated.94 For these reasons, we recommend testing all adults with a triple panel at least once in their lifetime and vaccinating all triple-panel-negative adults (Figure).
Figure.
The 5-line guidelines for HBV in adults.
It is generally agreed that the current HBV treatment guidelines by the European Association for the Study of the Liver, AASLD, and Asian Pacific Association for the Study of the Liver are complex and impractical to implement32,95 (Table 4). Additionally, a recent multicenter longitudinal cohort study in North America found that lower percentages of African American (AA) or black participants met the AASLD treatment criteria compared to Asians and white participants.96 The treatment initiation rates were highest among Asians and lowest among AA or blacks.96 This may be due to Africa-born AA or black participants have a lower prevalence of HBeAg and genotype A2 compared to US-born participants.96 Similarly, AA or blacks had a lower presence of HBeAg and or lower ALT levels compared to Asian participants.96 The lower prevalence of HBeAg and lower HBV-DNA levels among those who are HBeAg negative lead to lower percentages of AA or blacks meeting the treatment criteria.96 This emphasizes the presence of racial disparities within the treatment guidelines. Therefore, simplified treatment guidelines are important to reach all races, to reduce morbidity and mortality due to HBV, and to eliminate HBV as a public health threat.
Table 4.
Chronic HBV Infection Characteristics, Monitoring Guidelines, and Current Treatment Recommendations
| HBeAg positive | HBeAg negative | |
|---|---|---|
| Chronic HBV infection8,10,11 | High HBsAg levels High HBV-DNA levels (typically > 20,000 IU/mL) Normal ALT levels Monitoring guidelines
|
Anti-HBe antibodies positive Low HBsAg levels HBV-DNA levels 2000–20,000 IU/mL Normal ALT levels Monitoring guidelines
|
JSH, Japan Society of Hepatology; ULN, upper limit of normal.
Recently, a simplified treatment plan was recommended by Dieterich et al. They recommended antiviral treatment for those without cirrhosis who have HBV-DNA ≥2000 IU/mL and are 30 years of age or older or younger than 30 years with an ALT greater than the upper limit of normal (> 30U/L for males, > 19 U/L for females).95 They recommend cessation of treatment when all the following criteria are met: HBsAg loss, completion of at least 1 year of treatment, maintaining persistently normal ALT levels, undetectable HBV-DNA levels, and available for HBsAg monitoring for at least 2 years.95
Although this is a step in the right direction, we argue for a more simplified approach of treating all those who are HBV-DNA positive until there is HBsAg loss for at least 12 months (Figure). This is due to several factors. First, antiviral therapy has been shown to reduce inflammation and fibrosis; therefore, it will be beneficial even for those with normal liver enzymes.32 Second, there is still an increased risk for HCC- or liver-related death even for those with normal serum ALT levels and low HBV-DNA levels.97 Antiviral therapies have been shown to reduce integration of HBV-DNA, thereby reducing direct carcinogenesis.97 Additionally, early HBV treatment has been shown to reduce disease progression, improve inflammation, and even reverse cirrhosis, even in those with normal ALT levels.97 Furthermore, transmission can still occur with as little as 16 viral copies, which is equivalent to 3 IU.32 As mentioned above, there is a risk of transmission with blood exposures, MTCT, and sexual transmission, even with low HBV-DNA levels.
Several studies have estimated that the cost of expanding CHB therapy may be offset by the reduction in medical costs resulting from the disease progression of CHB. China has the largest CHB burden in the world. A recent study conducted in China investigated the effect of expanding CHB therapy to those with elevated ALT levels vs HBsAg-positive individuals.98 The study found that treating HBsAg positive, regardless of ALT levels, with 80% coverage not only allowed them to achieve WHO’s 2030 goal of a 65% reduction in HBV-related mortality but was also the most cost-effective in the long run by 2050.98 The study also found that this approach had the highest reduction in HBV-related complications and death, with the highest quality-adjusted life years compared to ALT level-based treatments.98
As mentioned above, there are additional poor outcomes associated with CHB. Those with CHB are at risk for extrahepatic manifestations (Table 1), coinfection with HDV with associated complications, reduced quality of life, and stigma. For these reasons, we urge the treatment of all who are HBV-DNA positive with an antiviral therapy until they are HBsAg negative for at least 12 months (Figure). Although expanding treatment guidelines may be difficult logistically, we urge government officials and the medical community to support this approach, as it will decrease morbidity and mortality due to HBV, improve the quality of life of patients with CHB, help achieve WHO’s goal of eliminating HBV as a health threat by 2030, and be cost-effective in the end.
Acute HBV Infection
Most adult patients who acquire HBV acutely do not require therapy as >95% of immunocompetent adult patients will recover spontaneously. It may be reasonable to treat in the context of severe liver injury or acute liver failure. Severe liver injury is defined by total bilirubin >3 mg/dL (or direct bilirubin >1.5 mg/dL), international normalized ratio >1.5, encephalopathy, or ascites. The preferred antiviral treatments are ETV, TAF, or TDF. INF-alpha is avoided due to the risk of decompensation. The treatment should be continued until HBsAg is no longer detectable, or indefinitely if the patient is to undergo liver transplantation.11
Resolved HBV Infection
Resolved HBV infection (ie, functional cure) is defined as HBsAg negative, anti-HBc total positive, HBeAg negative, and undetectable HBV-DNA. Spontaneous resolution of CHB with HBsAg loss is possible but only occurs in about 1% of patients per year. The progression of liver damage to cirrhosis or in patients with cirrhosis is halted with the loss of HBsAg unless the HBsAg loss occurs in individuals who are older than 50 years of age, already have cirrhosis, or have coinfection with HCV or HDV. Luckily, even in these patients, the disease progression is often halted. Once the patient achieves sustained HBsAg seroclearance, routine ALT and HBV-DNA surveillance is no longer needed. However, surveillance for HCC should continue if the patient has cirrhosis, has a first-degree relative with HCC, or has a long duration of infection (>40 years for males, >50 years for females).11,53
HBV in Pregnancy, Postpartum, and Newborn
All pregnant patients should be screened for HBV with HBsAg, core total antibody, and surface antibody testing. However, if the pregnant person has a history of appropriately timed triple-panel screening without subsequent exposure risk, only HBsAg screening is needed.16 If necessary, HBV vaccination is recommended and is safe and efficacious during pregnancy. Although CHB infection typically does not confer teratogenicity, the mother should receive antiviral therapy in the third trimester if serum HBV-DNA is greater than 200,000 IU/mL. TDF is the preferred choice due to its antiviral potency and concerns about resistance with other antiviral agents.11 There was no difference in rates of prematurity, congenital malformations, or Apgar scores between babies birthed to TDF-treated and untreated women.99, 100, 101 Clinical studies have shown safety in breastfeeding when receiving antiviral treatments.102,103 Additionally, a newborn from a mother with HBV infection should receive HBIG and HBV vaccine within 12 hours after delivery.104,105
Coinfection With HDV
These authors agree with the European Association for the Study of the Liver guidelines and the Hepatitis B Foundation’s stance that all patients who are HBsAg positive should be tested for HDV with antibody tests. We disagree with the AASLD guidance that recommends testing only if patients are considered at risk. Risk-based testing has failed for HIV, HCV, and HDV, as well as HBV. The risk factors that would stimulate repeat testing include patients with HIV and or HCV, IVDU, MSM, those with multiple sexually transmitted infections, patients born in high HDV-endemic areas, and those with ongoing risk behaviors.
