Abstract
Background
Mechanical thrombectomy is a promising treatment option for deep vein thrombosis; however, long-term data are lacking. Here, we report for the first time the 1-year clinical outcomes from the completely enrolled ClotTriever Outcomes (CLOUT) registry evaluating mechanical thrombectomy with the ClotTriever System (Inari Medical).
Methods
The CLOUT registry (NCT03575364) is a prospective, multicenter, single-arm study that enrolled 500 patients with proximal lower extremity deep vein thrombosis. Prespecified 1-year outcomes include Villalta score and corresponding postthrombotic syndrome (PTS) severity, duplex ultrasound findings of patency (defined as the presence of flow with normal or partial compressibility), Revised Venous Clinical Severity Score, and quality of life (QoL).
Results
In CLOUT, the median age was 61.9 years and 50.5% of patients were women. A total of 310 patients completed the 1-year visit. The 1-year PTS rate (Villalta score ≥ 5) was 19.3% and the moderate-to-severe PTS rate (Villalta score ≥ 10) was 8.8%. Median Villalta score decreased from 9.0 (IQR, 5.0-14.0) at baseline to 1.0 (IQR, 0.0-4.0) at 1 year (P < .0001). Similar rates of PTS and moderate-to-severe PTS were observed among limbs assessed at all study time points. Patency was observed in 94.2% of limbs. Median Revised Venous Clinical Severity Score was 6.0 (IQR, 3.0-9.0) at baseline and 3.0 (IQR, 1.0-4.0) at 1 year (P < .0001). Additionally, 90.4% of patients experienced improvements in QoL.
Conclusions
One-year outcomes from the CLOUT registry demonstrate low PTS rates and preserved patency accompanied by improved symptom relief and QoL. Study follow-up through 2 years is ongoing.
Keywords: deep vein thrombosis, intervention, lower extremity, mechanical thrombectomy, postthrombotic syndrome, prospective studies, quality of life
Introduction
Patients frequently experience unfavorable long-term outcomes following treatment for deep vein thrombosis (DVT), with up to 50% developing postthrombotic syndrome (PTS).1 PTS is a chronic condition associated with profound and enduring clinical, economic, and quality of life (QoL) implications. In the aftermath of an initial acute DVT episode, the emergence of PTS significantly contributes to work-related disability.2 PTS manifestations can vary from mild discomfort, swelling, itching, or heaviness during physical activity to severe edema and pain even at rest. As many as 10% of patients progress to severe PTS, which may involve the development of venous leg ulcers.3 The QoL of these patients is comparable to that of individuals with congestive heart failure or cancer.3
Anticoagulation (AC) is the standard of care for DVT treatment, with intervention recommended for more severe or complex manifestations.4, 5, 6, 7 Catheter-directed thrombolysis (CDT) is an interventional approach that has been evaluated in randomized controlled trials, aiming to reduce the incidence and severity of PTS following DVT in comparison to AC treatment.8, 9, 10 Although these trials have demonstrated certain benefits, they have not established superiority over AC in terms of long-term PTS reduction. The ClotTriever System (Inari Medical) is a mechanical thrombectomy device that has been the subject of frequent investigation.11,12 Unlike CDT, the ClotTriever System operates without the use of thrombolytics. Consequently, such mechanical thrombectomy aims to rapidly restore patency within a single session, while mitigating bleeding risks.13
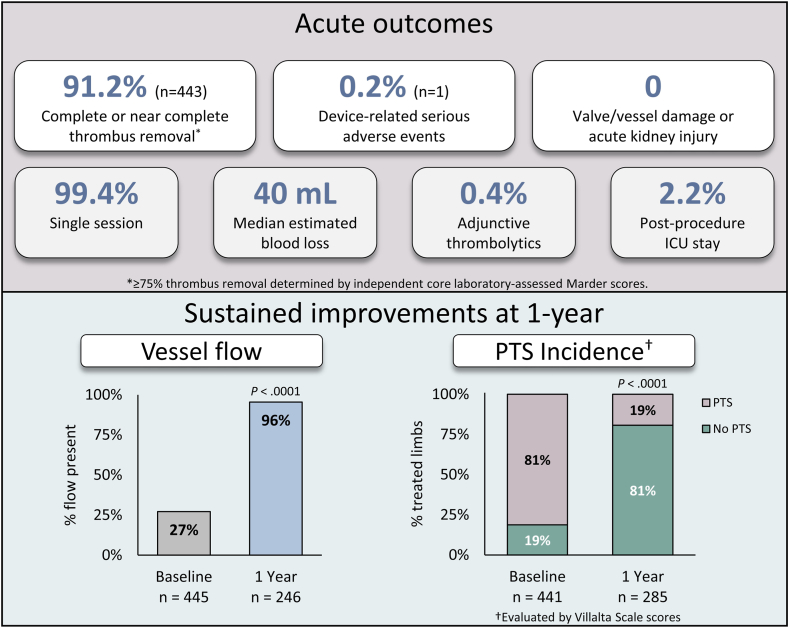
The ClotTriever Outcomes (CLOUT) registry (ClinicalTrials.gov identifier: NCT03575364) is a prospective, multicenter, single-arm study designed to assess patient outcomes over a 2-year period following mechanical thrombectomy with the ClotTriever System for proximal lower extremity DVT. In-hospital and 6-month outcomes for the fully enrolled cohort of 500 patients have demonstrated near-complete or complete thrombus removal in 91.2% of limbs, with a low device-related serious adverse event rate of 0.2% and sustained clinical improvements through the 6-month follow-up period.11,13 Despite these promising results, the long-term clinical outcomes of the ClotTriever System have yet to be evaluated. Here we report the 1-year clinical outcomes from the CLOUT registry for the first time.
