Introduction
Since its development and release in 2019, the Society for Cardiovascular Angiography and Interventions (SCAI) shock stage classification for adult patients has been widely cited and increasingly incorporated, owing to its simplicity across all clinical settings, easily understood and visualized framework, and notable endorsement by relevant societies and organizations that manage cardiogenic shock (CS).1 Ensuing validation studies over the course of the subsequent 2 years documented both its ease and rapidity of use as well as its ability to meaningfully discriminate patient risk across the spectrum of CS, including various phenotypes, presentations, and health care settings. Nonetheless, several areas of potential refinement have been identified to make the classification scheme more applicable across all settings and clinical time points, given that data from validation studies have provided useful information not previously available that could serve to significantly refine the classification. With this background, a clinical expert consensus writing group of all relevant stakeholders was reconvened to re-evaluate and refine the SCAI SHOCK stage classification based on the existing literature and clinician feedback from real-world experience.
Key summary points
-
1.
The SCAI SHOCK stage is an indication of shock severity and comprises one component of mortality risk prediction in patients with CS, along with etiology/phenotype and other risk modifiers; a 3-axis model of risk stratification in CS has been proposed to position the SCAI SHOCK stage in context.
-
2.
Validation studies have underscored the correlation of the SCAI SHOCK stage with mortality across all clinical subgroups, including CS with and without acute coronary syndrome (ACS), cardiac intensive care unit (CICU) patients, and those presenting with out-of-hospital cardiac arrest (OHCA).
-
3.
Progression across the SCAI SHOCK stage continuum is a dynamic process, incorporating new information as available, and patient trajectories are important both for communication among clinicians and for decision-making regarding the next level of care and therapeutics.
-
4.
A hub and spoke model for transfer of higher-risk patients including those with a deteriorating SCAI SHOCK stage has been proposed.
-
5.
Cardiac arrest (CA) as described herein relates to that accompanied by coma, defined as the inability to respond to verbal stimuli, most commonly associated with Glasgow Coma Scale <9, where there is concern for significant anoxic brain injury.
-
6.
The SCAI SHOCK pyramid and associated figure now reflect gradations of severity within each stage and pathways by which patients progress or recover.
-
7.
A streamlined table incorporating variables that are most typically seen, and the revised CA modifier definition, is also provided and incorporates lessons learned from validation studies and clinician experience.
-
8.
The lactate level and thresholds have been highlighted to detect hypoperfusion but may be dissociated from hemodynamics in cases such as chronic heart failure (HF). In addition, patients may demonstrate other manifestations of end-organ hypoperfusion with a normal lactate level, and there are also important causes of an elevated lactate level other than shock.
Development methodology
This statement has been developed as per SCAI Publications Committee policies for writing group composition, disclosure and management of relationships with industry, internal and external review, and organizational approval.2
The writing group has been organized to ensure diversity of perspectives and demographics, multistakeholder representation, and appropriate balance of relationships with industry. Relevant author disclosures are included in Supplemental Table S1. Before appointment, members of the writing group were asked to disclose financial and intellectual relationships from the 12 months before their nomination. A majority of the writing group disclosed no relevant, significant financial relationships. Financial and intellectual disclosure information was periodically reviewed by the writing group during document development and updated as needed. SCAI policy requires that writing group members with a current, relevant financial interest are recused from participating in related discussions or voting on recommendations. The work of the writing committee was supported exclusively by the SCAI, a nonprofit medical specialty society, without commercial support. Writing group members contributed to this effort on a volunteer basis and did not receive payment from the SCAI.
Narrative literature searches were performed by group members designated to lead each section, and initial findings were synthesized in section drafts authored primarily by the section leads in collaboration with other members of the writing group. Recommendations were iteratively discussed by the full writing group in a series of virtual consensus meetings until a majority of group members agreed on the text and qualifying remarks. In addition, all recommendations are supported by a short summary of the evidence or specific rationale.
The draft manuscript was peer reviewed in October 2021, and the document was revised to address pertinent comments. The writing group unanimously approved the final recommendations and updated classification. The SCAI Publications Committee and Executive Committee endorsed the document as official society guidance in December 2021.
SCAI statements are primarily intended to help clinicians make decisions about treatment alternatives. Clinicians also must consider the clinical presentation, setting, and preferences of individual patients to make judgments about the optimal approach.
