Abstract
Annexin-V (PAP-I, lipocortin-V) acts as a potent anticoagulant in vitro by binding to negatively charged phospholipids with higher affinity than vitamin K-dependent proteins, with a Kd in the 10(-10) M range. The purpose of the present study was to use annexin-V as a probe to assess the catalytic potential of phospholipids in pro- and anti-coagulant reactions in purified systems and at the surface of endothelial cells in culture after stimulation. Procoagulant tissue factor and anticoagulant thrombomodulin activities were compared by using specific two-stage amidolytic assays performed with purified proteins. Procoagulant activity was estimated by the generation of Factor Xa by the Factor VII(a)-tissue factor complex. Anticoagulant activity was estimated by the generation of activated protein C by either the thrombin-thrombomodulin complex or Factor Xa. Annexin-V induced a decrease of 70% of thrombomodulin activity when thrombomodulin (5.4-214 nM) was reconstituted into phosphatidylcholine/phosphatidylserine (1:1, mol/mol) vesicles at 37.5 or 75 microM-phospholipid concentration, the apparent Ki being 0.5 microM at 75 microM-lipid. The saturating concentration of annexin-V was dependent on phospholipid concentration, but was independent of the phospholipid/thrombomodulin ratio. By contrast, when thrombomodulin was not reconstituted in vesicles, annexin-V had no effect. At 2 microM, annexin-V totally inhibited the generation of activated protein C by Factor Xa in the presence of 75 microM-lipid, the saturating inhibitory concentration being dependent on phospholipid concentration. At 0.1 microM, annexin-V totally inhibited tissue-factor activity present in crude brain thromboplastin. In the absence of stimulation, human endothelial cells in culture expressed significant thrombomodulin activity and no detectable tissue-factor activity. Basal thrombomodulin activity was only slightly inhibited (less than 15%) by 0.5 microM-annexin-V. Phorbol myristate acetate (PMA) induced the expression of tissue-factor activity and decreased thrombomodulin activity at the endothelial-cell surface. Annexin-V, at a concentration of 16 microM, caused an 80% decrease of tissue-factor activity induced by PMA at 10 ng/ml, whereas it inhibited thrombomodulin activity by only 15% on the same stimulated cells. Our results confirm that annexin-V inhibits, in vitro, procoagulant tissue-factor activity and anticoagulant activities (activation of protein C by the thrombin-thrombomodulin complex and by Factor Xa), through phospholipid-dependent mechanisms.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


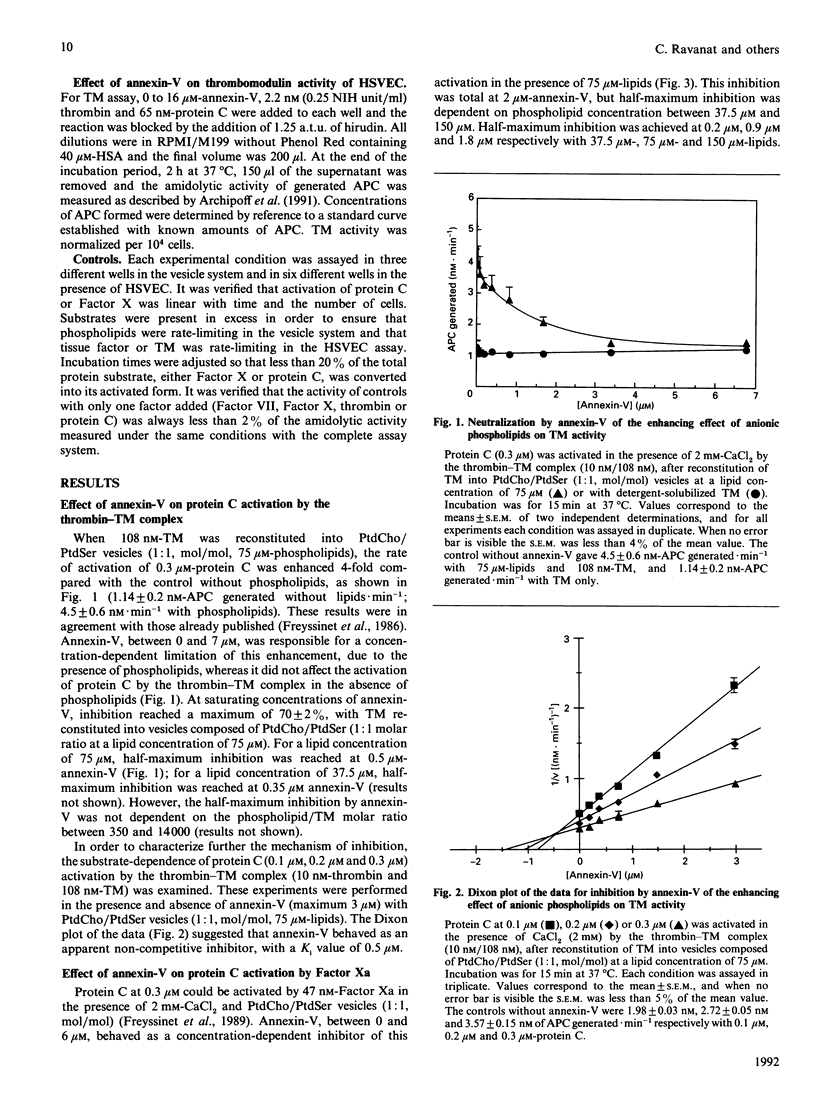
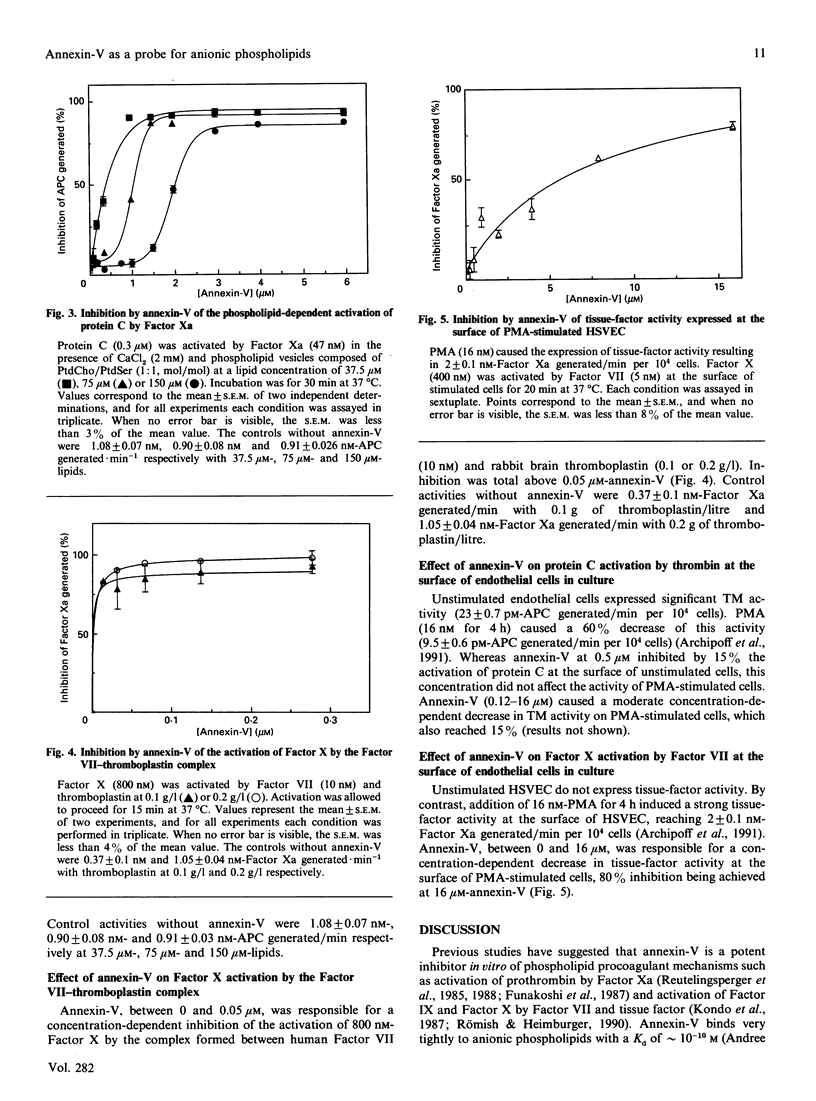