Methods
Study overview
The CLOUT registry began enrolling patients with DVT in September 2018. In February 2022, the registry completed enrollment of 500 patients at 43 study sites (Supplemental Table S1). Enrolled patients underwent interventional treatment using the ClotTriever System, a mechanical thrombectomy system specifically designed for the extraction of large thrombi from peripheral veins. Detailed inclusion and exclusion criteria for the registry have been previously published.13 Enrolled patients were required to be ≥18 years of age and present with either unilateral or bilateral proximal lower extremity DVT, regardless of the duration of symptoms, prior treatment failure for the current DVT event, or contraindications to thrombolytic therapy. The index DVT needed to involve the femoral vein, common femoral vein, iliac vein, and/or inferior vena cava. Patients were excluded if they were ineligible for AC therapy or if they had a prior stent in the target venous segment or an inferior vena cava filter in place at the time of the planned procedure. Follow-up assessments were scheduled at 30 days, 6 months, 1 year, and 2 years following the intervention, and data were analyzed per the study protocol.
Outcomes
Duplex ultrasound
Duplex ultrasound (DUS) was strongly recommended and performed in accordance with standard procedures at each study site. The examination included assessment of flow (categorized as present, absent, or not evaluable), compressibility (categorized as normal, partially compressible, incompressible, or not evaluable), and patency (defined as the presence of flow with normal or partial compressibility).
Villalta and PTS severity
The Villalta Scale stratifies the severity of PTS in lower extremity DVT based on 5 patient-rated symptoms and 6 clinician-rated clinical signs, each on a scale of 0 to 3, for a combined score ranging from 0 to 33.14 Villalta Scale scores were collected, and corresponding PTS severity was assessed. PTS was defined by a Villalta Scale score of ≥5 and further categorized into mild (Villalta 5-9), moderate (Villalta 10-14), and severe (Villalta ≥15) subgroups. Additionally, Villalta and PTS rates for patients with complete data across all time points were evaluated to analyze PTS progression over time.
Revised Venous Clinical Severity Score
The Revised Venous Clinical Severity Score (rVCSS) was used to assess the severity of venous disease. It involves examination and subsequent categorization of 10 components on a scale of 0 (none) to 3 (severe). These components include pain, varicose veins, venous edema, skin pigmentation, inflammation, induration, the use of compression therapy, and active ulcer number, duration, and size. The cumulative score, ranging from 0 to 30, is derived from the summation of these individual component scores.
Symptom relief and QoL
Symptom relief was determined by evaluating changes in leg edema and pain. Edema reduction in treated limbs was measured by midthigh and midcalf circumference. For unilateral DVT patients, the midthigh edema ratio and midcalf edema ratio were calculated by dividing the circumference of the treated limb by the corresponding circumference of the untreated limb.
Pain levels were documented using the numeric pain rating scale (NPRS), in which patients indicated their pain level on a scale ranging from 0 (indicating no pain) to 10 (representing the worst possible pain). Patient-reported QoL was assessed using the EuroQoL Group 5-dimension questionnaire (EQ-5D), which comprises 5 aspects of QoL: mobility, self-care, activity, pain, and anxiety. The EQ-5D cumulative score ranges from 0.0 (death) to 1.0 (best possible health).
Statistical analysis
Continuous variables were presented as means with their corresponding SDs or as medians with IQR, as appropriate. Categorical variables were presented as frequencies and percentages. Wilcoxon signed-rank and McNemar-Bowker tests were applied to test the changes from baseline for continuous and categorical outcomes, respectively, using available paired values. P values of <.05 were considered significant. All analyses were performed using SAS version 9.4 (SAS Institute) and R version 4.1.2 (R Foundation for Statistical Computing).15
Post hoc exploratory analyses included reporting of the following subgroups: iliofemoral (IF) DVT and isolated femoral-popliteal (FP) DVT, evaluation of patients with prior treatment for their current index DVT event, and patients with contraindications to thrombolytic agents.