Review of published SCAI SHOCK stage validation studies
Summary of published SCAI SHOCK validation studies
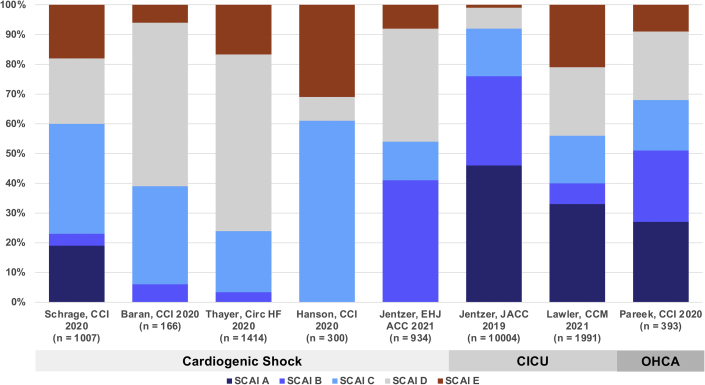
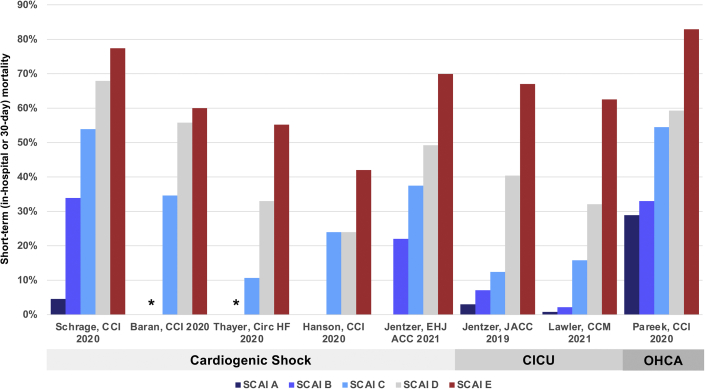
Since the publication of the SCAI SHOCK stage classification in 2019, several groups have produced observational validation studies ranging in size from 166 to 10004 patients that uniformly demonstrate an association between the SCAI SHOCK stage and mortality risk in a variety of populations (Table 1).1,3, 4, 5, 6, 7, 8, 9, 10, 11 Although several studies have focused on patients with CS,3, 4, 5, 6, 7 others have included a broader mix of CICU patients8, 9, 10 or those with OHCA.11 As expected, the prevalence of each SCAI SHOCK stage varied with the population studied and the definitions used in each study (Fig. 1). The observed short-term (in-hospital or 30-day) mortality also varied depending on the population, and higher SCAI SHOCK stages were consistently associated with higher short- and long-term mortality (Fig. 2).4,8,11 Furthermore, the SCAI SHOCK stages provided stepwise mortality risk stratification within the subgroups of ACS/acute myocardial infarction (AMI), HF, and those with and without CA.5,8,11 Most studies classified the SCAI SHOCK stage at a single time point, precluding an analysis of serial changes in stage over time. Importantly, real-time assignment of the SCAI SHOCK stage by the treating team was feasible and allowed for serial assessments.4 Stratification of mortality risk in the cited studies despite different criteria, populations, and therapies remained consistent, underscoring the strength of the classification scheme.
Table 1.
Characteristics of studies validating the association between the SCAI SHOCK stage and mortality.
| Study | Years included | Population | Design | Patients, n | Primary outcome |
|---|---|---|---|---|---|
| Schrage et al 2020a | 2009-2017 | CS or large MI | Retrospective single-center | 1007 | 30-day survival |
| Baran et al 2020 | 2019-2020 | CS | Prospective single-center | 166 | 30-day survival |
| Thayer et al 2020 | 2016-2019 | CS | Prospective multicenterb | 1414 | In-hospital mortality |
| Hanson et al 2020 | 2016-2019 | AMICS | Prospective multicenterb | 300 | Survival to discharge |
| Jentzer et al 2021a | 2007-2015 | CS | Retrospective single-center | 934 | 30-day survival |
| Jentzer et al 2019 | 2007-2015 | CICU | Retrospective single-center | 10,004 | In-hospital mortality |
| Lawler et al 2021 | 2017-2019 | CICU or CS | Retrospective multicenter | 1991 | In-hospital mortality |
| Jentzer et al 2020 | 2007-2015 | CICU survivors | Retrospective single-center | 9096 | Postdischarge survival |
| Pareek et al 2020 | 2012-2017 | OHCA | Retrospective single-center | 393 | 30-day mortality |
Duplicate data from the same cohort are not shown.
