Abstract
A 51-year-old male patient with alcoholic cirrhosis visited our hospital for a scheduled gastrostomy replacement. During the gastrostomy replacement, he suddenly experienced a massive hemorrhage from the fistula site. Based on enhanced computed tomography findings, we concluded that collateral blood vessels from the left gastroepiploic vein had flowed into the varices near the gastrostomy as the main origin of the bleeding. The patient received treatment with percutaneous transhepatic occlusion for the varices, which halted blood flow to the varices. This case suggests the possibility of such a complication in patients with worsening portal hypertension and the effectiveness of percutaneous transhepatic occlusion treatment.
Introduction
Percutaneous endoscopic gastrostomy is frequently used for nutrition management in patients with difficulty in swallowing. However, several complications have been associated with percutaneous endoscopic gastrostomy, 1,2 including bleeding from collateral blood vessels in the abdominal wall in patients with portal hypertension.3,4 Clinicians should therefore be aware of possible bleeding from collateral vessels formed during abdominal puncture and gastrostomy placement. Gastrostomy catheters can be periodically replaced following the initial placement procedure, with no reports of serious hemorrhage from collateral blood vessels extending into the abdominal wall during gastrostomy replacement to date. We herein present an extremely rare instance of massive bleeding from collateral vessels during gastrostomy replacement that was managed successfully with percutaneous transhepatic occlusion (PTO).
Case Report
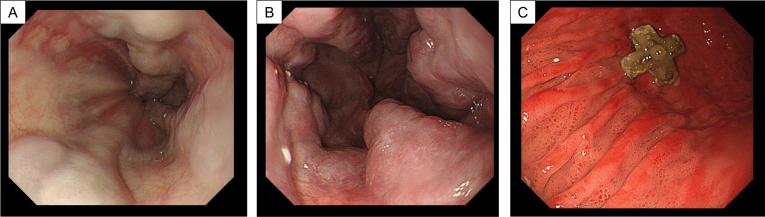
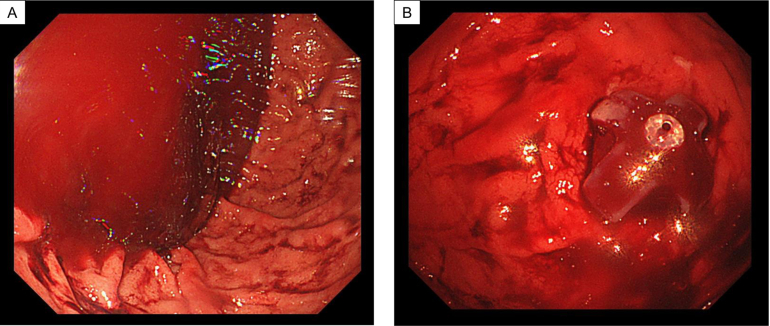
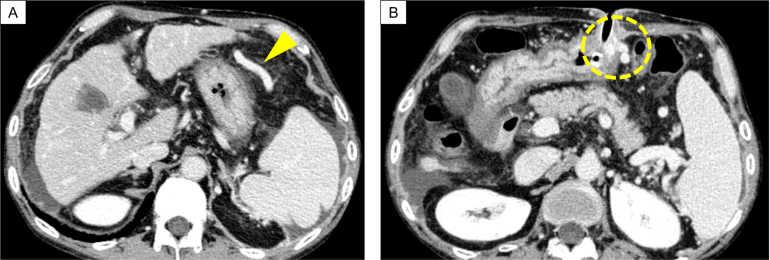
A 51-year-old man visited our hospital for a scheduled fifth gastrostomy replacement. He initially underwent gastrostomy placement before surgery for tongue cancer 3 years prior, after which he received gastrostomy replacement every 6 months. He had been treated with diuretics for complications of alcoholic cirrhosis with portal hypertension, including ascites and edema, for 3 years. He also had a history of asthma and interstitial pneumonia. Furosemide, ursodeoxycholic acid, and prednisolone were regularly prescribed. He had smoked one pack of cigarettes per day for 20 years before quitting 10 years prior. His ethanol intake had been over 200 g/day before the tongue cancer surgery, which was subsequently decreased to 20–40 g/day. Compared with esophagogastroduodenoscopy findings 6 months earlier (Figure 1A), his esophageal varices were considered to have deteriorated (Figure 1B) at the present gastrostomy replacement. Gastric mucosa findings just before the gastrostomy exchange showed portal hypertensive gastropathy (Figure 1C). Sudden massive bleeding occurred after gastrostomy catheter removal (Figure 2A). The endoscopist quickly inserted the new gastrostomy catheter and applied pressure to the site to temporarily stop the hemorrhage (Figure 2B). The patient was immediately admitted to our hospital for closer examination and treatment. He did not show any worsening of vital signs, such as hypotension, tachycardia, or hypoxemia. Laboratory data revealed no anemia progression from the postincident level of 14.3 g/dL, although thrombocytopenia (platelet count: 5.0×104/μL) and low albumin level (3.2 g/dL) were evident. Enhanced abdominal computed tomography (CT) showed findings consistent with liver cirrhosis, including an irregular liver surface, splenomegaly, and the new appearance of ascites (Figure 3A). Based on these findings, he was diagnosed as having decompensated cirrhosis Child-Pugh class B (score: 8 points). Collateral blood vessels from the left gastroepiploic vein were found to flow to the varices near the gastrostomy, which was presumed as the origin of the bleeding (Figure 3A and B). No new portal vein thromboses were noted. We presumed that the hemorrhage had been induced by the gastrostomy replacement from collateral blood vessel emergence due to his worsening portal hypertension. Therefore, treatment for esophageal varices was necessary as well. To resolve these problems, we planned to halt the gastrostomy bleeding by PTO first, followed next by endoscopic treatment of the esophageal varices.