Results
The full analysis population of the CLOUT registry consists of 499 patients with a total of 521 treated limbs. Comprehensive baseline characteristics and procedural outcomes for the entire CLOUT cohort have been previously published, with a summary available in Supplemental Table S2 and the Central Illustration.11 In brief, the median patient age was 61.9 years, with 50.5% (n = 252) of patients being women, and 30.7% (n = 153) having either an absolute or relative contraindication to thrombolytics. Among the treated limbs, 78.6% (n = 389) were affected by IF DVT, whereas 21.4% (n = 106) were diagnosed with isolated FP DVT. Notably, the target DVT had been previously treated in 23.2% (n = 120) of limbs and in most cases, AC was the prior treatment method (n = 106).
Central Illustration.
Summary of acute procedural outcomes and vessel flow and postthrombotic syndrome (PTS) at 1 year.
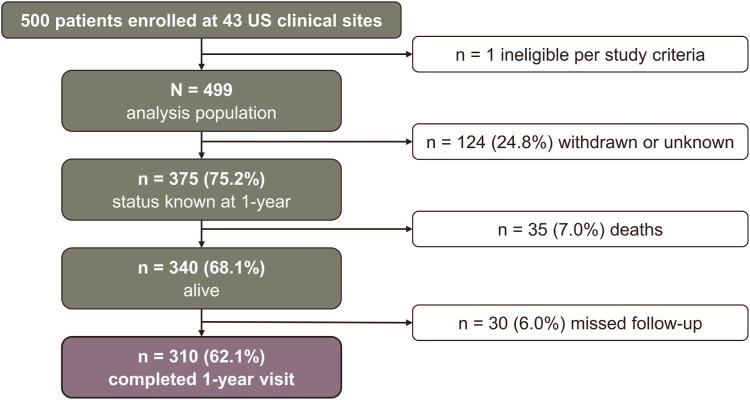
The enrollment flowchart illustrating patient inclusion is presented in Figure 1. One-year follow-up visits were successfully completed by 310 patients (62.1%), with 30 patients missing their scheduled follow-up. Thirty-five patients (7%) passed away during the course of the study, and 124 patients (24.8%) were either withdrawn or had an unknown disposition.
Figure 1.
Patient disposition flowchart. One year following treatment for proximal lower extremity deep vein thrombosis.
DUS
Results of DUS interrogation are provided in Table 1. At 1 year, 95.5% (n = 235) of treated limbs demonstrated the presence of flow and 96.7% (n = 234) exhibited normal or partial compressibility, representing significant improvements from baseline (P < .0001 each). Patency was present in 94.2% (n = 227) of limbs at 1 year compared with 17.3% (n = 72) of limbs at baseline (P < .0001).
Table 1.
Duplex ultrasound flow, compressibility, and patency outcomes from baseline to 1-year follow-up in the overall population, iliofemoral deep vein thrombosis, and isolated femoral-popliteal deep vein thrombosis.
| Outcomes | Overall population |
IF DVT |
Isolated FP DVT |
|||
|---|---|---|---|---|---|---|
| Baseline | 1 y | Baseline | 1 y | Baseline | 1 y | |
| Flow | n = 445 | n = 246 | n = 337 | n = 188 | n = 88 | n = 48 |
| Present, % | 27.2 | 95.5 | 27.9 | 95.7 | 22.7 | 93.8 |
| Changea | 68.1 | 67.8 | 70.2 | |||
| P valuea | <.0001 | <.0001 | <.0001 | |||
| Compressibility | n = 453 | n = 242 | n = 338 | n = 185 | n = 96 | n = 47 |
| Normal/partial, % | 28.0 | 96.7 | 28.4 | 96.8 | 22.9 | 95.7 |
| Incompressible, % | 72.0 | 3.3 | 71.6 | 3.2 | 77.1 | 4.3 |
| Changea | 68.2 | 67.8 | 72.6 | |||
| P valuea | <.0001 | <.0001 | <.0001 | |||
| Patency | n = 416 | n = 241 | n = 315 | n = 184 | n = 85 | n = 47 |
| Yes, % | 17.3 | 94.2 | 18.4 | 94.0 | 11.8 | 93.6 |
| Changea | 76.2 | 75.0 | 80.9 | |||
| P valuea | <.0001 | <.0001 | <.0001 | |||
DVT, deep vein thrombosis; FP, femoral-popliteal; IF, iliofemoral.
Absolute change and P values derived from the McNemar-Bowker test calculated with paired baseline and 1-y follow-up visit values.