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Williamson P., Schlegel R. A. Phosphatidylserine as a determinant of reticuloendothelial recognition of liposome models of the erythrocyte surface. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8067–8071. doi: 10.1073/pnas.85.21.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andree H. A., Reutelingsperger C. P., Hauptmann R., Hemker H. C., Hermens W. T., Willems G. M. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J Biol Chem. 1990 Mar 25;265(9):4923–4928. [PubMed] [Google Scholar]
- Archipoff G., Beretz A., Freyssinet J. M., Klein-Soyer C., Brisson C., Cazenave J. P. Heterogeneous regulation of constitutive thrombomodulin or inducible tissue-factor activities on the surface of human saphenous-vein endothelial cells in culture following stimulation by interleukin-1, tumour necrosis factor, thrombin or phorbol ester. Biochem J. 1991 Feb 1;273(Pt 3):679–684. doi: 10.1042/bj2730679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach R., Gentry R., Nemerson Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: induction of cooperativity by phosphatidylserine. Biochemistry. 1986 Jul 15;25(14):4007–4020. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- Bach R., Rifkin D. B. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretz A., Freyssinet J. M., Gauchy J., Schmitt D. A., Klein-Soyer C., Edgell C. J., Cazenave J. P. Stability of the thrombin-thrombomodulin complex on the surface of endothelial cells from human saphenous vein or from the cell line EA.hy 926. Biochem J. 1989 Apr 1;259(1):35–40. doi: 10.1042/bj2590035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona R., Lee E., Rickles F. Tissue factor apoprotein: intracellular transport and expression in shed membrane vesicles. Thromb Res. 1987 Nov 15;48(4):487–500. doi: 10.1016/0049-3848(87)90405-1. [DOI] [PubMed] [Google Scholar]
- Carson S. D., Ross S. E. Effects of lipid-binding proteins apo A-I, apo A-IL, beta 2-glycoprotein I, and C-reactive protein on activation of factor X by tissue factor--factor VIIa. Thromb Res. 1988 Jun 1;50(5):669–678. doi: 10.1016/0049-3848(88)90325-8. [DOI] [PubMed] [Google Scholar]
- Comfurius P., Senden J. M., Tilly R. H., Schroit A. J., Bevers E. M., Zwaal R. F. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochim Biophys Acta. 1990 Jul 24;1026(2):153–160. doi: 10.1016/0005-2736(90)90058-v. [DOI] [PubMed] [Google Scholar]
- Connor J., Bucana C., Fidler I. J., Schroit A. J. Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc Natl Acad Sci U S A. 1989 May;86(9):3184–3188. doi: 10.1073/pnas.86.9.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Ruf W., Morrissey J. H., Edgington T. S. Functional tissue factor is entirely cell surface expressed on lipopolysaccharide-stimulated human blood monocytes and a constitutively tissue factor-producing neoplastic cell line. J Cell Biol. 1989 Jul;109(1):389–395. doi: 10.1083/jcb.109.