AMICS, CS from acute myocardial infarction; CICU, cardiac intensive care unit; CS, cardiogenic shock; MI, myocardial infarction; OHCA, out-of-hospital cardiac arrest; SCAI, Society for Cardiovascular Angiography and Interventions.
Patients with CS from the Schrage 2020 study were included in the Jentzer 2021 study, so only the nonduplicated patients are reported for the Jentzer 2021 study.
Patient enrollment in these studies was prospective, but the SCAI SHOCK stage was assigned retrospectively.
Fig. 1.
Distribution of SCAI SHOCK stages in each study. CICU, cardiac intensive care unit; OHCA, out-of-hospital cardiac arrest; SCAI, Society for Cardiovascular Angiography and Interventions.
Fig. 2.
Short-term mortality as a function of SCAI SHOCK stages in each study. ∗denotes that no deaths were observed in patients with SCAI stage B in these studies. CICU, cardiac intensive care unit; OHCA, out-of-hospital cardiac arrest; SCAI, Society for Cardiovascular Angiography and Interventions.
Variables used to define SCAI SHOCK stages in the validation studies
Each study used different criteria to define the SCAI SHOCK stages (Supplemental Tables S2-S7), including various combinations of clinical variables based on the availability of data.3, 4, 5, 6,8,10,11 Five groups3,5,8,10,11 developed study-specific SCAI SHOCK stage criteria, whereas two groups4,6 used physician assessment of the stage without study-specific criteria. Apart from the study by Baran et al which involved real-time prospective assignment of the stage by the treating team, each study assigned the stage retrospectively.
The definitions of the SCAI SHOCK stages used in individual studies range from simple to complex.5,8 For studies including patients with SCAI SHOCK stage B, this group was defined using vital sign abnormalities (Supplemental Table S4), and there was variability with respect to whether patients receiving vasopressors were classified as SCAI SHOCK stage B or C.3, 4, 5,7,8,10,11 Most studies used elevated lactate levels (≥2 mmol/L) to define hypoperfusion as stage C (Supplemental Table S5); impaired renal function was often used to define hypoperfusion, but few studies distinguished between acute and chronic renal dysfunction. Stage D shock was commonly defined as rising lactate and/or increasing vasopressor or mechanical circulatory support (MCS) requirements (Supplemental Table S6). Definitions of SCAI SHOCK stage E varied (Supplemental Table S7), with criteria including a high lactate level (≥5-10 mmol/L), a low pH (≤7.2), the need for multiple vasopressors/MCS devices, or the need for cardiopulmonary resuscitation (CPR). Despite the importance of physical examination and invasive hemodynamic assessment in defining CS clinically, these variables were not used in most studies because of retrospective data collection. Jentzer et al examined different definitions of shock and preshock in CICU patients and identified that hypoperfusion was associated with mortality to a greater extent than hypotension.12
To date, no published study has directly compared the performance of different SCAI SHOCK stage classification schemes in the same population for risk stratification. Importantly, the heterogeneity in mortality in each of the different stages across various studies likely reflects the dissimilar populations and different definitions used; more objective definitions and placing the SCAI SHOCK stage in the context of etiology, phenotype, and other nonmodifiable risk modifiers will help to optimize risk assessment in the future. However, the consistent stratification of risk (using different combinations of variables) suggests that refining and streamlining the criteria for the SCAI SHOCK stage as a categorization of shock severity will facilitate prospective assignment in clinical practice.