At the 1-year follow-up, significant improvements in DUS flow and compressibility compared with baseline were observed in limbs affected by both IF DVT and isolated FP DVT (Table 1; P < .0001). DUS outcomes in limbs affected by IF DVT and isolated FP DVT were similar to one another and consistent with the overall findings among patients with DUS assessments at the 1-year follow-up. In limbs treated for IF DVT, patency was restored in 94.0% (n = 173) of cases at 1 year, a significant increase from the 18.4% patency rate (n = 58) observed at baseline (P < .0001). Similarly, in limbs treated for isolated FP DVT, patency was present in 93.6% of cases (n = 44) at 1 year, significantly improved from the baseline rate of 11.8% (n = 10) (P < .0001).
Villalta and PTS severity
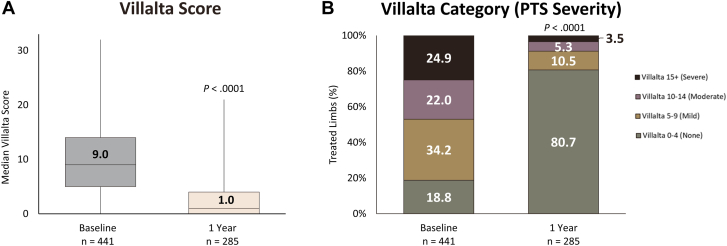
The median Villalta score decreased significantly from 9.0 (IQR, 5.0-14.0) at baseline to 1.0 (IQR, 0.0-4.0) at the 1-year visit (P < .0001; Figure 2A). The median reduction in Villalta score between baseline and the 1-year visit was 6.0 points (IQR, 2.0-10.0). As illustrated in Figure 2B, 80.7% (n = 230) of limbs were free from PTS at 1 year (P < .0001 compared with baseline). The rate of moderate or severe PTS at the 1-year visit was 8.8% (n = 25). Assessment of the change in limb Villalta category (PTS severity) from baseline to 1 year revealed that 88.3% (n = 218) improved or maintained their status of no PTS, whereas 5.7% (n = 14) retained their baseline Villalta category, and 6.1% (n = 15) experienced a worsening in PTS severity.
Figure 2.
Summary of Villalta and postthrombotic syndrome (PTS) severity outcomes. At 1-year follow-up, patients in the CLOUT registry had significant reductions in (A) median Villalta score and (B) PTS severity when compared with baseline. Most patients (80.7%) had no PTS 1 year posttreatment, whereas 8.8% had moderate or severe PTS. Boxes represent IQR (Q1, Q3) with horizontal bars representing median values and vertical lines representing 1.5× (Q1-Q3). P values comparing values between baseline and 1-year visit derived from the Wilcoxon signed-rank test for the Villalta score and the McNemar-Bowker test for PTS severity.
The change in median Villalta score and Villalta category (PTS severity) from baseline to the 1-year visit is presented separately for limbs affected by IF and isolated FP DVT in Table 2. The median reduction in Villalta score between baseline and the 1-year visit was 6.0 points (IQR, 3.0-10.0) for IF DVT and 6.0 points (IQR, 2.0-11.0) for isolated FP DVT. These findings closely mirrored those observed in the overall patient population who completed the 1-year follow-up visit.
Table 2.
Median Villalta score and postthrombotic syndrome severity rate from baseline to 1-year follow-up for iliofemoral deep vein thrombosis vs isolated femoral-popliteal deep vein thrombosis.
| Outcomes | IF DVT |
Isolated FP DVT |
||
|---|---|---|---|---|
| Baseline | 1 y | Baseline | 1 y | |
| Villalta score | n = 332 | n = 213 | n = 87 | n = 59 |
| Median [IQR] | 9.0 [6.0-14.0] | 1.0 [0.0-4.0] | 10.0 [6.0-15.0] | 2.0 [0.0-4.0] |
| P valuea | <.0001 | <.0001 | ||
| PTS severity | n = 332 | n = 213 | n = 87 | n = 59 |
| Severe PTS, % | 24.7 | 3.3 | 28.7 | 5.1 |
| Moderate PTS, % | 20.5 | 4.7 | 27.6 | 8.5 |
| Mild PTS, % | 37.0 | 10.8 | 28.7 | 10.2 |
| No PTS, % | 17.8 | 81.2 | 14.9 | 76.3 |
| P valuea | <.0001 | .0001 | ||
| Change in PTS severity from baseline | n = 186 | n = 49 | ||
| Improved or maintained no PTS, % | 89.8 | 81.6 | ||
| Maintained baseline severity, % | 4.8 | 10.2 | ||
| Worsened, % | 5.4 | 8.2 | ||
DVT, deep vein thrombosis; FP, femoral-popliteal; IF, iliofemoral; PTS, postthrombotic syndrome.
P values derived from the Wilcoxon signed-rank test for the Villalta score and the McNemar-Bowker test for PTS severity between baseline and 1-y follow-up visit.
