Abstract
Background and Aims
Children with very early onset inflammatory bowel disease (VEO-IBD) are uniquely at risk of inadequate infliximab (IFX) exposure. We studied the association between standard body weight (BW)-based and body surface area (BSA)-based dosing strategies and outcomes.
Methods
We identified VEO-IBD patients treated with IFX before 9 years at a single center. Patients were separated into those that received a BSA-based dose (200 mg/m2) and standard BW dosing (5 mg/kg). IFX drug levels, dose intensification, time on steroids, and long-term outcomes were compared. Receiver operator characteristic curves determined the optimal BW- and BSA-based dose to achieve a trough ≥10 μg/ml at dose 4 (IFX#4).
Results
Forty-three children with VEO-IBD were identified. Receiver operator characteristic curves demonstrated optimal BW- and BSA-based doses to achieve IFX trough ≥10 μg/ml at IFX#4 were 7.5 mg/kg and 180mg/m2. Children were classified to standard BW dosing (22/43) and BSA dosing (10/43). IFX#4 trough was significantly higher in those who received BSA dosing (BSA 18.6 μg/ml [interquartile range 10.8–28.1] vs BW 5.1 μg/ml [interquartile range 2.6–10.7], P = .04). BSA dosing was more likely to achieve a target drug level >10 μg/ml at IFX#4 (BSA 70% vs BW 18%, P = .02). BW dosing was associated with a greater likelihood of dose escalation (BW 82% vs BSA 30%, P < .01) and a shorter time to first escalation. BSA dosing was associated with shorter time spent on steroids (P = .02).
Conclusion
Young children require higher IFX dosing to achieve adequate drug exposure. Our data support the use of a BSA-based dose of 200 mg/m2 or, if a BW-based approach is used, 7.5 mg/kg. BSA dosing allows the use of a consistent dose over the age and weight spectrum.
Keywords: Infliximab, Very Early Onset Inflammatory Bowel Disease, Body Surface Area
Background
The incidence of inflammatory bowel disease (IBD) is increasing fastest in the youngest children diagnosed < 6 years,1 considered as very early onset IBD (VEO-IBD). Infliximab (IFX), an anti-tumor necrosis factor-α biologic, is an established treatment for IBD which uses weight-based dosing, with a recommended standard 5mg/kg at induction and subsequently during maintenance. Body weight (BW)-based (mg/kg) dosing may not be optimal for all children. Young children have distinct pharmacokinetics which may affect drug exposure and therefore treatment efficacy. Dotan et al showed a nonlinear correlation between BW and IFX clearance, with lower BWs having 35% lower drug exposure than patients with double their weight given 5 mg/kg.2 Compared to older children, young children have higher rates of IFX failure during induction and maintenance phases3 and require more IFX per kg to achieve target serum drug levels.4
Weight-stratified dosing may lead to better exposure than BW-based dosing, as has been used with other monoclonal antibodies such as tocilizumab, which uses a higher per kg dosage for patients <30kg (12mg/kg) than for patients ≥30kg (8mg/kg).5 Recent studies have attempted to optimize IFX dosing using model-based tools6 and dashboard-guided dosing strategies.7 However, the ideal dosing regimen has not yet been defined.
Young children have a larger body surface area (BSA) relative to their weight with general population data demonstrating an exponential rise in BSA relative to weight in young children, transitioning to a more linear relationship between BSA and weight at older ages and higher weights that approximate adult weights (Figure A1).8 In the phase 3 study of adalimumab in juvenile idiopathic arthritis, BSA dosing was found to be effective with younger children having slightly higher steady-state troughs than older children. IgG-based therapeutic proteins are predominantly eliminated by proteolytic catabolism, and therefore BSA-based dosing may lead to less variable exposure as catabolism in mammalian organisms is essentially constant when expressed per unit of BSA.5
We hypothesized that VEO-IBD patients are uniquely at risk of inadequate IFX exposure using standard weight-based dosing and that BSA-based dosing results in better drug exposure and superior short-term outcomes.