SCAI SHOCK validation studies in patients with CS with and without AMI
The National Cardiogenic Shock Initiative reported on 300 patients with CS from AMI (AMICS) and determined the SCAI SHOCK stage by retrospective chart review, assigning the worst shock stage on admission and at 24 hours. The authors found an incremental but strong association between the shock stage and mortality at both time points.6 Analyses from the Cardiogenic Shock Working Group included a broader group of patients with CS and defined the maximum shock stage during hospitalization, finding a stepwise increase in mortality with a higher shock stage in both patients with AMI and HF.5 Schrage et al reported on 1007 patients with mixed etiologies of CS and demonstrated mortality risk stratification across the shock stages (including at-risk patients with large AMI).3 Patients with CS from this cohort were combined with patients with CS from the Mayo Clinic cohort and reported similar findings.7 Baran et al reported the first prospective validation study in patients with CS by having the treating physician assign the SCAI SHOCK stage in real time based on available clinical data.4 The studies by Hanson et al and Baran et al demonstrated that a rising or persistently elevated SCAI SHOCK stage was associated with substantially worse outcomes.4,6
SCAI SHOCK validation studies in CICU and OHCA patients
Jentzer et al first validated the SCAI SHOCK stages using data from 10004 consecutive CICU patients at the Mayo Clinic, finding that each higher stage was associated with an incrementally higher risk of in-hospital mortality, even after adjustment for known predictors of mortality.8 Hospital survivors with a higher SCAI SHOCK stage on admission had increased postdischarge mortality.9 Patients with CA had a higher risk of dying at each SCAI SHOCK stage; both the location in which CA occurred (in-hospital versus out-of-hospital) and the rhythm of CA affected the risk of mortality.13 A subsequent multicenter study from the CCCTN database in 1991 CICU patients with ACS or HF also demonstrated that the SCAI SHOCK stage was associated with in-hospital mortality; a diagnosis of CS was required for patients in SCAI stages C, D, and E.10 In a distinct cohort of 393 OHCA patients, Pareek et al found that the observed short-term mortality was higher at each SCAI SHOCK stage than that in other studies, with clear mortality risk stratification as per shock stage.11
Studies examining risk modifiers within the SCAI SHOCK stage classification
The SCAI SHOCK stage classification has been leveraged to examine other aspects of mortality risk stratification across the spectrum of shock severity (Table 2). In the Mayo Clinic CICU cohort, age, the presence of systemic inflammatory response syndrome, acute kidney injury, and other noncardiac organ failure, severe acidosis, and echocardiographic findings were found to improve mortality risk stratification beyond SCAI SHOCK stages alone.14, 15, 16, 17, 18 The importance of age as a risk factor for mortality, independent of shock stage, was likewise reported in CS cohorts.4,7 Thayer et al and Garan et al showed the importance of pulmonary artery catheter use, congestion profile, and invasive hemodynamic data (particularly an elevated right atrial pressure) as risk modifiers independent of the shock stage in patients with CS.5,19 Worsening shock, either defined by rising shock stage over time or late deterioration, has been consistently associated with higher mortality.4,6,8,9 Jentzer et al demonstrated that an increasing number of abnormal markers of hypotension and hypoperfusion was associated with incrementally higher mortality risk in CICU patients; an elevated lactate level or an elevated shock index (the ratio of heart rate to systolic blood pressure) was more strongly associated with mortality.12 Biochemical phenotypes were identified in a large multicenter registry of patients with CS, highlighting the variability in observed mortality with the different phenotypes across the shock stages.20 Finally, SCAI SHOCK stages have been used to evaluate the association between certain treatments and outcomes in patients with CS.21
Table 2.
Studies examining potential risk modifiers on top of the SCAI SHOCK stages for mortality risk stratification.
| Study | Population | Design | Patients, n | Variable of interest | Conclusions |
|---|---|---|---|---|---|
| Jentzer et al 2019 | CICU | Retrospective single-center | 10,004 | CA | CA and late deterioration were associated with higher mortality |
| Baran et al 2020 | CS | Prospective single-center | 166 | Change in the SCAI stage | An increasing SCAI stage is associated with higher mortality |
| Garan et al 2020 | CS | Prospective multicenter | 1414 | Invasive hemodynamics | Higher mortality with higher RAP and HR or lower MAP, lower with PAC |
| Hanson et al 2020 | AMICS | Prospective multicenter | 300 | Change in the SCAI stage | An increasing SCAI stage is associated with higher mortality |
| Jentzer et al 2020 | CICU | Retrospective single-center | 9898 | CA type | Non-VF CA is associated with higher mortality |
| Jentzer et al 2020 | CICU | Retrospective single-center | 8995 | SIRS on admission | SIRS is associated with higher mortality |
| Padkins et al 2020 | CICU | Retrospective single-center | 10,004 | Age | Higher age is associated with higher mortality |
| Thayer et al 2020 | CS | Prospective multicenter | 1414 | Invasive hemodynamics | Higher RAP is associated with higher mortality |
| Jentzer et al 2021 | CS | Retrospective multicenter | 1749 | Age | Higher age is associated with lower survival |
| Jentzer et al 2021 | CICU | Retrospective single-center | 5453 | Echo hemodynamics | Low SVI and high E/e' are associated with higher mortality |
| Jentzer et al 2021 | CICU | Retrospective single-center | 9311 | AKI during hospitalization | Worse AKI is associated with higher mortality across SCAI stages |
| Jentzer et al 2021 | CICU | Retrospective single-center | 1065 | Severe acidosis | Severe acidosis associated with higher mortality across SCAI stages |
| Zweck et al 2021 | CS | Prospective multicenter | 1959 | Biochemical phenotype | “Cardiometabolic” phenotype associated with higher mortality |
Several of these study populations overlap with those presented in Table 1.