At the 1-year visit, 81.2% (n = 173) of limbs affected by IF DVT were free from PTS, whereas 76.3% (n = 45) of patients with isolated FP DVT experienced a PTS-free outcome (Table 2). The rate of moderate or severe PTS was 13.6% (n = 8) in the isolated FP group and 8.0% (n = 17) in the IF group.
Progression over time
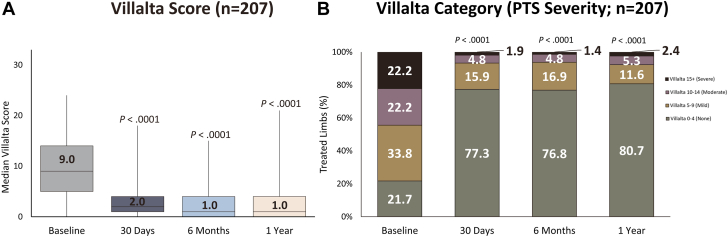
There were 207 limbs that had available Villalta scores at all study time points through 1 year. When evaluating this cohort, the Villalta score and Villalta category (PTS severity) outcomes remained consistent with those observed in the overall population (Figure 3). The median Villalta score decreased significantly from 9.0 (IQR, 5.0-14.0) at baseline to 1.0 (IQR, 0.0-4.0) at the 1-year visit (P < .0001; Figure 3A). The median reduction in Villalta score between baseline and the 1-year visit was 6.0 points (IQR, 2.0-10.0). Once again, at the 1-year mark, 80.7% (n = 167) of limbs were found to be free from PTS (Figure 3B). Notably, the rate of moderate or severe PTS at the 1-year visit (7.7%, n = 16) remained relatively consistent with the rate observed at the 6-month visit (6.2%, n = 13). An assessment of the change in Villalta category (PTS severity) from baseline to 1 year revealed that 88.9% (n = 184) of patients improved or maintained a status of no PTS, whereas 5.8% (n = 12) maintained their baseline level Villalta category, and 5.3% (n = 11) experienced a worsening in PTS severity.
Figure 3.
Progression in Villalta and postthrombotic syndrome (PTS) severity among the same population. Improvements in (A) median Villalta score and (B) PTS severity from baseline were observed at 30 days, 6 months, and 1 year among limbs assessed at all study time points. Boxes represent IQR (Q1, Q3) with horizontal bars representing median values and vertical lines representing 1.5× (Q1-Q3). P values comparing follow-up and baseline assessment values were derived from the Wilcoxon signed-rank test for the Villalta score and the McNemar-Bowker test for PTS severity.
Thrombolytic contraindications and prior treatment
Patients with baseline contraindications to thrombolytics had numerically similar median Villalta score reductions from baseline to the 1-year visit (10.0 [IQR, 6.0-16.0] to 2.0 [IQR, 0.0-3.0], P < .0001), as well as PTS severity outcomes when compared with the overall population of patients with available Villalta score assessments at the 1-year visit (Supplemental Table S3). Patients who had received prior treatment for their current DVT (Supplemental Table S3) demonstrated significant reductions in median Villalta score from baseline to the 1-year visit (P < .0001) and improvements in the Villalta category from baseline to 1-year (P = .0002).
At 1-year, the PTS rate was 12.8% (n = 10) among patients with contraindications to thrombolytic agents and 29.9% (n = 20) among patients who had received prior treatment for their current DVT. Furthermore, 1-year posttreatment, the Villalta PTS severity category was either improved or maintained at the level of no PTS in 90.9% of patients (n = 60) with contraindications to thrombolytics and in 78.3% of patients (n = 47) with a history of failed prior DVT treatment.
Revised Venous Clinical Severity Score
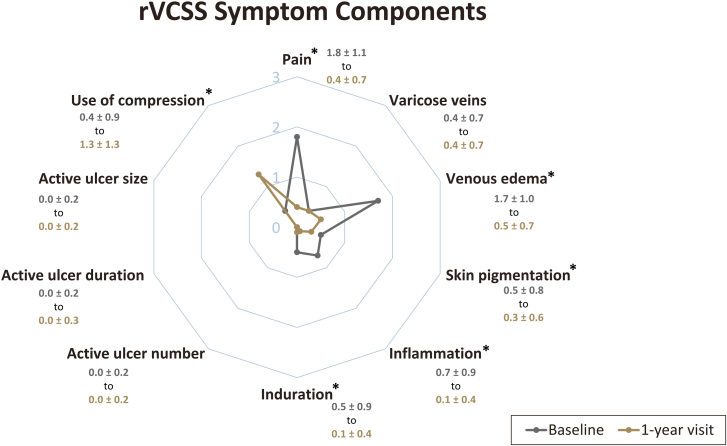
Improvements in the Revised Venous Clinical Severity Score (rVCSS) were observed, with the median overall scores decreasing from 6.0 (IQR, 3.0-9.0) at baseline to 3.0 (IQR, 1.0-4.0) at the 1-year visit (P < .0001). Among patients with recorded rVCSS scores >0 at baseline (n = 229), 75.1% (n = 172) experienced at least a 1-point improvement in their rVCSS at 1 year. The mean symptom severity scores for the individual components of the rVCSS are included in Figure 4. Notably, at both baseline and 1 year, the median rVCSS component scores for active venous ulcer number, duration, and size remained at 0.0 (IQR, 0.0-0.0), with no significant change observed (P > .05 for all components). Similarly, there was no significant change in the median varicose veins component scores between baseline (0.0 [IQR, 0.0-1.0]) and 1 year (0.0 [IQR, 0.0-1.0]).