Methods
We performed a retrospective single-center (SickKids, Toronto, Canada) cohort study of VEO-IBD patients diagnosed at <6 years and treated with IFX before 9 years from January 2010 to July 2021, with a minimum 6-month follow-up. Patients were excluded if they had a known monogenic cause for their IBD. Demographic and clinical data at diagnosis and IFX initiation were collected including age, disease location, behavior (as per Paris classification), and anthropometrics. Clinical disease activity was described by Physician Global Assessment as none, mild, moderate, or severe. Combination immunomodulator therapy was defined as azathioprine or methotrexate used in combination with IFX that was started within 3 months of IFX and continued for a minimum of 3 months. Adverse events during treatment and reasons for IFX withdrawal were recorded. IFX trough levels were measured as part of clinical care using a drug-sensitive assay. This was carried out routinely before the fourth dose of IFX with a standard dosing schedule at our center of weeks 0, 2, 6, and 12, which may differ from centers using weeks 0, 2, 6, and 14 dosing. Patients were censored at IFX cessation, surgery, or transfer to adult care.
All dosing, including induction dosing, treatment intensification by dose escalation, and/or interval shortening, was done based on physician discretion. Importantly, in 2014, physicians at our center started adopting a strategy of dosing IFX in VEO-IBD patients based on BSA, rather than using conventional weight-based dosing. Specifically, patients dosed per BSA were given 200 mg/m2. This dose was selected because it roughly equals 5 mg/kg in average-sized adults based on Centres for Disease Control and Prevention and World Health Organisation normative anthropometric data; 5mg/kg is the recommended standard dosing for IFX regardless of age and is the standard dose used in our center before the use of BSA dosing. Using the Mosteller formula to calculate BSA and given the average weight and height of an adult (>20 years) as per the Centres for Disease Control and Prevention (male: height 175cm, weight 90kg, BSA 2.09; female height 161.3cm, weight 77.5kg, BSA 1.9), a dose of 5 mg/kg translates to a BSA-based dose of 215mg/m2 and 204mg/m2, respectively. World Health Organisation growth charts 50th centile adult (19 years) male and female anthropometrics (male 176.8cm, 70.5kg, BSA 1.85; female 163cm, 58kg, BSA 1.6) yield a BSA dosing of 189mg/m2 and 181mg/m2, respectively. To further validate the choice of a BSA-based dose of 200 mg/m2, we graphically compared the absolute dose yielded by 200mg/m2 to that obtained with a standard weight-based dose (5mg/kg) using recorded heights and weights at induction of IFX in a separate 436-patient pediatric IBD cohort treated at SickKids from 2001 to 2018.
We first summarized clinical outcomes for all VEO-IBD patients in our cohort and examined the association between BSA-based and standard weight-based dosing and drug exposure. We constructed receiver operator characteristic (ROC) curves to determine the optimal BSA-based dose to obtain a drug level ≥10 μg/ml prior to the first maintenance dose7 (ie, predose 4).
Next, we separated the VEO-IBD cohort into 2 groups: 1) patients who received a BSA-based dose of 200 mg/m2 and 2) patients who received standard weight-based IFX dosing of 5 mg/kg (both rounded up to the nearest 100 mg). Patients not falling into either category or falling into both were excluded from this part of the analysis. We compared the following outcomes between the 2 groups: time on steroids, IFX dose intensification, corticosteroid-free clinical remission, surgery, IFX durability, development of antidrug antibodies (ADAs), and adverse events.
Continuous variables were summarized as medians with interquartile range (IQR) and categorical variables as frequencies with proportions. We compared continuous variables using the Mann-Whitney U test and categorical variables using the ꭕ2 test or Fisher exact test. Kaplan-Meier survival analysis was performed to evaluate time to escalation of IFX and time on steroids from induction. Optimal dosing to achieve target IFX levels was based on ROC curve analysis and Youden’s index. A P value < .05 was considered statistically significant. Analyses were performed using SPSS (IBM, Armonk, NY). This study was conducted with research ethics board approval from The Hospital for Sick Children.