The initial screening test for HDV is anti-hepatitis Delta virus total antibody; if positive, HDV-RNA quantification should be checked. If there is a diagnosis of HDV infection with polymerase chain reaction testing, treatment should be considered promptly. The preferred treatment for chronic HDV infection is pegINF-alpha for 12–18 months with no difference in efficacy between pegINF-alpha-2a and -2b11,92 in patients with no evidence of hepatic decompensation. The treatment should be continued for up to 6 months after HDV-RNA becomes undetectable, which is seen in almost 25% of patients. After 12–18 months, treatment for chronic HDV can be stopped with ALT normalization and decline of HDV-RNA.92 In some cases, HBV-DNA levels can increase during the treatment. If the HBV-DNA levels increase after stopping INF, we recommend health-care providers continue or start nucleos(t)ide analogs (ETV, TDF, or TAF)11 in all HBV-DNA-positive patients.
There are many novel treatments being developed for HBV-HDV infection, such as small interfering RNAs (siRNAs), nucleic acid polymers, and the entry-blocking antiviral bulevirtide (BLV). BLV inhibits the binding of HBsAg to sodium taurocholate cotransporting polypeptide. This blocks the entry of both HBV and HDV.106,107 BLV was conditionally approved for the treatment of HDV by the European Medicines Agency in 2020108 as a chronic suppressive therapy. Given its novelty, we do not have clear clinical guidance on the drug, optimal dose, duration, the need for combination therapy, accepted virological efficacy marker, or criteria for stopping therapy.109
Coinfection With HCV
Every patient with positive HBsAg should be tested for HCV. HBV-associated HCV coinfections in many countries are in the 0.9%–5% range and are often seen in patients with a history of IVDU, HIV, end-stage renal disease receiving hemodialysis, and frequent blood transfusions. Those with coinfection are at increased risk for progression to cirrhosis, HCC, and liver-related deaths. The coinfection may occur simultaneously or as a superinfection (infection of HCV after HBV infection).110 HCV treatment should be started when HCV-RNA levels are detectable. The disease progression can be monitored by measuring HBV-DNA and HCV-RNA levels.111 Due to the risk of HBV flares during HCV treatment, HBV-DNA should be monitored every 4–8 weeks during and for 3 months after treatment of HCV.112 Providers can consider prophylactic therapy with nucleos(t)ide analogs. If liver enzymes, especially ALT, fail to normalize or increase despite declining HCV-RNA, it is recommended to test for HBsAg and HBV-DNA to check for HBV reactivation in anti-HBc-positive patients. If laboratory findings suggest HBV reactivation, HBV antiviral therapy should be initiated.11 If the patient is found to meet the criteria for both HBV and HCV therapies, HBV antiviral therapy should be started, along with direct antiviral therapy for HCV. There are no known drug-to-drug interactions between HBV and HCV antiviral therapy.11
Emerging Therapies
New therapies are being studied that would permit discontinuation of nucleos(t)ide analogs and possible cure. There are several new drugs that are currently under development that target HBV replication cycle or enhance human immune response (Table 5).
Table 5.
HBV Therapy Targets and Associated Drugs
| HBV therapy targets | Associated drugs |
|---|---|
| HBV life cycle | |
| Blocking entry | BLV |
| Blocking protein synthesis (siRNA, LNA, ASO) | ARC520 RG6004, RO7062931 GSK3389404 Bepirovirsen |
| Inactivating cccDNA (CRISPR-Cas9) | |
| Blocking core synthesis (CpAMs) | NVR3-778 JNJ-6379 Vebicorvir (ABI-H0731) |
| Blocking release and formation of virions (HBsAg) | REP-2139 |
| Directly inhibit HBsAg with monoclonal antibody | Lenvervimab VIR-3434 |
| Vaccines | |
| Mediate T-cell response | GS-4774 |
| Stimulate innate immune response | |
| TLR7 agonist | Vestaolimod (GS-9620) |
| TLR8 agonist | GS-9688 |
| RIG-1 agonist | SB-9200 |
| PD-1 and PD-1 inhibitors | |
LNA, locked nucleic acid.
HBV Life Cycle
There are many modes by which an antiviral can disrupt the HBV life cycle: blocking HBV entry, blocking HBV protein synthesis, blocking core synthesis, inactivating closed covalent circular DNA (cccDNA), and blocking the release and formation of virions.
-
a.
Blocking entry: BLV is an entry-blocking antiviral that competitively and irreversibly binds to the large HBsAg envelope protein, preventing entry of the HBV into healthy cells.92,113,114 It has been approved by the European Union for HDV-RNA-positive chronic HDV hepatitis. It has shown to improve HDV-RNA clearance when used synergistically with other antivirals.115
-
b.