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon N. L., Esmon C. T. Protein C and the endothelium. Semin Thromb Hemost. 1988 Apr;14(2):210–215. doi: 10.1055/s-2007-1002779. [DOI] [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Freyssinet J. M., Beretz A., Klein-Soyer C., Gauchy J., Schuhler S., Cazenave J. P. Interference of blood-coagulation vitamin K-dependent proteins in the activation of human protein C. Involvement of the 4-carboxyglutamic acid domain in two distinct interactions with the thrombin-thrombomodulin complex and with phospholipids. Biochem J. 1988 Dec 1;256(2):501–507. doi: 10.1042/bj2560501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssinet J. M., Gauchy J., Cazenave J. P. The effect of phospholipids on the activation of protein C by the human thrombin-thrombomodulin complex. Biochem J. 1986 Aug 15;238(1):151–157. doi: 10.1042/bj2380151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssinet J. M., Wiesel M. L., Grunebaum L., Pereillo J. M., Gauchy J., Schuhler S., Freund G., Cazenave J. P. Activation of human protein C by blood coagulation factor Xa in the presence of anionic phospholipids. Enhancement by sulphated polysaccharides. Biochem J. 1989 Jul 15;261(2):341–348. doi: 10.1042/bj2610341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T., Heimark R. L., Hendrickson L. E., McMullen B. A., Fujikawa K. Human placental anticoagulant protein: isolation and characterization. Biochemistry. 1987 Aug 25;26(17):5572–5578. doi: 10.1021/bi00391a053. [DOI] [PubMed] [Google Scholar]
- Galvin J. B., Kurosawa S., Moore K., Esmon C. T., Esmon N. L. Reconstitution of rabbit thrombomodulin into phospholipid vesicles. J Biol Chem. 1987 Feb 15;262(5):2199–2205. [PubMed] [Google Scholar]
- Gramzinski R. A., Broze G. J., Jr, Carson S. D. Human fibroblast tissue factor is inhibited by lipoprotein-associated coagulation inhibitor and placental anticoagulant protein but not by apolipoprotein A-II. Blood. 1989 Mar;73(4):983–989. [PubMed] [Google Scholar]
- Hamilton K. K., Hattori R., Esmon C. T., Sims P. J. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990 Mar 5;265(7):3809–3814. [PubMed] [Google Scholar]
- Huang C., Thompson T. E. Preparation of homogeneous, single-walled phosphatidylcholine vesicles. Methods Enzymol. 1974;32:485–489. doi: 10.1016/0076-6879(74)32048-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Suda M., Nakao H., Nagoya T., Saino Y., Arai K., Mizoguchi T., Sato F., Yoshizaki H., Hirata M. Structure and expression of cDNA for an inhibitor of blood coagulation isolated from human placenta: a new lipocortin-like protein. J Biochem. 1987 Nov;102(5):1261–1273. doi: 10.1093/oxfordjournals.jbchem.a122165. [DOI] [PubMed] [Google Scholar]