Figure 1.
(A) Esophageal varices demonstrated by esophagogastroduodenoscopy 6 months prior to gastrostomy replacement. (B) Exacerbation of esophageal varices shown by esophagogastroduodenoscopy at the time of gastrostomy replacement. (C) Portal hypertensive gastropathy just prior to gastrostomy replacement.
Figure 2.
(A) Massive amount of blood in the stomach after hemorrhage from the fistula site. Images during bleeding could not be obtained as hemostasis was a higher priority. (B) The endoscopist inserted a new gastrostomy catheter, applied pressure to the bleeding site, and was able to temporarily stop the bleeding.
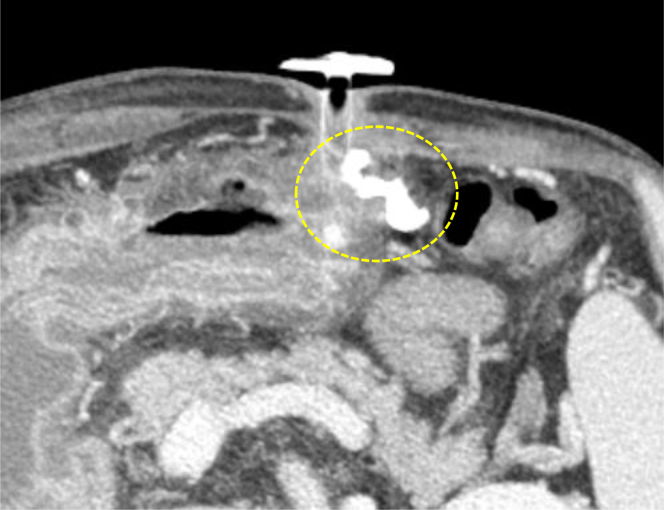
Figure 3.
(A) Collateral blood vessels from the left gastroepiploic vein (arrowhead). (B) Enhanced computed tomography of the bleeding site in the gastrostomy (dotted circle).
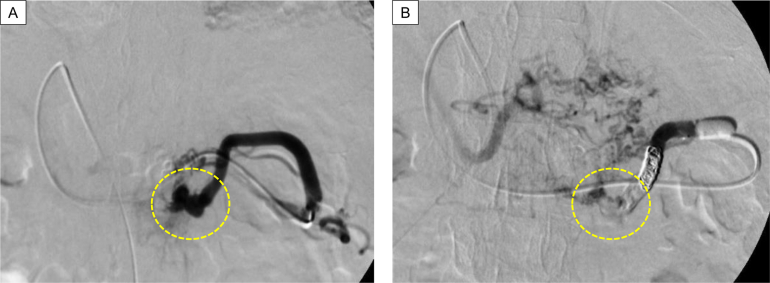
PTO was performed on day 6 of hospitalization. Percutaneous transhepatic puncture of the branch portal vein (P3) was made, and the left gastroepiploic vein was selected via the portal vein main channel and superior mesenteric vein. After digital subtraction angiography to confirm the targeted varices near the gastrostomy, he received 2 injections of 5 mL of 5% ethanolamine oleate. Since the varicose vein persisted, n-butyl-2-cyanoacrylate with lipiodol was administered in addition to coil embolization in the vicinity of the varicose vein (Figure 4A and B).