Blocking HBV protein synthesis (siRNA, locked nucleic acid, antisense oligonucleotide [ASO]): There are many antivirals that are being developed that target HBV synthesis: siRNA, locked nucleic acid, and ASO target specific gene products and cause degradation of mRNA. siRNA limitations include the lack of reduction in cccDNA, unmediated effects on other organs (ie, kidney), and problems with the mode of delivery.92,93 siRNA is administered parenterally as it is degraded in the gastrointestinal system. A new form in subcutaneous injection is also on the market with fewer side effects. The subcutaneous form targets the liver and requires less frequent dosing, reducing its negative effects on off-target sites and inducing host immune response. Pairing siRNA with N-acetylgalactosamine enhances liver cell uptake via the asialoglycoprotein receptor.116,117
An siRNA ARC520 can silence transcription of HBsAg by targeting cccDNA-derived pregenomic RNA (pgRNA) in HBeAg-positive patients.92,118 In a randomized multicenter study, high doses of ARC520 (2 mg/kg) showed significant reduction of HBsAg levels compared to placebo. HBsAg reduction persisted longer (>85 days) after the last dose in HBeAg-positive patients when compared to HBeAg-negative patients (85 days). This is likely due to the origin of the siRNA derivation. Overall, ARC520 was well tolerated.92,118
There are several ASO-based drugs that are approved for treatment of HBV infection. Previous N-acetylgalactosamine ASOs (RG6004, RO7062931 and GSK3389404) have shown to have variable HBsAg decline.119,120 Bepirovirsen is an unconjugated variant of GSK3389404 and is delivered mainly to liver cells, liver sinusoidal endothelial cells, and Kupffer cells, unlike GSK3389404, which is delivered primarily to hepatocytes. It has shown significant reduction of HBsAg and HBV-DNA in treatment-naive patients with CHB121 with direct effects on mRNA as well as immune stimulation. There was a dose-dependent HBsAg reduction regardless of baseline HBsAg, with 9% of participants maintaining HBsAg and HBV-DNA loss 24 weeks after the end of treatment.119
-
c.
Inactivating cccDNA (CRISPR-Cas9): The only way to decrease the risk of reactivation is to deactivate or eliminate cccDNA. cccDNA clearance is also needed for complete and sterilizing cure of HBV. Within bacteria, short DNA sequences from the virus are inserted into the CRISPR within the bacterial genome. This serves as a memory function to remember previous infections. The mechanism of viral clearance is through triggers of CRISPR-associated nuclease to cause double-stranded breaks at the viral DNA sequence, causing deactivation of the targeted genes. In humans, CRISPR/Cas9 has been studied for hereditary gene editing. In vitro CRISPR/Cas9 experiments have shown rapid reduction of cccDNA and HBV proteins. Combining siRNA with CRISPR/Cas9 has shown improved reduction and even disappearance of cccDNA.122,123
There are a few challenges to using CRISPR-Cas9 for patients with CHB. There is a risk of cross-reactivity with human genetic material and a need for a delivery system specific to hepatocytes. Currently, there are no standardized assays for cccDNA, which limits the measurement of end points (changes in the level of cccDNA and their proteins). If even 1 residual cccDNA survives, it could result in persistent HBV infection.123
-
d.
Blocking core synthesis (capsid assembly modulators [CpAMs]): The CpAMs target capsid assembly or cause disassembly, creating aberrant capsids or empty capsids.124 This process inhibits formation and release of new viruses, reduces spread of the virus to uninfected cells, and prevents relaxed-circular DNA transformation to cccDNA.92,125 Unfortunately, several CpAMs have been discontinued due to liver toxicity (AB-506, AB-836, and ABI-H2173).126 Currently, there are several core protein allosteric modulators being studied.
NVR3-778 is a class I CpAM that is being studied alone or in combination with pegINF or ETV. Combination therapy with pegINF was found to reduce HBV-DNA levels more so than with CpAM alone but did not reduce HBsAg levels, and viral rebound was observed after treatment cessation.92,127
JNJ-6379 is a class II CpAM that is currently being studied in a phase 2 trial93,128 as a combination therapy. This drug has so far shown dose-dependent antiviral activity, with decreases in HBV-DNA and HBV-RNA. However, this drug is limited in its efficacy since it does not target HBsAg, and subviral particles (SVPs) of HBsAg can still be released into the blood.
One promising class II CpAM was vebicorvir (ABI-H0731); however, development of this drug was recently discontinued in March 2021 as the phase 2b trial did not achieve the desired antiviral effect in patients with CHB infection.129 Although recent data showed 100% of HBeAg-negative patients with CHB achieved virological suppression of HBV-DNA plus pgRNA <20 IU/mL after 48 weeks of treatment when vebicorvir was combined with ETV, TDF, or TAF, the study did not demonstrate improvements in viral antigen levels.93,129
-
e.
Blocking release and formation of virions (HBsAg): Most of the HBsAg that is circulating in the blood is assembled into noninfectious HBV SVPs.130, 131, 132 HBsAg can be targeted by preventing its release from infected hepatocytes. REP-2139 is a nucleic acid polymer that targets the assembly and or secretion of SVPs, reducing circulating HBsAg levels.133 A phase 2 trial showed that REP-2139 in combination with TDF or pegINF in HBeAg-negative patients resulted in significantly reduced HBsAg levels in 60% of patients and functional cure in 35% of patients at follow-up after 48 weeks without treatment.133
-
f.
HBsAg inhibition with monoclonal antibody: HBsAg can also be targeted directly with a recombinant human monoclonal antibody. Lenvervimab can bind to HBsAg directly and neutralize the antigen by forming ICs, thereby preventing entry into hepatocytes. In a prospective trial, there was a correlation between baseline HBsAg and sustained HBsAg loss.134
When a monoclonal antibody targeting HBsAg (VIR-3434) and siRNA was combined, a significant reduction in HBsAg levels in all participants and HBsAg <10 IU/mL in most participants were observed. The HBsAg levels were reduced more in combination therapy than either alone, affirming the complimentary effects.135
Therapeutic vaccines
The patient’s own immune response to HBV infection, if robust enough, can clear HBV infection. The goal of therapeutic vaccines is to stimulate host immune system to restore HBV-specific immune control and suppress HBV activity while ultimately inducing HBsAg loss.
One vaccine known as GS-4774 has shown to mediate T-cell response in 90% of volunteers with very few of the volunteers developing low-level anti-HBsAg. This vaccine uses a recombinant cerevisiae yeast to express surface, core, and X proteins. Unfortunately, it did not produce significant reduction in HBsAg levels.136 Another vaccine that is being studied is INO-1800, made from multiple HBV proteins that contain the HBsAg and HBV core antigen encoding.93
Stimulation of Innate Immune Response
TLR7
Vestaolimod (GS-9620) is an oral toll-like receptor (TLR) 7 agonist currently being studied. TLR7 influences both innate and adaptive immune response as well as antiviral cytokine responses. In mammals, vestaolimod resulted in a reduction of HBV-DNA levels and a loss of HBsAg. In 2 randomized trials, weekly administration led to viral suppression but had no effect on HBsAg levels in treatment-naive patients with CHB.92
TLR8
Myeloid cells (myeloid dendritic cells, monocytes, and Kupffer cells) express toll-like receptor (TLR) 8. Activation of TLR8 causes maturation of professional antigen cells, mostly in the gut lymphoid tissue and the liver. In animals, TLR8 activation induced innate immune response without adverse systemic INF-alpha. Currently, the TLR8 agonist, GS-9688, is being studied.93
RIG-1
Retinoic acid-inducible protein (RIG-1) is a cytosolic sensor of RNA that activates interferon regulatory factor 3 and nuclear factor kappa light chain enhancer of activated B cells. RIG-1 has been shown to bind HBV pgRNA and interfere with HBV replication while inducing INF and cytokine production. SB-9200, a RIG-1 activator, is a prodrug, a relative of dinucleotide SB900 that resulted in INF-mediated antiviral immune response in infected cells.137 The development of this drug was stopped after unexpected liver toxicity.