Results
We identified 43 children with VEO-IBD treated with IFX (patient characteristics shown in the first column of Table 1). The median IFX induction dose over the first 3 infusions for the entire cohort was median 7.0 (IQR 5.4–10.4) mg/kg or 175.9 (IQR 130.7–259.6) mg/m2. This did not change significantly over one and 2 years of follow-up. The median time from dose 1 to dose 4 was 69 (IQR 56–84) days, which reflects accelerated induction in some children. IFX trough level prior to IFX#4 was median 12.0 (IQR 4.6–28.0) μg/ml. Clinical outcomes are shown in column 1 of Table 2.
Table 1.
Baseline Characteristics
| Baseline characteristics | Overall (n = 43) | 5mg/kg (n = 22) | 200mg/m2 (n = 10) | P values |
|---|---|---|---|---|
| Male | 22 (51.2%) | 13 (40.6%) | 4 (12.5%) | .32 |
| Age (y) at diagnosis, median (IQR) | 3.5 (2.3, 5.2) | 3.3 (2.1, 5.1) | 2.5 (1.8, 4.3) | .45 |
| Age (y) at IFX start, median (IQR) | 5.7 (3.7, 7.3) | 6.2 (3.7, 7.5) | 5.0 (3.3, 5.9) | .15 |
| Weight at IFX start (kg), median (IQR) | 19 (15, 21.5) | 19.5 (15.8, 21.7) | 16 (11.7, 19.2) | .04a |
| BSA at IFX start (mg/m2), median (IQR) | 0.77 (0.64, 0.85) | 0.78 (0.68, 0.85) | 0.67 (0.54, 0.77) | .25 |
| Albumin at IFX start (g/L), median (IQR) | 35 (32, 41) | 37 (33, 40) | 33 (29, 37) | .80 |
| Hemoglobin at IFX start, median (IQR) | 100 (84, 115) | 107 (79, 117) | 96 (79, 115) | .80 |
| IBD type | ||||
| CD | 15 (34.9%) | 7 (31.8%) | 4 (40%) | .85 |
| L2 | 12 (27.9%) | 6 (27.2%) | 4 (40%) | .43 |
| L3 | 3 (7%) | 1 (4.5%) | 0 (0%) | .43 |
| UC/IBDU | 28 (65.1%) | 14 (63.6%) | 6 (60%) | .84 |
| Perianal disease | 3 (7%) | 0 (0%) | 0 (0%) | 1.00 |
| Physician Global Assessment at IFX start | ||||
| Mild | 1 (2.4%) | 1 (4.5%) | 0 (0%) | .48 |
| Moderate | 28 (65.1%) | 14 (63.6%) | 5 (50%) | .37 |
| Severe | 13 (30.2%) | 6 (27.2%) | 5 (50%) | .24 |
| Global Endoscopic Severity at IFX start | ||||
| Mild | 1 (2.4%) | 1 (4.5%) | 0 (0%) | .48 |
| Moderate | 15 (34.8%) | 7 (31.8%) | 5 (50%) | .28 |
| Severe | 9 (20.9%) | 7 (31.8%) | 2 (20%) | .42 |
| Unknown | 18 (41.8%) | 7 (31.8%) | 3 (30%) | .92 |
| Combination immunomodulator therapy | 11 (25.6%) | 7 (31.8%) | 1 (10%) | .19 |
| Steroids at infliximab start | 27 (62.8%) | 15 (68.1%) | 9 (90%) | .19 |
CD, Crohn’s disease; IBD-U, IBD-unclassified; UC, ulcerative colitis.
P < .05.
Table 2.