Figure 4.
(A) Varices as the bleeding source in the gastrostomy were distal to collateral vessels from the left gastroepiploic vein. Dotted circle indicates the therapeutically targeted bleeding source. (B) After treatment, blood flow to the varices disappeared. Dotted circle indicates the area where variceal blood flow was present before treatment.
Enhanced CT on day 9 of hospitalization showed the disappearance of blood flow in the collateral vessels and sclerosing varices (Figure 5). Endoscopic variceal ligation of the esophageal varices was performed on admission days 13 and 20. No further rebleeding incidents from the gastrostomy occurred during hospitalization, and he was later discharged on day 27.
Figure 5.
Enhanced computed tomography after percutaneous transhepatic occlusion showed the absence of blood flow in the collateral vessels and sclerosing varices. Dotted circle indicates lipiodol deposition in the area where variceal blood flow was present before treatment.
Discussion
We encountered a rare case of massive hemorrhage from collateral vessels during gastrostomy replacement. To the best of our knowledge, there have been no such reports to date.
Increased portal pressure causes portal blood to escape in other directions instead of toward the liver. This collateral circulation results in venous irritation of the abdominal wall and varices in the esophagus and stomach,5 which can lead to massive bleeding and even death in cirrhotic patients with portal hypertension.6 Therefore, the prevention and treatment of collateral hemorrhage are important in the management of cirrhotic patients.
Gastrostomy can cause hemorrhage complications, especially in cirrhotic patients who may be at even higher risk for reasons of coagulation abnormalities and thrombocytopenia.7 The present case suggests that endoscopists should be aware of complicating hemorrhage not only at the time of gastrostomy placement, but also during replacement, in patients with liver cirrhosis. Due to worsening portal hypertension, new collateral blood vessels may have developed by the time of replacement that were absent at the initial procedure. To prevent bleeding during gastrostomy replacement, it may be beneficial to evaluate for collateral blood vessels by CT and other imaging exams before the replacement if signs of portal hypertension, such as gastroesophageal varices, portal hypertensive gastropathy, ascites, splenomegaly, and visible collateral veins of the abdominal wall, have worsened.
Eligible diseases for PTO treatment include esophageal varices difficult to treat endoscopically, gastric varices difficult to treat with balloon-occluded retrograde obliteration, duodenal varices, colorectal varices, and hepatic encephalopathy from portal-arterial shunt.8 The reported case was considered an appropriate indication for PTO because of the inability to perform balloon-occluded retrograde obliteration and endoscopic treatment due to the absence of gastro-renal shunt and inability of recognition by endoscopy, respectively. Prior literature provides no evidence-based treatment owing to the rarity of massive hemorrhage from collateral blood vessels in gastrostomy replacement. However, our case may provide novel insights on PTO as an effective treatment option for this condition.
In conclusion, clinicians should be aware of the possibility of bleeding from collateral blood vessels when replacing a gastrostomy in patients with worsening portal hypertension and consider imaging exams beforehand to evaluate for collateral blood vessels. This report provides new clinical information on precautions and treatment for this rare condition.
Acknowledgments:
The authors would like to acknowledge Trevor Ralph for his English proofreading.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: The data that support the findings of this study are available from the corresponding author, Takefumi Kimura and Takeji Umemura, upon reasonable request.
Reporting Guidelines: Helsinki Declaration, CARE.
Contributor Information
Takefumi Kimura, Email: kimuratakefumii@yahoo.co.jp.
Takeji Umemura, Email: tumemura@shinshu-u.ac.jp.
References
- 1.Suzuki H., et al. Sci Rep. 2020;10(1):20551. doi: 10.1038/s41598-020-77553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderloni A., et al. Dig Liver Dis. 2019;51(10):1380–1387. doi: 10.1016/j.dld.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Cho K.C., et al. Radiographics. 1995;15(3):609–622. doi: 10.1148/radiographics.15.3.7624566. [DOI] [PubMed] [Google Scholar]
- 4.Horoldt B.S., et al. Dig Liver Dis. 2005;37(9):709–712. doi: 10.1016/j.dld.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama H., et al. Quant Imaging Med Surg. 2021;11(8):3867–3881. doi: 10.21037/qims-20-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Amico G., et al. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Pih G.Y., et al. BMC Gastroenterol. 2018;18(1):101. doi: 10.1186/s12876-018-0825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ninoi T., et al. AJR Am J Roentgenol. 2004;183(2):369–376. doi: 10.2214/ajr.183.2.1830369. [DOI] [PubMed] [Google Scholar]