PD-1 and PD-1 inhibitors
Programmed death-1 (PD-1) receptor is the most expressed in HBV-specific T cells in the liver. A recent clinical trial of anti-PD-1 in patients with chronic HDV showed a significant decrease in HBsAg, and 1 patient achieved complete seroconversion. However, more studies are needed to determine the right dosage to avoid triggering autoimmune conditions and immune-mediated HBV flares.92,138 Oral liver-targeted PD-1 inhibitors are in development as well.
With these new therapies, we are headed toward HBV treatment with combination therapy. There will likely be 3–4 medications and or antivirals used together to achieve synergistic or additive results. We are hopeful that these will lead to increased functional cure of HBV with a finite course of therapy.
Linkage to Care
Access to HBV testing, diagnosis, and treatment are all limited by access to care. Even for those who are aware of their HBV status, access to clinical services may be limited. Unfortunately, the limited number of specialists available in LMICs and the location of specialists only in major cities in high-income countries2 make it hard to access testing and treatment in both developing and developed countries. Therefore, there needs to be more public and political efforts to improve HBV awareness and access to care if we are to eliminate HBV as a public threat. One of the best success stories is the San Francisco Hep B Free Campaign: it used wide community and political efforts to destigmatize HBV while increasing awareness and access to care.139
Conclusion
In conclusion, HBV is a global health issue that disproportionately affects people from LMICs, with the highest prevalence in Africa, Western Pacific, East Asia, and Southeast Asia regions.1,4 In the United States, HBV is most frequently seen in Asian immigrants.89,90 The prevalence of HBV is partly due to the limited access to care, laboratory tests, and understanding of HBV transmission in the community. Although there are treatments for HBV, there still is no definitive cure. This review summarized different presentations of HBV infection (HDV, extrahepatic manifestations, and HCC), social barriers to care, current treatments, and promising future therapies that may lead to the cure of CHB. There is strong effort globally to simplify the guidelines and expand testing and treatment to all patients who are HBV-DNA positive because of the benefit on infectivity, quality of life, stigma, HCC, and cirrhosis risk as well as decreased mortality in all stages of HBV infection. We propose a simplified 5-line guideline for tackling CHB infection worldwide.
Acknowledgments:
We thank Kelly Schrank, MA, ELS, of Bookworm Editing Services LLC for her editorial services in preparing the manuscript for publication.
Authors' Contributions:
Katerina Roma - initial draft, revision of the article, and approval of the final draft submitted. Toni-Marie Chandler - initial draft, revision of the article, and approval of the final draft submitted. Zahra Dossaji - revision of the article, and approval of the final draft submitted. Ankoor Patel - revision of the article, and approval of the final draft submitted. Kapil Gupta - revision of the article, and approval of the final draft submitted. Vinod Rustgi - study design, revision of the article, and approval of the final draft submitted. Robert Gish - study design, revision of the article, and approval of the final draft submitted.
Footnotes
Conflicts of Interest: This author discloses the following: Vinod Rustgi is a member of the Board of Editors. Their paper was handled in accordance with our conflict-of-interest policy. See https://www.ghadvances.org/content/authorinfo#conflict_of_interest_policy for full details. The remaining authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The study did not require the approval of an institutional review board.
Reporting Guidelines: Not applicable for this article type.
References
- 1.World Health Organization Hepatitis B. 2021. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- 2.Polaris Observatory HCV at-a-Glance. Regions - database. CDA foundation website. https://cdafound.org/polaris-regions-database/
- 3.Kawanaka M., Nishino K., Kawamoto H., et al. Hepatitis B: who should be treated?-managing patients with chronic hepatitis B during the immune-tolerant and immunoactive phases. World J Gastroenterol. 2021;27(43):7497–7508. doi: 10.3748/wjg.v27.i43.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim J.K., Nguyen M.H., Kim W.R., et al. Prevalence of chronic hepatitis B virus infection in the United States. Am J Gastroenterol. 2020;115(9):1429–1438. doi: 10.14309/ajg.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 5.Tang L.S.Y., Covert E., Wilson E., et al. Chronic hepatitis B infection: a review. JAMA. 2018;319(17):1802–1813. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 6.Im Y.R., Jagdish R., Leith D., et al. Prevalence of occult hepatitis B virus infection in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(10):932–942. doi: 10.1016/S2468-1253(22)00201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drafting committee for hepatitis management guidelines, the Japan society of Hepatology. Japan society of Hepatology guidelines for the management of hepatitis B virus infection: 2019 update. Hepatol Res. 2020;50(8):892–923. doi: 10.1111/hepr.13504. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Martin P., Nguyen M.H., Dieterich D.T., et al. Treatment algorithm for managing chronic hepatitis B virus infection in the United States: 2021 Update. Clin Gastroenterol Hepatol. 2022;20(8):1766–1775. doi: 10.1016/j.cgh.2021.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Sarin S.K., Kumar M., Lau G.K., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terrault N.A., Lok A.S.F., McMahon B.J., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moini M., Fung S. HBsAg loss as a treatment endpoint for chronic HBV infection: HBV cure. Viruses. 2022;14(4):657. doi: 10.3390/v14040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song A., Lin X., Chen X. Functional cure for chronic hepatitis B: accessibility, durability, and prognosis. Virol J. 2021;18(1):114. doi: 10.1186/s12985-021-01589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolders E.J., Burger D.M., Feld J.J., et al. Review article: clinical pharmacology of current and investigational hepatitis B virus therapies. Aliment Pharmacol Ther. 2020;51(2):231–243. doi: 10.1111/apt.15581. [DOI] [PubMed] [Google Scholar]
- 15.Toy M., Hutton D., Harris A.M., et al. Cost-effectiveness of 1-time universal screening for chronic hepatitis B infection in adults in the United States. Clin Infect Dis. 2022;74(2):210–217. doi: 10.1093/cid/ciab405. [DOI] [PubMed] [Google Scholar]