Infliximab Dosing Details and Outcomes
| Infliximab dosing details and outcomes | Overall (n = 43) | 5mg/kg (n = 22) | 200mg/m2 (n = 10) | P values |
|---|---|---|---|---|
| Dose/kg | ||||
| Induction-median dose 1–3 (IQR) | 6.9 (5.4,10.4) | 5.6 (5.1, 6) | 11.9 (9.7, 17.1) | .001a |
| 1 y (IQR) | 8.2 (6.4, 10) | 7.4 (6.1, 9.8) | 8.7 (7.5, 8.7) | 1.00 |
| 2 y (IQR) | 8.7 (6.3, 9.7) | 8 (6, 9.6) | 10.9 (10.9) | .47 |
| Dose/m2 | ||||
| Induction-median dose 1–3 (IQR) | 177.4 (130.7, 283.9) | 136 (124,161.5) | 283.9 (236.8370.8) | .001a |
| 1 y (IQR) | 211.4 (170.6, 263.9) | 200 (159.8, 247.2) | 231.3 (188.5, 231.3) | 1.00 |
| 2 y (IQR) | 224.8 (164.1, 257.7) | 215.3 (161.4, 247.8) | 314.1 (314.1) | .47 |
| Infliximab level predose 4 (μg/ml) (IQR) | 12 (4.6, 28) | 5.1 (2.6, 10.7) | 18.6 (10.8, 28.1) | .04a |
| Interval dose 1 – dose 4 IFX (d), median (IQR) | 69 (56, 84) | 70 (56,87) | 58 (56, 71) | .11 |
| Infliximab cessation | 19 (44.1%) | 12 (54.5%) | 5 (50%) | 1.00 |
| Infliximab escalation | 26 (60.5%) | 18 (81.8%) | 3 (30%) | .004a |
| CSF remission | ||||
| Post induction | 13 (30.2%) | 6 (27.3%) | 3 (30%) | .87 |
| 6 mo | 20 (54.1%) | 8 (42.1%) | 4 (50%) | .71 |
| 1 y | 20 (71%) | 12 (75%) | 4 (67%) | .70 |
| 2 y | 12 (63.2%) | 10 (67%) | 2 (100%) | .33 |
| Time to stopping steroids (d), (IQR) | 67.5 (39.8, 138.5) | 106 (49.5, 178) | 53.5 (32, 99) | .65 |
| Colectomy | 6 (13.9%) | 5 (22.7%) | 1 (10%) | .39 |
CSF, corticosteroid free.
P < .05.
The ability to achieve a target drug level of ≥10 μg/ml prior to IFX#4 was associated with a significantly higher median IFX induction dose (weight-based: median 10.1 [IQR 7.2–15.8] vs 5.8 [IQR 5.6–6.8] mg/kg, P = .012; BSA-based: median 249.6 [IQR 185.2–379.8] vs 151.9 [IQR 128.6–220.3] mg/m2, P = .02). There was no difference in albumin levels at induction between those who did or did not achieve this target drug level (IFX level≥10 μg/ml: median albumin 35 [IQR 32–41] vs IFX level<10μg/ml: median albumin 34 [IQR 31–41] g/L, P = 1.00).