- Jackson C. M. Characterization of two glycoprotein variants of bovine factor X and demonstration that the factor X zymogen contains two polypeptide chains. Biochemistry. 1972 Dec 19;11(26):4873–4882. doi: 10.1021/bi00776a001. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. V., Kline M. D., Owen W. G. The effect of bovine thrombomodulin on the specificity of bovine thrombin. J Biol Chem. 1986 Mar 15;261(8):3876–3882. [PubMed] [Google Scholar]
- Kisiel W., Ericsson L. H., Davie E. W. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976 Nov 2;15(22):4893–4900. doi: 10.1021/bi00667a022. [DOI] [PubMed] [Google Scholar]
- Klein-Soyer C., Beretz A., Millon-Collard R., Abecassis J., Cazenave J. P. A simple in vitro model of mechanical injury of confluent cultured endothelial cells to study quantitatively the repair process. Thromb Haemost. 1986 Oct 21;56(2):232–235. [PubMed] [Google Scholar]
- Komiyama Y., Pedersen A. H., Kisiel W. Proteolytic activation of human factors IX and X by recombinant human factor VIIa: effects of calcium, phospholipids, and tissue factor. Biochemistry. 1990 Oct 9;29(40):9418–9425. doi: 10.1021/bi00492a016. [DOI] [PubMed] [Google Scholar]
- Kondo S., Noguchi M., Funakoshi T., Fujikawa K., Kisiel W. Inhibition of human factor VIIa-tissue factor activity by placental anticoagulant protein. Thromb Res. 1987 Nov 15;48(4):449–459. doi: 10.1016/0049-3848(87)90402-6. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Esmon C. T., Esmon N. L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989 Jan;73(1):159–165. [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsestuen G. L., Kisiel W., Di Scipio R. G. Interaction of vitamin K dependent proteins with membranes. Biochemistry. 1978 May 30;17(11):2134–2138. doi: 10.1021/bi00604a017. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. I. Characterization of the reaction products formed during the activation of bovine prothrombin. J Biol Chem. 1974 Jan 25;249(2):594–605. [PubMed] [Google Scholar]
- Preissner K. T. Anticoagulant potential of endothelial cell membrane components. Haemostasis. 1988;18(4-6):271–300. doi: 10.1159/000215813. [DOI] [PubMed] [Google Scholar]
- Radcliffe R., Nemerson Y. Mechanism of activation of bovine factor VII. Products of cleavage by factor Xa. J Biol Chem. 1976 Aug 25;251(16):4749–4802. [PubMed] [Google Scholar]
- Reutelingsperger C. P., Hornstra G., Hemker H. C. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur J Biochem. 1985 Sep 16;151(3):625–629. doi: 10.1111/j.1432-1033.1985.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Reutelingsperger C. P., Kop J. M., Hornstra G., Hemker H. C. Purification and characterization of a novel protein from bovine aorta that inhibits coagulation. Inhibition of the phospholipid-dependent factor-Xa-catalyzed prothrombin activation, through a high-affinity binding of the anticoagulant to the phospholipids. Eur J Biochem. 1988 Apr 5;173(1):171–178. doi: 10.1111/j.1432-1033.1988.tb13981.x. [DOI] [PubMed] [Google Scholar]