- 16.Conners E.E., Panagiotakopoulos L., Hofmeister M.G., et al. Screening and testing for hepatitis B virus infection: CDC recommendations — United States, 2023. MMWR Recomm Rep. 2023;72(1):1–25. doi: 10.15585/mmwr.rr7201a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A., Quigg R.J. Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis. 2015;22(5):343–351. doi: 10.1053/j.ackd.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Su T.H., Liu C.J., Tseng T.C., et al. Chronic hepatitis B is associated with an increased risk of B-cell non-Hodgkin's lymphoma and multiple myeloma. Aliment Pharmacol Ther. 2019;49(5):589–598. doi: 10.1111/apt.15132. [DOI] [PubMed] [Google Scholar]
- 19.Adjei C.A., Stutterheim S.E., Naab F., et al. Chronic hepatitis B stigma in Ghana: a qualitative study with patients and providers. BMJ Open. 2019;9(6) doi: 10.1136/bmjopen-2018-025503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asadi-Lari M., Tamburini M., Gray D. Patients' needs, satisfaction, and health related quality of life: towards a comprehensive model. Health Qual Life Outcomes. 2004;2:32. doi: 10.1186/1477-7525-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younossi Z.M., Stepanova M., Younossi I., et al. Development and validation of a hepatitis B-specific health-related quality-of-life instrument: CLDQ-HBV. J Viral Hepat. 2021;28(3):484–492. doi: 10.1111/jvh.13451. [DOI] [PubMed] [Google Scholar]
- 22.Castellanos-Fernández M.I., Borges-González S.A., Stepanova M., et al. Health-related quality of life in Cuban patients with chronic liver disease: a real-world experience. Ann Hepatol. 2021;22 doi: 10.1016/j.aohep.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Prinsen C.A.C., Mokkink L.B., Bouter L.M., et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–1157. doi: 10.1007/s11136-018-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho I.Y., Chang Y., Sung E., et al. Depressive symptoms and risk of liver-related mortality in individuals with hepatitis B virus infection: a cohort study. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-77886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon A.M., Singal A.G., Tapper E.B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinman S.H., Lelie N., Busch M.P. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion. 2009;49(11):2454–2489. doi: 10.1111/j.1537-2995.2009.02322.x. [DOI] [PubMed] [Google Scholar]
- 27.McCullough J., Alter H.J., Ness P.M. Interpretation of pathogen load in relationship to infectivity and pathogen reduction efficacy. Transfusion. 2019;59(3):1132–1146. doi: 10.1111/trf.15103. [DOI] [PubMed] [Google Scholar]
- 28.Tabuchi A., Tanaka J., Katayama K., et al. Titration of hepatitis B virus infectivity in the sera of pre-acute and late acute phases of HBV infection: transmission experiments to chimeric mice with human liver repopulated hepatocytes. J Med Virol. 2008;80(12):2064–2068. doi: 10.1002/jmv.21320. [DOI] [PubMed] [Google Scholar]
- 29.Yotsuyanagi H., Yasuda K., Moriya K., et al. Frequent presence of HBV in the sera of HBsAg-negative, anti-HBc-positive blood donors. Transfusion. 2001;41(9):1093–1099. doi: 10.1046/j.1537-2995.2001.41091093.x. [DOI] [PubMed] [Google Scholar]
- 30.Bremer C.M., Saniewski M., Wend U.C., et al. Transient occult hepatitis B virus infection in a blood donor with high viremia. Transfusion. 2009;49(8):1621–1629. doi: 10.1111/j.1537-2995.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 31.Allain J.P., Mihaljevic I., Gonzalez-Fraile M.I., et al. Infectivity of blood products from donors with occult hepatitis B virus infection. Transfusion. 2013;53(7):1405–1415. doi: 10.1111/trf.12096. [DOI] [PubMed] [Google Scholar]
- 32.McNaughton A.L., Lemoine M., van Rensburg C., et al. Extending treatment eligibility for chronic hepatitis B virus infection. Nat Rev Gastroenterol Hepatol. 2021;18(3):146–147. doi: 10.1038/s41575-020-00398-x. [DOI] [PubMed] [Google Scholar]
- 33.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34(Suppl 1):S1–S3. doi: 10.1016/s1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 34.Jonas M.M. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29(Suppl 1):133–139. doi: 10.1111/j.1478-3231.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen C.J., Iloeje U.H., Yang H.I. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11(4):797–816. doi: 10.1016/j.cld.2007.08.005. viii. [DOI] [PubMed] [Google Scholar]
- 36.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 37.Goyal A., Murray J.M. The impact of vaccination and antiviral therapy on hepatitis B and hepatitis D epidemiology. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vodkin I., Patton H. Management of hepatitis B virus infection during pregnancy. Minerva Gastroenterol Dietol. 2014;60(4):205–214. [PubMed] [Google Scholar]
- 39.Okada K., Kamiyama I., Inomata M., et al. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976;294(14):746–749. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- 40.Beasley R.P., Trepo C., Stevens C.E., et al. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105(2):94–98. doi: 10.1093/oxfordjournals.aje.a112370. [DOI] [PubMed] [Google Scholar]
- 41.Yi P., Chen R., Huang Y., et al. Management of mother-to-child transmission of hepatitis B virus: propositions and challenges. J Clin Virol. 2016;77:32–39. doi: 10.1016/j.jcv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Wiseman E., Fraser M.A., Holden S., et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190(9):489–492. doi: 10.5694/j.1326-5377.2009.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 43.Zou H., Chen Y., Duan Z., et al. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19(2):e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 44.Nelson N.P., Jamieson D.J., Murphy T.V. Prevention of perinatal hepatitis B virus transmission. J Pediatric Infect Dis Soc. 2014;3(Suppl 1):S7–S12. doi: 10.1093/jpids/piu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han G.R., Xu C.L., Zhao W., et al. Management of chronic hepatitis B in pregnancy. World J Gastroenterol. 2012;18(33):4517–4521. doi: 10.3748/wjg.v18.i33.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alter M.J. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39(Suppl 1):S64–S69. doi: 10.1016/s0168-8278(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 47.Carl M., Blakey D.L., Francis D.P., et al. Interruption of hepatitis B transmission by modification of a gynaecologist's surgical technique. Lancet. 1982;1(8274):731–733. doi: 10.1016/s0140-6736(82)92636-8. [DOI] [PubMed] [Google Scholar]