Figure 4.
Summary of changes in Revised Venous Clinical Severity Score (rVCSS) components. Assessments of mean ± SD symptom severity scores (0 = none, 1 = mild, 2 = moderate, 3 = severe) for each rVCSS component indicate significant improvements in pain, venous edema, skin pigmentation, inflammation, and induration at 1 year. The use of compression therapy significantly increased from baseline to 1-year visit. n = 433-442 at baseline and n = 283-285 at 1-year visit. ∗P < .0001; P values are derived from the Wilcoxon signed-rank test with comparison against baseline values.
As depicted in Figure 4, the significant improvement in total rVCSS from baseline to 1 year was primarily driven by reductions in pain (median score from 2.0 [IQR, 1.0-3.0] to 0.0 [IQR, 0.0-1.0]; P < .0001) and venous edema (median score from 2.0 [IQR, 1.0-3.0] to 0.0 [IQR, 0.0-1.0]; P < .0001). Additionally, significant improvements were also observed for the skin pigmentation, inflammation, and induration components (P < .0001 for all components). Furthermore, the utilization of compression therapy increased from baseline to 1 year (P < .0001).
Symptom relief and QoL
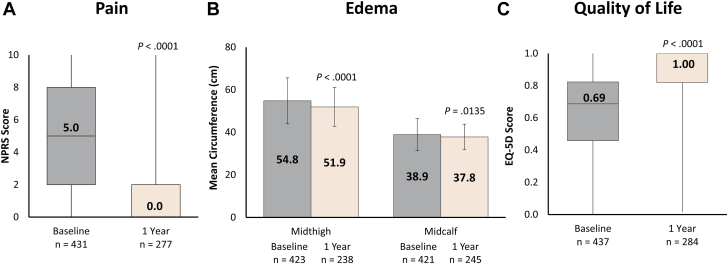
Significant improvement in pain and edema was observed at 1 year (Figure 5A, B, respectively). The median NPRS score decreased from 5.0 (IQR, 2.0-8.0) at baseline to 0.0 (IQR, 0.0-2.0) at 1 year (P < .0001). A majority of patients (86.5%, n = 180) experienced an improvement in their NPRS score from baseline to the 1-year visit. Similarly, the mean midthigh and midcalf circumferences demonstrated significant reductions at the 1-year visit when compared with baseline (51.9 vs 54.8 cm; P < .0001 and 37.8 vs 38.9 cm; P = .0135, respectively). At 1 year, 78.5% of patients (n = 139) exhibited improvements in the midthigh edema ratio, and 81.2% (n = 147) displayed improvements in the midcalf edema ratio.
Figure 5.
Summary of symptom relief and quality of life (QoL) outcomes. At 1-year follow-up, patients had significant improvements from baseline in (A) median numeric pain rating scale (NPRS) score indicating pain relief, (B) mean midcalf and midthigh measurements showing edema reduction, and (C) median EuroQoL Group 5-dimension questionnaire (EQ-5D) score demonstrating improved QoL. Boxes represent IQR (Q1, Q3) with horizontal bars representing median values and vertical lines representing 1.5 × (Q1-Q3). P values are derived from the Wilcoxon signed-rank test with comparison against baseline values.
QoL improvements were reflected in the median EQ-5D score, which increased from 0.687 (IQR, 0.458-0.823) at baseline to 1.000 (IQR, 0.820-1.000) at the 1-year visit (P < .0001; Figure 5C). The majority of patients (90.4%, n = 206) experienced an improvement in their EQ-5D score from baseline to the 1-year visit.
Discussion
This study presents the first report of 1-year outcomes derived from the fully enrolled CLOUT registry, which includes patients with proximal lower extremity DVT treated with the ClotTriever System (Central Illustration). The results revealed that 1 year after treatment, the rate of moderate-to-severe PTS was 8.8% and venous patency was observed in 94.2% of limbs. Patients experienced a significant median reduction of 3 points in rVCSS at 1 year when compared with baseline. These clinical benefits were complemented by notable improvements in patients' symptoms and QoL. Specifically, 86.5% of patients reported improvements in their NPRS scores and 90.4% reported enhanced EQ-5D scores. Furthermore, 78.5% and 81.2% of patients with unilateral DVT noted improvements in midthigh and midcalf edema ratios, respectively.