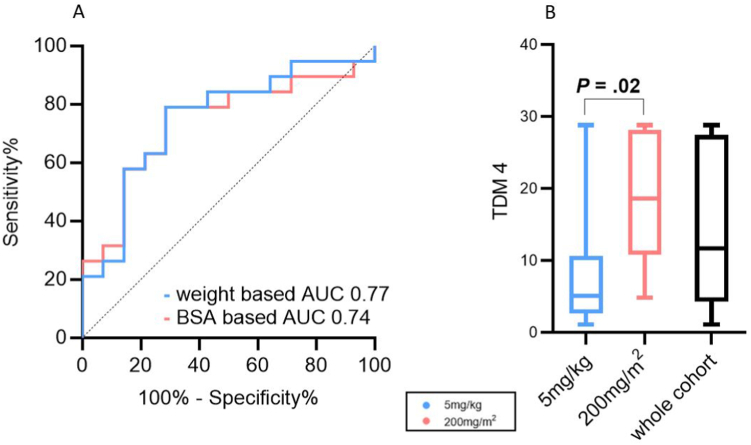
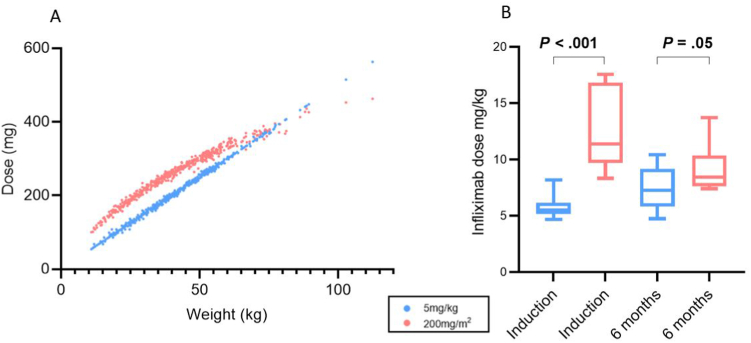
Based on ROC curves (Figure 1A), the optimal weight-based dose to achieve an IFX trough ≥ 10 μg/ml at dose 4 was 7.5mg/kg; the positive predictive value for a dose of 5mg/kg was low at 59% (sensitivity 100%, specificity 7%) (area under the curve 0.77; 95% confidence interval [CI] 0.60–0.95). The optimal BSA-based dose for a trough of 10μg/ml at dose 4 was 180 mg/m2 (area under the curve 0.74; 95% CI 0.56–0.91), with a positive predictive value of 79% (sensitivity 79%, specificitiy 71%). This supported our use of 200 mg/m2 (180mg rounded up) as the BSA-based dose. Moreover, in examining anthropometric data from a separate cohort of 436 IFX-treated children at our center, with an age range of 1.6-18 years at induction, we found that 200 mg/m2 resulted in higher absolute doses than 5 mg/kg up until about 70 kg, or adult weight, at which point the 2 converged (Figure 2A).
Figure 1.
Infliximab trough levels at dose 4 (A) ROC of dosing to achieve target infliximab trough at dose 4. (B) Infliximab trough levels at dose 4 by dosing regimen.
Figure 2.
Infliximab dosing by body weight and body surface area (A) Correlation between 5mg/kg and 200mg/m2 dosing in a pediatric IBD cohort of 436 patients. (B) Infliximab dosing across time points in a very early onset–IBD cohort.
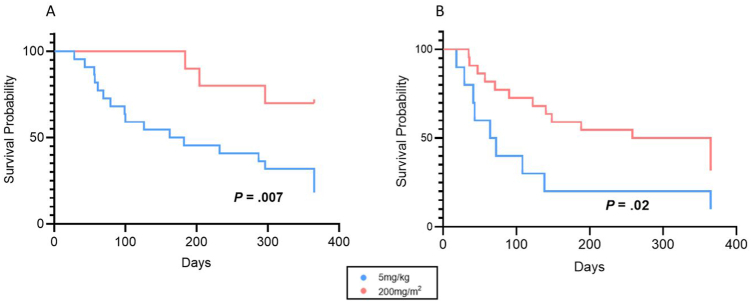
In part 2 of the analysis, 22/43 were classified to the standard weight-based group (BW) (5mg/kg), and 10/43 to the BSA-based group (BSA) (200mg/m2). Patient characteristics for both groups are shown in columns 2 and 3 of Table 1. Median induction dosing (per kg) was significantly higher in the group dosed according to BSA compared to those receiving standard weight-based dosing (BSA 11.9 [IQR 9.7–17.1] vs BW median 5.6 [IQR 5.1–6.0] mg/kg, P = .001), but the induction time interval (from dose #1 to #4) was similar in both groups. IFX trough level predose #4 was significantly higher in those who received BSA dosing (BSA 18.6 μg/ml [IQR 10.8–28.1] vs BW median 5.1 μg/ml [IQR 2.6–10.7], P = .04) (Figure 1B). Children dosed according to BSA were also significantly more likely to achieve a target drug level greater than 10 μg/ml at the start of maintenance phase (BSA 70% vs BW 18%, P = .02). Standard weight-based dosing was associated with a greater likelihood of dose escalation (BW 82% vs BSA 30%, P < .01) and a shorter time to first escalation (Figure 3A). For children who were on steroids when IFX was started, patients dosed according to BSA had shorter time on steroids (Figure 3B).