AKI, acute kidney injury; AMICS, CS from acute myocardial infarction; CA, cardiac arrest; CICU, cardiac intensive care unit; CS, cardiogenic shock; HR, heart rate; MAP, mean arterial pressure; PAC, pulmonary artery catheter; RAP, right atrial pressure; SCAI, Society for Cardiovascular Angiography and Interventions; SIRS, systemic inflammatory response syndrome; VF, ventricular fibrillation.
Collectively, these studies have demonstrated that higher-risk and lower-risk subgroups exist within each SCAI SHOCK stage, and a higher-risk subgroup within a lower SCAI SHOCK stage might have a mortality risk that exceeds a lower-risk subgroup within a higher SCAI SHOCK stage. Clearly, shock severity assessment is a central component of overall mortality risk stratification in patients with CS, yet other clinical variables modify the predicted mortality risk.22 Established CS-specific mortality risk prediction scores combine lactate and renal function (markers of hypoperfusion and shock severity) with patient-level variables to provide mortality risk stratification and should be considered distinct from shock severity classification algorithms.23,24 Recently, a newer CS-specific mortality risk prediction score based on biomarkers has been presented, suggesting that such biomarkers may be integrated into future risk assessment strategies.25
Lessons learned from the SCAI SHOCK validation studies
Shortcomings of original classification
The original SCAI SHOCK classification
In brief, SCAI SHOCK stage A is broad and represents the myriad of stable patients who have acute cardiac diagnoses that place them at risk for CS but fail to meet the criteria for preshock (stage B) or shock (stages C-E). Stage B represents patients who have intact systemic perfusion with evidence of hemodynamic instability, such as hypotension or compensatory tachycardia; patients with preserved perfusion despite significantly abnormal invasive hemodynamics (such as reduced cardiac output) are also classified as SCAI stage B. Stage C represents the more classic patients with CS who present with hypoperfusion either untreated or requiring hemodynamic support through pharmacologic or mechanical intervention. Stage D represents the failure of an adequate trial of an initial supportive intervention and, therefore, captures a different shock state than stage C, requiring some element of time. Stage E is reserved for refractory shock with actual or impending cardiovascular collapse despite high and escalating levels of support (including arrest-in-progress). In the opinion of the writing group, SCAI SHOCK stage E is usually a transient state that is easy to recognize in clinical practice (typically a peri-code situation or need to rapidly escalate hemodynamic support) but can be difficult to define retrospectively for the purpose of research.
Retrospective vs real-time classification
The original SCAI SHOCK stage classification was designed to be simple, recognizing that complete clinical information for grading shock severity is not always available. Physical examination, laboratory, and hemodynamic findings were provided to guide the clinician in assigning a specific SCAI SHOCK stage, in the order in which these variables are typically available, while allowing flexibility for application in different care environments. This approach works well for teaching the classification system and for applying it prospectively, but it presents challenges when applying it retrospectively to existing data sets with missing or inappropriately timed data. As a result, the assignment of the SCAI SHOCK stage in published studies has contributed to heterogeneity across studies, and future studies should ideally use consistent staging criteria.
Differentiating preshock (stage B) vs classic shock (stage C)
The distinction between SCAI SHOCK stage B (hemodynamic instability with preserved perfusion, ie, preshock) and SCAI SHOCK stage C (hypoperfusion with or without overt hemodynamic instability, ie, classic shock) is critical and requires the integration of multiple clinical and laboratory data points. Patients with hypoperfusion in the absence of hypotension are at higher risk of dying than patients with hypotension and preserved perfusion.12 Biomarkers such as lactate are commonly used to detect hypoperfusion but may be dissociated from hemodynamics in cases such as chronic HF where patients may have a normal lactate level with a depressed cardiac index. The authors suggest that a lactate level of >2 mmol/L is consistent with at least SCAI SHOCK stage C, although some patients may demonstrate other manifestations of end-organ hypoperfusion with a normal lactate level, and there are also causes of an elevated lactate level other than shock, such as mesenteric ischemia or compartment syndrome.