- 48.Alter M.J., Margolis H.S. The emergence of hepatitis B as a sexually transmitted disease. Med Clin North Am. 1990;74(6):1529–1541. doi: 10.1016/s0025-7125(16)30493-x. [DOI] [PubMed] [Google Scholar]
- 49.Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 50.Petruzziello A. Epidemiology of hepatitis B virus (HBV) and hepatitis C virus (HCV) related hepatocellular carcinoma. Open Virol J. 2018;12:26–32. doi: 10.2174/1874357901812010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Omata M., Cheng A.L., Kokudo N., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simonetti J., Bulkow L., McMahon B.J., et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51(5):1531–1537. doi: 10.1002/hep.23464. [DOI] [PubMed] [Google Scholar]
- 53.Heimbach J.K., Kulik L.M., Finn R.S., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 54.EASL Clinical Practice Guidelines Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Tran S., Jeong D., Henry L., et al. Initial evaluation, long-term monitoring, and hepatocellular carcinoma surveillance of chronic hepatitis B in routine practice: a nationwide US Study. Am J Gastroenterol. 2021;116(9):1885–1895. doi: 10.14309/ajg.0000000000001271. [DOI] [PubMed] [Google Scholar]
- 56.Rizzo G.E.M., Cabibbo G., Craxì A. Hepatitis B virus-associated hepatocellular carcinoma. Viruses. 2022;14(5):986. doi: 10.3390/v14050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tayob N., Kanwal F., Alsarraj A., et al. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): a phase 3 biomarker study in the United States. Clin Gastroenterol Hepatol. 2023;21(2):415–423.e4. doi: 10.1016/j.cgh.2022.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J.H., Kim Y.D., Lee M., et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. 2018;69(5):1066–1073. doi: 10.1016/j.jhep.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 59.Johnson P.J., Pirrie S.J., Cox T.F., et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23(1):144–153. doi: 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]
- 60.Schotten C., Ostertag B., Sowa J.P., et al. GALAD score detects early-stage hepatocellular carcinoma in a European cohort of chronic hepatitis B and C patients. Pharmaceuticals (Basel) 2021;14(8):735. doi: 10.3390/ph14080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teng W., Liu Y.C., Jeng W.J., et al. Tertiary prevention of HCC in chronic hepatitis B or C infected patients. Cancers (Basel) 2021;13(7):1729. doi: 10.3390/cancers13071729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J.C., Huang Y.H., Chau G.Y., et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51(5):890–897. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Hung I.F., Poon R.T., Lai C.L., et al. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103(7):1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 64.Kubo S., Hirohashi K., Tanaka H., et al. Effect of viral status on recurrence after liver resection for patients with hepatitis B virus-related hepatocellular carcinoma. Cancer. 2000;88(5):1016–1024. doi: 10.1002/(sici)1097-0142(20000301)88:5<1016::aid-cncr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 65.Yin J., Li N., Han Y., et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31(29):3647–3655. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 66.Giri S., Agrawal D., Afzalpurkar S., et al. Tenofovir versus entecavir for tertiary prevention of hepatocellular carcinoma in chronic hepatitis B infection after curative therapy: a systematic review and meta-analysis. J Viral Hepat. 2023;30:108–115. doi: 10.1111/jvh.13766. [DOI] [PubMed] [Google Scholar]
- 67.Mazzaro C., Adinolfi L.E., Pozzato G., et al. Extrahepatic manifestations of chronic HBV infection and the role of antiviral therapy. J Clin Med. 2022;11(21):6247. doi: 10.3390/jcm11216247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gayraud M., Guillevin L., le Toumelin P., et al. Long-term follow-up of polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: analysis of four prospective trials including 278 patients. Arthritis Rheum. 2001;44(3):666–675. doi: 10.1002/1529-0131(200103)44:3<666::AID-ANR116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 69.Guillevin L., Lhote F., Cohen P., et al. Polyarteritis nodosa related to hepatitis B virus. A prospective study with long-term observation of 41 patients. Medicine (Baltimore) 1995;74(5):238–253. doi: 10.1097/00005792-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Cacoub P., Saadoun D., Bourlière M., et al. Hepatitis B virus genotypes and extrahepatic manifestations. J Hepatol. 2005;43(5):764–770. doi: 10.1016/j.jhep.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 71.Cacoub P., Terrier B. Hepatitis B-related autoimmune manifestations. Rheum Dis Clin North Am. 2009;35(1):125–137. doi: 10.1016/j.rdc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Hočevar A., Tomšič M., Perdan Pirkmajer K. Clinical approach to diagnosis and therapy of polyarteritis nodosa. Curr Rheumatol Rep. 2021;23(3):14. doi: 10.1007/s11926-021-00983-2. [DOI] [PubMed] [Google Scholar]
- 73.Cacoub P., Comarmond C., Domont F., et al. Cryoglobulinemia vasculitis. Am J Med. 2015;128(9):950–955. doi: 10.1016/j.amjmed.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 74.Ferri C., Zignego A.L., Giuggioli D., et al. HCV and cryoglobulinemic vasculitis. Cleve Clin J Med. 2002;69(Suppl 2):SII20–SII23. doi: 10.3949/ccjm.69.suppl_2.sii20. [DOI] [PubMed] [Google Scholar]
- 75.Roccatello D., Saadoun D., Ramos-Casals M., et al. Cryoglobulinaemia. Nat Rev Dis Primers. 2018;4(1):11. doi: 10.1038/s41572-018-0009-4. [DOI] [PubMed] [Google Scholar]
- 76.Silva F., Pinto C., Barbosa A., et al. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105 doi: 10.1016/j.jaut.2019.102313. [DOI] [PubMed] [Google Scholar]
- 77.Cacoub P., Asselah T. Hepatitis B virus infection and extra-hepatic manifestations: a systemic disease. Am J Gastroenterol. 2022;117(2):253–263. doi: 10.14309/ajg.0000000000001575. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y.L., Jing J., Mo Y.Q., et al. Presence of hepatitis B virus in synovium and its clinical significance in rheumatoid arthritis. Arthritis Res Ther. 2018;20(1):130. doi: 10.1186/s13075-018-1623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu F., Li G., Hao W., et al. Hepatitis B virus-related glomerulonephritis with positive and negative serum HBsAg: different clinicopathologic characteristics of two clinical subtypes. Int J Gen Med. 2021;14:3069–3077. doi: 10.2147/IJGM.S318087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lai K.N., Lai F.M. Clinical features and the natural course of hepatitis B virus-related glomerulopathy in adults. Kidney Int Suppl. 1991;35:S40–S45. [PubMed] [Google Scholar]