Figure 3.
Survival curves of infliximab escalation and time to steroid cessation. (A) Survival curve of time to first escalation of IFX by dosing regimen. (B) Survival curve of time to steroid cessation from start of IFX by dosing regimen.
BSA-based dosing did not differ from BW-based dosing in rates of corticosteroid free remission at 6 months, 1 year or 2 years, nor in overall rates of IFX cessation (Table 2). Importantly, by 6 months, dosing was more similar across the groups due to interim dose escalation in the children originally treated with standard weight-based dosing (Figure 2B). ADAs were identified in 4 children, all of whom were in the standard weight-based dosing group. The median time to develop ADAs was 707 (IQR 236–1230) days.
Discussion
In our VEO-IBD cohort, standard weight-based IFX dosing resulted in inadequate drug exposure as supported by low trough levels at the end of induction, more frequent ADA development, and longer time to wean steroids. Interestingly, longer-term outcomes such as rates of IFX cessation, corticosteroid-free remission rates, and colectomy did not differ between groups, likely due to dose convergence in both groups over time. This was evidenced by the higher rate of dose optimization in the weight-based dosing group with comparable dosing by 1 and 2 years. Similarly, DeBruyn et al9 and Jongsma et al4 showed that children under 10 years had higher rates of IFX optimization.
Although IFX dosing is weight based, previous studies have shown that the correlation between IFX clearance and BW is not linear.2,10 In their pharmacokinetic model based on serum IFX concentration data from 692 patients, including 112 children (>6 years), Fasanmade et al10 demonstrated that the volume of distribution in the peripheral compartment was higher in the pediatric population. Weight emerging as a covariate suggests inadequate correction of body size by the per-kilogram dosing scheme. This may lead to underdosing in children or low-weight adults. This model was further used by Frymoyer et al to determine IFX troughs during maintenance in children with Crohn’s disease. They suggested an optimized dosing strategy of 7.5 or 10 mg/kg every 4 weeks for albumin levels of 3 g/dl; and 5 mg/kg every 4 weeks or 10 mg/kg every 6 weeks for albumin levels of 4 g/dl in children with a weight of 32kg.
Our data similarly support the use of routine higher IFX dosing in young children, specifically 7.5 mg/kg at induction, if using a weight-based approach, based on our ROC analysis targeting a drug level of at least 10μg/ml at the start of maintenance (predose 4). Serum albumin, although well described in other studies as influencing trough levels,11, 12, 13 was not found to be a factor in our cohort with no difference seen in median albumin levels in those who did or did not achieve the target drug level.
Previous studies exploring pharmacokinetics and ideal dosing of IFX in children have focused mainly on children > 6 years of age.10,14 Our study looks at the youngest cohort of IBD patients who due to their low weight are most vulnerable to under dosing. In adults, a standard weight-based (5 mg/kg) IFX dose is approximately equal to a BSA-based dose of 200 mg/m2. The same is not true in young children, in whom a standard weight-based dose systematically results in a lower absolute dose than a BSA-based dose. BSA dosing has been previously explored in other monoclonal antibodies, including adalimumab which, like IFX, targets tumor necrosis factor-α. Although BSA dosing was found to be effective, tiered fixed dosing is used largely due to the complexity of the formula for calculating BSA and practitioner preference.5 However, with the widespread use of electronic medical record systems, BSA can be automatically calculated. In comparison to stratified dosing, BSA dosing has the advantage of a consistent dose/m2 over the age and weight spectrum.