Shock classification based on required therapeutic interventions: differentiating stages C and D
As described by the National Cardiogenic Shock Initiative and Cardiogenic Shock Working Group, the intensity of therapies required to achieve hemodynamic stability and restore systemic perfusion can be used to define the SCAI SHOCK stage, but this approach will be most informative when the same escalation strategy is used by clinicians (such as with an institutional CS protocol) given practice variability in MCS patient selection and implantation.26,27 We suggest that if a patient requires vasoactive drugs or MCS to reverse hypoperfusion or hemodynamic compromise, they should be assigned SCAI SHOCK stage C. If this initial therapy is ineffective, evidenced by the need to add one or more additional vasoactive drugs or MCS devices, then SCAI SHOCK stage D is present. If perfusion cannot be restored using multiple vasoactive drugs and/or MCS devices, or if extremely high vasoactive drug doses are required, then SCAI SHOCK stage E is present. An important limitation of this approach is the variability in vasoactive drug dosing which affects the prognosis. For example, a patient who has stabilized on low doses of two vasoactive drugs may be classified as stage C, whereas a patient who is failing a high dose of a single vasoactive drug might be classified as stage D. Differentiating the CS stage and prognosis based on dose escalation as opposed to additional pharmacotherapy or mechanical support will require further data.
Cardiac arrest modifier clarification
Another area of controversy with the original classification is the “A” modifier representing an episode of CA. Clearly CA events are heterogeneous, and single defibrillation for a brief ventricular arrhythmia without CPR and normal neurologic function does not change the prognosis of a patient with CS.7 Instead, the two aspects of greatest relevance are the neurologic status (awake or comatose) and physiologic impact of the arrest, as prolonged CA may fundamentally change the patient trajectory if ischemia-reperfusion heralds multiorgan failure. At this time, there is no clearly defined CPR duration that would qualify a patient for the “A” modifier, and we believe that the “A” modifier should refer to patients with potential anoxic brain injury. This may be evidenced by a decreased Glasgow Coma Scale, where a value less than 9 typically defines coma; alternatively, the absence of a motor response to voice (ie, not following commands) is a useful definition.
Whether to include age as a modifier
One of the strongest findings from multiple studies is the effect of increasing age on mortality. Age is a well-known continuous risk factor for adverse outcomes in patients with CS and was highlighted in the seminal shock trial, the IABP-SHOCK II trial, and subsequent risk scores.23,24 The ability to overcome the stress of CS declines with advancing age, irrespective of other comorbidities. Older patients are more likely to die, and this added risk is likely to guide clinicians in determining candidacy for specific therapies for CS at each SCAI SHOCK stage.7,16 A significant challenge with using age as a modifier is that as a continuous variable there is no clearly defined binary threshold of risk. Overall, age functions more as a marker of comorbidities, frailty, and candidacy for (or futility of) therapeutic interventions. Therefore, although age is clearly a major risk factor for adverse outcomes that modifies risk across the SCAI SHOCK stages and should be taken into account by clinicians, too much uncertainty exists regarding how best to apply this prognostic information to incorporate age directly into the SCAI SHOCK classification.