Historically, symptomatic proximal DVT has been linked to poor long-term patient outcomes, primarily due to the development of PTS. Although a direct conclusion cannot be made by comparing rates across independent studies, for illustrative purposes, the 1-year PTS rate observed in the CLOUT study (19%; n/N = 55/285) is lower than that reported in previous randomized controlled trials.9,10,16 Moreover, among patients with complete data across all time points, the 1-year PTS rate remained consistent at 19%. Like CLOUT, the ATTRACT study enrolled patients with DVT involving the femoral or more proximal veins. In ATTRACT, the PTS rate in the overall population at the 1-year follow-up visit was 34% (n/N = 92/272) in the pharmacomechanical CDT arm and 34% (n/N = 88/258) in the AC arm.10 In the subgroup of ATTRACT patients with IF DVT (ie, any involvement of the iliac veins or common femoral vein), the PTS rate at the 1-year visit was 37% (n/N = 58/155) in the CDT arm and 36% (n/N = 49/137) in the AC arm with a moderate-to-severe PTS rate of 12% (n/N = 18/155) and 18% (n/N = 24/137), respectively.16 Further, a recent propensity score matching analysis compared CLOUT with the ATTRACT pharmacomechanical CDT arm and showed that PTS rates at 1 year were 17% for CLOUT and 36% for ATTRACT.17 The CAVA study included only patients with IF DVT, and the 1-year PTS rate was 29% (n/N = 22/77) in the ultrasound-accelerated CDT arm and 35% (n/N = 26/75) in the AC arm with a moderate-to-severe PTS rate of 16% (n/N = 12/77) and 21% (n/N = 16/75), respectively.9 In the subgroup of CLOUT patients with IF DVT, the 1-year PTS rate was 19% (n/N = 40/213) with a moderate-to-severe PTS rate of 8% (n/N = 17/213). Notably, the CLOUT study included patients with limited treatment options, including those contraindicated for thrombolytics and those with a history of failed prior treatment for their current DVT.
Although current guidelines continue to recommend AC by itself as first-line treatment for IF DVT,4, 5, 6, 7 a recent position statement from the Society for Interventional Radiology recommends that interventional treatment is a reasonable option in carefully selected patients with acute IF DVT.18 Studies focusing on patients with IF DVT have demonstrated potential benefits with CDT compared with AC alone at 2-year follow-up.8,16 For instance, the CaVenT study reported a 2-year PTS rate of 41% (n/N = 37/90) in the CDT arm vs 56% (n/N = 55/99) in the AC arm (P = .047); the rate of PTS at the 1-year follow-up was not assessed separately.8 Additionally, in the IF DVT ATTRACT subgroup, although there was not a significant difference in the rate of moderate-to-severe PTS at the 1-year visit, the cumulative proportion of patients with moderate-to-severe PTS over 2 years of follow-up was significantly lower in the CDT arm compared with the AC arm (18% vs 28%, respectively; P = .021).16
Despite the encouraging real-world prospective outcomes evident in the CLOUT registry, there remains a gap in randomized controlled data for mechanical thrombectomy. To that end, the recently initiated randomized controlled DEFIANCE trial (clinicaltrials.gov identifier: NCT05701917) will compare clinical outcomes following treatment of symptomatic IF DVT with the ClotTriever System to the standard of care AC. The evidence generated from the DEFIANCE trial will help determine the therapeutic value of mechanical thrombectomy in treating acute IF DVT when compared with AC alone.4, 5, 6, 7
Even with the robust enrollment of 500 patients across 43 study sites and the planned follow-up period extending to 2 years, it is important to acknowledge that CLOUT is a single-arm registry study and, as such, is inherently constrained by its study design. Consequently, the results should be interpreted with caution. Furthermore, the attrition of registry participants during follow-up exceeds what would typically be expected in a randomized controlled trial. Additionally, it is worth noting that the study has limitations related to the absence of blinded evaluation and core-lab adjudication for ultrasound assessments. The 2-year follow-up of the CLOUT registry will provide valuable data to corroborate the 1-year outcomes presented in this report.
Conclusions
One-year outcomes from the single-arm, multicenter, all-comer CLOUT registry suggest preserved venous patency and low-PTS occurrence following treatment with the ClotTriever System. These outcomes were complemented by symptom relief and enhancements in patient QoL. Study follow-up through 2 years is ongoing.
Acknowledgments
The authors acknowledge medical writing and editorial support from Kelly Koch, Jarrod Collins, and Prashanthi Vandrangi.