This study is limited by its retrospective design. Dosing was at the individual physician’s discretion which increased the risk for bias and may have led to population differences between our 2 groups. Those who received BSA dosing were lighter, had lower albumin levels, and shorter dosing intervals. Although not statistically significant, lower albumin levels have a potential negative impact on trough levels, while shorter dosing intervals may have contributed to the higher troughs seen. However, a prospective study in this relatively small population would require a long time. In addition, a drug-sensitive assay was used for measuring ADAs which may underestimate the rate of antibody formation in this cohort.
Conclusion
BSA-based IFX dosing offers a novel approach to IFX dosing in young children with IBD. Should weight-based dosing be used, our data support the routine use of a dose higher than standard 5 mg/kg, specifically 7.5 mg/kg, targeting a drug level of ≥10μg/ml at the start of maintenance. However, given the nonlinear relationship between BSA and weight in young children, this weight-based dose may need to be reassessed over time as patients age and grow. BSA-based dosing allows the use of a consistent dose over the age and weight spectrum.
Acknowledgments
Authors' Contributions:
Amanda Ricciuto and Aleixo M. Muise were the principal investigators of the study. Lorraine Stallard, Nathaniel Frost, Luca Scarallo, Eric I. Benchimol, Thomas D. Walters, and Peter C. Church contributed to the data collection and statistical analysis of the data. Amanda Ricciuto, Aleixo M. Muise, Anne M. Griffiths, Karen Frost, and Lorraine Stallard conceived the study design. Lorraine Stallard drafted the initial manuscript and all authors contributed equally to revising the work.
Footnotes
Conflicts of Interest: These authors disclose the following: Anne M Griffiths has served as a speaker or consultant or advisory board member for AbbVie, Amgen, Bristol Meyers Squibb, Celgene, Janssen, Lilly, Merck, Nestle, Pfizer, and Roche, and has received a research grant from AbbVie. Eric Benchimol has acted as a legal consultant for Hoffman La-Roche Limited and Peabody & Arnold LLP for matters unrelated to medications used to treat inflammatory bowel disease. He has also acted as a consultant for McKesson Canada and the Dairy Farmers of Ontario for matters unrelated to medications used to treat inflammatory bowel disease. Peter C. Church has received educational grants from AbbVie, Amgen, Janssen, Takeda, Viatris, speaker fees from AbbVie, Amgen and consultant fees from AbbVie, Amgen, Ferring, and Merck. The remaining authors disclose no conflicts.
Funding: Aleixo M Muise is funded by the Leona M. and Harry B. Helmsley Charitable Trust, NIH (RC2DK122532 and RC2DK118640), Canada Research Chair (Tier 1) in Pediatric IBD, and CIHR Foundation. Lorraine Stallard is in receipt of a Canadian Association of Gastroenterology resident research award.
Ethical Statement: This study was conducted with research ethics board approval (REB# 1000078961) at the Hospital for Sick Children, Toronto, Canada. Figure A1 reprinted with permission from The American Physiological Society, license number 5487240982555.
Data Transparency Statement: Data, analytic methods, and study materials will be made available to other researchers upon request to corresponding authors on reasonable request.
Reporting Guidelines: STROBE.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2023.11.004.
Contributor Information
Aleixo M. Muise, Email: aleixo.muise@utoronto.ca.
Amanda Ricciuto, Email: amanda.ricciuto@sickkids.ca.
Supplementary Materials
Figure A1.