- 81.Xie Q., Li Y., Xue J., et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol. 2015;41(4-5):345–353. doi: 10.1159/000431331. [DOI] [PubMed] [Google Scholar]
- 82.Rong X., Wang H., Ma J., et al. Chronic hepatitis B virus infection is associated with a poorer prognosis in diffuse large B-cell lymphoma: a meta-analysis and systemic review. J Cancer. 2019;10(15):3450–3458. doi: 10.7150/jca.31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Urban S., Neumann-Haefelin C., Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 2021;70(9):1782–1794. doi: 10.1136/gutjnl-2020-323888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miao Z., Zhang S., Ou X., et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J Infect Dis. 2020;221(10):1677–1687. doi: 10.1093/infdis/jiz633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stockdale A.J., Kreuels B., Henrion M.Y.R., et al. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol. 2020;73(3):523–532. doi: 10.1016/j.jhep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen H.Y., Shen D.T., Ji D.Z., et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68(3):512–521. doi: 10.1136/gutjnl-2018-316601. [DOI] [PubMed] [Google Scholar]
- 87.Weller M.L., Gardener M.R., Bogus Z.C., et al. Hepatitis delta virus detected in salivary glands of Sjögren's Syndrome patients and recapitulates a Sjögren's Syndrome-like phenotype in vivo. Pathog Immun. 2016;1(1):12–40. doi: 10.20411/pai.v1i1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Philipp T., Obermayer-Straub P., Manns M.P. Autoantibodies in hepatitis delta. Biomed Pharmacother. 1995;49(7-8):344–349. doi: 10.1016/0753-3322(96)82663-1. [DOI] [PubMed] [Google Scholar]
- 89.Cotler S.J., Cotler S., Xie H., et al. Characterizing hepatitis B stigma in Chinese immigrants. J Viral Hepat. 2012;19(2):147–152. doi: 10.1111/j.1365-2893.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 90.Dam L., Cheng A., Tran P., et al. Hepatitis B stigma and knowledge among Vietnamese in Ho chi Minh city and Chicago. Can J Gastroenterol Hepatol. 2016;2016 doi: 10.1155/2016/1910292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buster E.H., Flink H.J., Cakaloglu Y., et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135(2):459–467. doi: 10.1053/j.gastro.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen M.H., Wong G., Gane E., et al. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev. 2020;33(2) doi: 10.1128/CMR.00046-19. e00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee H.W., Lee J.S., Ahn S.H. Hepatitis B virus cure: targets and future therapies. Int J Mol Sci. 2020;22(1):213. doi: 10.3390/ijms22010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weng M.K., Doshani M., Khan M.A., et al. Hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the advisory committee on immunization practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:477–483. doi: 10.15585/mmwr.mm7113a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dieterich D., Graham C., Wang S., et al. It is time for a simplified approach to hepatitis B elimination. Gastro Hep Adv. 2023;2(2):209–218. [Google Scholar]
- 96.Khalili M., Leonard K.R., Ghany M.G., et al. Racial disparities in treatment initiation and outcomes of chronic hepatitis B virus infection in North America. JAMA Netw Open. 2023;6(4) doi: 10.1001/jamanetworkopen.2023.7018. e237018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim Y.-S., Kim W.R., Dieterich D., et al. Evidence for benefits of early treatment initiation for chronic hepatitis B. Viruses. 2023;15(4):997. doi: 10.3390/v15040997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang S., Wang C., Liu B., et al. Cost-effectiveness of expanded antiviral treatment for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Reg Health West Pac. 2023;35 doi: 10.1016/j.lanwpc.2023.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacobson D.L., Patel K., Williams P.L., et al. Growth at 2 years of age in HIV-exposed uninfected children in the United States by trimester of maternal antiretroviral initiation. Pediatr Infect Dis J. 2017;36(2):189–197. doi: 10.1097/INF.0000000000001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jao J., Abrams E.J., Phillips T., et al. In utero tenofovir exposure is not associated with fetal long bone growth. Clin Infect Dis. 2016;62(12):1604–1609. doi: 10.1093/cid/ciw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nachega J.B., Uthman O.A., Mofenson L.M., et al. Safety of tenofovir disoproxil fumarate-based antiretroviral therapy regimens in pregnancy for HIV-infected women and their infants: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2017;76(1):1–12. doi: 10.1097/QAI.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benaboud S., Pruvost A., Coffie P.A., et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55(3):1315–1317. doi: 10.1128/AAC.00514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mirochnick M., Taha T., Kreitchmann R., et al. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr. 2014;65(1):33–41. doi: 10.1097/QAI.0b013e3182a921eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weinbaum C.M., Williams I., Mast E.E., et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- 105.Mast E.E., Weinbaum C.M., Fiore A.E., et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR-16):1–33. quiz CE1-4. [PubMed] [Google Scholar]
- 106.Blanchet M., Sureau C. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J Virol. 2007;81(11):5841–5849. doi: 10.1128/JVI.00096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ni Y., Lempp F.A., Mehrle S., et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146(4):1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 108.Buti M., Wedemeyer H., Aleman S., et al. Bulevirtide improves health related quality life measured by EQ-5D VAS in patients with chronic hepatitis delta: an exploratory analysis of a phase 3 trial at 48 weeks. Hepatology. 2022;76:S224–S225. [Google Scholar]
- 109.Ferenci P., Reiberger T., Jachs M. Treatment of chronic hepatitis D with bulevirtide-A fight against two foes-an update. Cells. 2022;11(22):3531. doi: 10.3390/cells11223531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Potthoff A., Manns M.P., Wedemeyer H. Treatment of HBV/HCV coinfection. Expert Opin Pharmacother. 2010;11(6):919–928. doi: 10.1517/14656561003637659. [DOI] [PubMed] [Google Scholar]