Declaration of competing interest
Mohannad Bisharat is a consultant for Inari Medical. Eugene Ichinose is a consultant for Inari Medical, Penumbra, and AngioDynamics and a speaker for Pfizer and Bristol Myers Squibb. Eric Trestman is a consultant for Inari Medical. Stuart Harlin is a consultant for Inari Medical, Endologix, and Bard. Ambarish Bhat is a consultant for Inari Medical. Ronald Winokur is a speaker/consultant for Inari Medical, BD/Bard, Medtronic, Tactile Medical, Penumbra, and Koya. David Dexter is a consultant for Inari Medical, Penumbra, Boston Scientific, and AngioDynamics. Kalyan Veerina, Vipul Khetarpaul, Ezana Azene, Adam Plotnik, Jeffrey Hnath, Shuo Li, Graham Long, David O’Connor, and Saqib Zia reported no financial interests.
Funding sources
The CLOUT registry is sponsored by Inari Medical.
Ethics statement and patient consent
The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, Good Clinical Practice principles, and ISO 14155:2011. Institutional review board approval was obtained at each site, and all patients provided written informed consent.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.101307.
Supplementary material
References
- 1.Kahn S.R., Galanaud J.P., Vedantham S., Ginsberg J.S. Guidance for the prevention and treatment of the post-thrombotic syndrome. J Thromb Thrombolysis. 2016;41(1):144–153. doi: 10.1007/s11239-015-1312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braekkan S.K., Grosse S.D., Okoroh E.M., et al. Venous thromboembolism and subsequent permanent work-related disability. J Thromb Haemost. 2016;14(10):1978–1987. doi: 10.1111/jth.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polak M.W., Siudut J., Plens K., Undas A. Prothrombotic clot properties can predict venous ulcers in patients following deep vein thrombosis: a cohort study. J Thromb Thrombolysis. 2019;48(4):603–609. doi: 10.1007/s11239-019-01914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaff M.R., McMurtry M.S., Archer S.L., et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 5.Mazzolai L., Aboyans V., Ageno W., et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39(47):4208–4218. doi: 10.1093/eurheartj/ehx003. [DOI] [PubMed] [Google Scholar]
- 6.Ortel T.L., Neumann I., Ageno W., et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–4738. doi: 10.1182/bloodadvances.2020001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens S.M., Woller S.C., Baumann Kreuziger L., et al. Executive Summary: antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(6):2247–2259. doi: 10.1016/j.chest.2021.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Enden T., Haig Y., Kløw N.E., et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31–38. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 9.Notten P., Ten Cate-Hoek A.J., Arnoldussen C.W.K.P., et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol. 2020;7(1):e40–e49. doi: 10.1016/S2352-3026(19)30209-1. [DOI] [PubMed] [Google Scholar]
- 10.Vedantham S., Goldhaber S.Z., Julian J.A., et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240–2252. doi: 10.1056/NEJMoa1615066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh A., Zybulewski A., Paulisin J., et al. Six-month outcomes of mechanical thrombectomy for treating deep vein thrombosis: analysis from the 500-patient CLOUT registry. Cardiovasc Intervent Radiol. 2023;46(11):1571–1580. doi: 10.1007/s00270-023-03509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jolly M.A., Lockhart M.M., Shah D., et al. Outcomes from a tertiary care center using A catheter thrombectomy system for managing acute iliofemoral deep venous thrombosis. J Vasc Surg Venous Lymph Disord. 2022;10(5):1044–1050. doi: 10.1016/j.jvsv.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Dexter D., Kado H., Shaikh A., et al. Safety and effectiveness of mechanical thrombectomy from the fully enrolled multicenter, prospective CLOUT registry. J Soc Cardiovasc Angiogr Interv. 2023;2(2) doi: 10.1016/j.jscai.2023.100585. [DOI] [Google Scholar]
- 14.Villalta S., Bagatella P., Piccioli A., Lensing A., Prins M., Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24(suppl 1):158a. [Google Scholar]
- 15.R Foundation for Statistical Computing R Core Team. R: A language and environment for statistical computing. https://www.R-project.org/
- 16.Comerota A.J., Kearon C., Gu C.S., et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139(9):1162–1173. doi: 10.1161/CIRCULATIONAHA.118.037425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramowitz S., Bunte M.C., Maldonado T.S., et al. Mechanical thrombectomy vs. pharmacomechanical catheter directed thrombolysis for the treatment of iliofemoral deep vein thrombosis: a propensity score matched exploratory analysis of 12 month clinical outcomes. Eur J Vasc Endovasc Surg. 2023 doi: 10.1016/j.ejvs.2023.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Vedantham S., Desai K.R., Weinberg I., et al. Society of Interventional Radiology position statement on the endovascular management of acute iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2023;34(2):284–299.e7. doi: 10.1016/j.jvir.2022.10.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.