- Rodgers G. M., Shuman M. A. Prothrombin is activated on vascular endothelial cells by factor Xa and calcium. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7001–7005. doi: 10.1073/pnas.80.22.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Römisch J., Heimburger N. Purification and characterization of six annexins from human placenta. Biol Chem Hoppe Seyler. 1990 May;371(5):383–388. doi: 10.1515/bchm3.1990.371.1.383. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T., Esmon C. T., Weiss H. J., Shattil S. J. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem. 1989 Oct 15;264(29):17049–17057. [PubMed] [Google Scholar]
- Solymoss S., Tucker M. M., Tracy P. B. Kinetics of inactivation of membrane-bound factor Va by activated protein C. Protein S modulates factor Xa protection. J Biol Chem. 1988 Oct 15;263(29):14884–14890. [PubMed] [Google Scholar]
- Stern D. M., Kaiser E., Nawroth P. P. Regulation of the coagulation system by vascular endothelial cells. Haemostasis. 1988;18(4-6):202–214. doi: 10.1159/000215808. [DOI] [PubMed] [Google Scholar]
- Stern D., Nawroth P., Handley D., Kisiel W. An endothelial cell-dependent pathway of coagulation. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2523–2527. doi: 10.1073/pnas.82.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan P., Tait J. F. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem. 1990 Oct 15;265(29):17420–17423. [PubMed] [Google Scholar]
- Torbet J., Freyssinet J. M. Neutron scattering determination of the binding of prothrombin to lipid vesicles. Biochemistry. 1987 Dec 1;26(24):7791–7798. doi: 10.1021/bi00398a039. [DOI] [PubMed] [Google Scholar]
- Tracy P. B., Eide L. L., Mann K. G. Human prothrombinase complex assembly and function on isolated peripheral blood cell populations. J Biol Chem. 1985 Feb 25;260(4):2119–2124. [PubMed] [Google Scholar]
- Zaccaï G., Jacrot B. Small angle neutron scattering. Annu Rev Biophys Bioeng. 1983;12:139–157. doi: 10.1146/annurev.bb.12.060183.001035. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Bevers E. M., Comfurius P., Rosing J., Tilly R. H., Verhallen P. F. Loss of membrane phospholipid asymmetry during activation of blood platelets and sickled red cells; mechanisms and physiological significance. 1989 Nov 23-Dec 19Mol Cell Biochem. 91(1-2):23–31. doi: 10.1007/BF00228075. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Comfurius P., van Deenen L. L. Membrane asymmetry and blood coagulation. Nature. 1977 Jul 28;268(5618):358–360. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Hemker H. C. Blood cell membranes and haemostasis. Haemostasis. 1982;11(1):12–39. doi: 10.1159/000214638. [DOI] [PubMed] [Google Scholar]