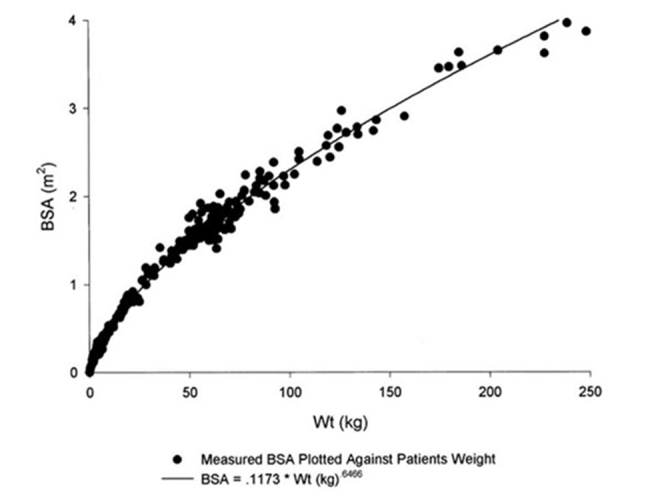
Nonlinear regression of estimated body surface area (BSA) and body weight, reproduced with permission from Livingston et al.
References
- 1.Kuenzig M.E., Fung S.G., Marderfeld L., et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. 2022;162:1147–1159.e4. doi: 10.1053/j.gastro.2021.12.282. [DOI] [PubMed] [Google Scholar]
- 2.Dotan I., Ron Y., Yanai H., et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 3.Bramuzzo M., Arrigo S., Romano C., et al. Efficacy and safety of infliximab in very early onset inflammatory bowel disease: a national comparative retrospective study. United European Gastroenterol J. 2019;7:759–766. doi: 10.1177/2050640619847592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jongsma M.M.E., Winter D.A., Huynh H.Q., et al. Infliximab in young paediatric IBD patients: it is all about the dosing. Eur J Pediatr. 2020;179:1935–1944. doi: 10.1007/s00431-020-03750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Davis H.M., Zhou H. Rational development and utilization of antibody-based therapeutic proteins in pediatrics. Pharmacol Ther. 2013;137:225–247. doi: 10.1016/j.pharmthera.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Kantasiripitak W., Wicha S.G., Thomas D., et al. A model-based tool for guiding infliximab induction dosing to maximise long-term deep remission in children with inflammatory bowel diseases. J Crohns Colitis. 2023;17:896–908. doi: 10.1093/ecco-jcc/jjad009. [DOI] [PubMed] [Google Scholar]
- 7.Dubinsky M.C., Mendiolaza M.L., Phan B.L., et al. Dashboard-driven accelerated infliximab induction dosing increases infliximab durability and reduces immunogenicity. Inflamm Bowel Dis. 2022;28:1375–1385. doi: 10.1093/ibd/izab285. [DOI] [PubMed] [Google Scholar]
- 8.Livingston E.H., Lee S. Body surface area prediction in normal-weight and obese patients. Am J Physiol Endocrinol Metab. 2001;281:E586–E591. doi: 10.1152/ajpendo.2001.281.3.E586. [DOI] [PubMed] [Google Scholar]
- 9.DeBruyn J.C., Jacobson K., El-Matary W., et al. Long-term outcomes of infliximab use for pediatric Crohn disease: a Canadian multicenter clinical practice experience. J Pediatr Gastroenterol Nutr. 2018;66:268–273. doi: 10.1097/MPG.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 10.Fasanmade A.A., Adedokun O.J., Blank M., et al. Pharmacokinetic properties of infliximab in children and adults with Crohn's disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33:946–964. doi: 10.1016/j.clinthera.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Frymoyer A., Piester T.L., Park K.T. Infliximab dosing strategies and predicted trough exposure in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2016;62:723–727. doi: 10.1097/MPG.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandse J.F., van den Brink G.R., Wildenberg M.E., et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149:350–355.e2. doi: 10.1053/j.gastro.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Fasanmade A.A., Adedokun O.J., Olson A., et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. doi: 10.5414/cpp48297. [DOI] [PubMed] [Google Scholar]
- 14.Adedokun O.J., Xu Z., Padgett L., et al. Pharmacokinetics of infliximab in children with moderate-to-severe ulcerative colitis: results from a randomized, multicenter, open-label, phase 3 study. Inflamm Bowel Dis. 2013;19:2753–2762. doi: 10.1097/01.MIB.0000435438.84365.f7. [DOI] [PubMed] [Google Scholar]