- 111.Hepatitis C Guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018;67(10):1477–1492. doi: 10.1093/cid/ciy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terrault N.A., Bzowej N.H., Chang K.M., et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Urban S., Bartenschlager R., Kubitz R., et al. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147(1):48–64. doi: 10.1053/j.gastro.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 114.Asselah T., Loureiro D., Tout I., et al. Future treatments for hepatitis delta virus infection. Liver Int. 2020;40(Suppl 1):54–60. doi: 10.1111/liv.14356. [DOI] [PubMed] [Google Scholar]
- 115.Kang C., Syed Y.Y. Bulevirtide: first approval. Drugs. 2020;80(15):1601–1605. doi: 10.1007/s40265-020-01400-1. [DOI] [PubMed] [Google Scholar]
- 116.Xu Z., Chavez D., Guerra M., et al. Treatment of chronically HBV-infected chimpanzees with RNA interference therapeutic ARC-520 LED to potent reduction of viral MRNA, DNA and proteins without observed drug resistance. J Hepatol. 2016;64:2. [Google Scholar]
- 117.Yuen M.F., Chan H.L.Y., Liu K., et al. Differential reductions in viral antigens expressed from CCCDNAVS integrated DNA in treatment naïve HBEAG positive and negative patients with chronic HBV after RNA interference therapy with ARC-520. J Hepatol. 2016;64(2):S390–S391. [Google Scholar]
- 118.Yuen M.F., Schiefke I., Yoon J.H., et al. RNA interference therapy with ARC-520 results in prolonged hepatitis B surface antigen response in patients with chronic hepatitis B infection. Hepatology. 2020;72(1):19–31. doi: 10.1002/hep.31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yuen M.F., Heo J., Kumada H., et al. Phase IIa, randomised, double-blind study of GSK3389404 in patients with chronic hepatitis B on stable nucleos(t)ide therapy. J Hepatol. 2022;77(4):967–977. doi: 10.1016/j.jhep.2022.05.031. [DOI] [PubMed] [Google Scholar]
- 120.Gane E., Yuen M.F., Kim D.J., et al. Clinical study of single-stranded oligonucleotide RO7062931 in healthy volunteers and patients with chronic hepatitis B. Hepatology. 2021;74(4):1795–1808. doi: 10.1002/hep.31920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yuen M.F., Heo J., Jang J.W., et al. Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: a phase 2 randomized controlled trial. Nat Med. 2021;27(10):1725–1734. doi: 10.1038/s41591-021-01513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J., Lu F. Dual-gRNAs and gRNA-microRNA (miRNA)-gRNA ternary cassette combined CRISPR/Cas9 system and RNAi approach promotes the clearance of HBV cccDNA. Hepatology. 2015;62:223A–224A. [Google Scholar]
- 123.Shi Y., Tu Z., Duan X.D., et al. The combination effects of CRISPR/Cas9 and APOBEC3B editing on HBV cccDNA formation. Hepatology. 2017;66:782A. [Google Scholar]
- 124.Hui R.W.-H., Mak L.-Y., Seto W.-K., et al. Role of core/capsid inhibitors in functional cure strategies for chronic hepatitis B. Curr Hepatol Rep. 2020;19(3):293–301. [Google Scholar]
- 125.Zoulim F., Zlotnick A., Buchholz S., et al. Nomenclature of HBV core protein-targeting antivirals. Nat Rev Gastroenterol Hepatol. 2022;19(12):748–750. doi: 10.1038/s41575-022-00700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuen M.F., Berliba E., Sukeepaisarnjaroen W., et al. Safety, pharmacokinetics, and antiviral activity of the capsid inhibitor AB-506 from Phase 1 studies in healthy subjects and those with hepatitis B. Hepatol Commun. 2022;6(12):3457–3472. doi: 10.1002/hep4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuen M.F., Gane E.J., Kim D.J., et al. Antiviral activity, safety, and pharmacokinetics of capsid assembly modulator NVR 3-778 in patients with chronic HBV infection. Gastroenterology. 2019;156(5):1392–1403.e7. doi: 10.1053/j.gastro.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 128.Zoulim F., Lenz O., Vandenbossche J.J., et al. JNJ-56136379, an HBV capsid assembly modulator, is well-tolerated and has antiviral activity in a Phase 1 study of patients with chronic infection. Gastroenterology. 2020;159(2):521–533.e9. doi: 10.1053/j.gastro.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 129.Yuen M.-F., Agarwal K., Ma X., et al. Safety and efficacy of vebicorvir in virologically suppressed patients with chronic hepatitis B virus infection. J Hepatol. 2022;77(3):642–652. doi: 10.1016/j.jhep.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 130.Franke C., Matschl U., Bruns M. Enzymatic treatment of duck hepatitis B virus: topology of the surface proteins for virions and noninfectious subviral particles. Virology. 2007;359(1):126–136. doi: 10.1016/j.virol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 131.Chai N., Chang H.E., Nicolas E., et al. Properties of subviral particles of hepatitis B virus. J Virol. 2008;82(16):7812–7817. doi: 10.1128/JVI.00561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu J., Liu K. Complete and incomplete hepatitis B virus particles: formation, function, and application. Viruses. 2017;9(3):56. doi: 10.3390/v9030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bazinet M., Pântea V., Placinta G., et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa-2a in patients with chronic HBV infection naïve to nucleos(t)ide therapy. Gastroenterology. 2020;158(8):2180–2194. doi: 10.1053/j.gastro.2020.02.058. [DOI] [PubMed] [Google Scholar]
- 134.Lee H.W., Park J.Y., Hong T., et al. Efficacy of lenvervimab, a recombinant human immunoglobulin, in treatment of chronic hepatitis B virus infection. Clin Gastroenterol Hepatol. 2020;18(13):3043–3045.e1. doi: 10.1016/j.cgh.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 135.Gane E., Jucov A., Dobryanska M., et al. Safety, tolerability, and antiviral activity of the siRNA VIR 2218 in combination with the investigational neutralizing monoclonal antibody VIR 3434 for the treatment of chronic hepatitis B virus infection: preliminary results from the phase 2 MARCH trial. Conference Report from the National AIDS Treatment Advocacy Project (NATAP) on the liver meeting digital experience - American Association for the Study of Liver Diseases (AASLD); November 4-8, 2022. https://www.natap.org/2022/AASLD/AASLD_19.htm
- 136.Gaggar A., Coeshott C., Apelian D., et al. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32(39):4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 137.Korolowicz K.E., Iyer R.P., Czerwinski S., et al. Antiviral efficacy and host innate immunity associated with SB 9200 treatment in the Woodchuck Model of chronic hepatitis B. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen J., Wang X.M., Wu X.J., et al. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm Res. 2011;60(1):47–53. doi: 10.1007/s00011-010-0233-1. [DOI] [PubMed] [Google Scholar]
- 139.Yoo G.J., Fang T., Zola J., et al. Destigmatizing hepatitis B in the asian American community: lessons learned from the san Francisco Hep B free Campaign. J Cancer Educ. 2012;27(1):138–144. doi: 10.1007/s13187-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]