Reasons to maintain a similar classification framework
Although there are reasons to modify the SCAI SHOCK classification as detailed previously, there are also reasons to avoid unnecessarily complex revisions that would make the classification more difficult to use and its distribution globally less rapid. Most importantly, it is simple for multidisciplinary teams to use the existing SCAI SHOCK schema across the spectrum of care in a prospective fashion. Indeed, real-world patient care reflects the fact that not all patients will have the comprehensive information that may be included in a more complex risk score or on a clinical research flow sheet. Moreover, the work of Baran et al shows that real-time prospective assignment of the SCAI SHOCK stage by a team achieves the same predictive value including mortality observed in several retrospective validation studies using complex criteria.4 Maintaining simplicity and flexibility will therefore allow the SCAI SHOCK stage classification to be used by clinicians with expertise in critical care medicine and emergency medicine, including prehospital clinicians, without losing significant prognostic impact. A system analogous to that used for STEMI may allow patients with shock to be selectively sent to “shock centers”, facilitated by a simplified prehospital SCAI SHOCK classification that relies on physical examination alone.28,29
Key aspects to emphasize in an updated classification
It is crucial to emphasize the distinction between the grading of shock severity and the prediction of mortality risk. Although shock severity is among the most potent predictors of mortality in patients with CS, numerous other risk modifiers can influence this risk, resulting in lower-risk and higher-risk patients at each SCAI SHOCK stage, as highlighted previously. In addition, the transition between stages is of significant prognostic value. Although the SCAI SHOCK stage can provide mortality risk stratification (particularly when risk modifiers are integrated), its greatest value is in standardization of shock severity assessment to enhance clinical communication and decision-making. In addition, re-evaluation of the clinical stage can guide further treatment options regarding escalation or de-escalation strategies and assist in prognosis. The SCAI SHOCK stage should be reassessed at intervals, the timing of which will differ based on the initial severity and response to therapy. The improvement of the SCAI SHOCK stage by even one category is a powerful favorable prognostic indicator, and conversely, a maintaining or declining SCAI SHOCK is a potent negative marker.6 Similarly, CA that results in neurologic injury or impacts peripheral organ function is an important concern that impacts mortality and the potential for recovery. Finally, the consideration of age along with the SCAI SHOCK stage is of value to the clinician while planning the next intervention, including the recognition of futility before care is rendered.28
To improve care, it will be important to recognize that most sites are not equipped with all modalities for the care of CS, and therefore, some patients will need to be transferred to a primary shock center or “hub” that has the ability and technology to care for all patients.28,29 However, given capacity constraints, it is important to have a classification system that allows identification of the sickest patients, but also ones where the possibility of survival is greatest; this is where an understanding of the distinction between shock severity and mortality risk is essential, and risk modifiers must be taken into account. Transfers for futile care in unrecoverable high-risk patients do not change outcomes and deny capacity to those who might otherwise benefit. One proposal would be for sites to classify their capabilities and organize into spokes and hubs. The spoke centers with MCS capabilities would manage SCAI SHOCK stage C (most patients) but are triggered to consider referral when progression to SCAI SHOCK stage D occurs (before development of SCAI SHOCK stage E). However, patient candidacy for advanced supportive therapies should always be a central consideration in these decisions, adding a layer of complexity.
Patients with CS represent a heterogeneous population including distinct phenotypes, which are challenging to define and may be independent from shock severity per se.20 Clinicians must recognize that patients at each SCAI SHOCK stage may appear or behave differently and may present with a spectrum of overall illness severity and mortality risk. Clinical decision-making for patients with CS must integrate not only shock severity but also the etiology of shock (particularly ischemic versus nonischemic and acute versus acute-on-chronic), the presence and reversibility of organ failure, degree of congestion, mixed or vasodilatory shock states, ventricular involvement (LV, RV, or biventricular dysfunction), and a multitude of factors influencing candidacy for supportive therapies such as age, CA, and important comorbidities. Therefore, using the SCAI SHOCK stages to provide a uniform assessment of shock severity is merely one important component of prognostication and management for these patients, and we believe that a consistent classification system that can be tailored to each care environment is more useful than a comprehensive one.
Updated SCAI SHOCK classification pyramid and table
Framework and criteria for shock stages
The framework emphasizing the domains of physical examination, biochemical, and hemodynamic criteria has been maintained, representing the availability of better data over time that can and should be integrated into assigning the SCAI SHOCK stage. Suggested criteria in each domain to define the SCAI SHOCK stages (Table 3) have been modified to be more succinct and data-driven, with the goal of optimizing sensitivity and specificity to enable increased incorporation into clinical practice. This remains a work in progress that is designed to be flexible and will continue to be refined as more and better data become available. Lactate thresholds have been modified to reflect the available data; although not all studies measured lactate levels routinely, this is important both therapeutically and prognostically and should be adopted as a standard practice going forward.
Table 3.
Descriptors of shock stages: Physical examination, biochemical markers, and hemodynamics.
| Stage | Description | Physical examination/bedside findings |
Biochemical markers |
Hemodynamics |
|||
|---|---|---|---|---|---|---|---|
| Typically includes | May include | Typically includes | May include | Typically includes | May include | ||
|
A At risk |
A patient who is not currently experiencing signs or symptoms of CS, but is at risk for its development. These patients may include those with large acute myocardial infarction or prior infarction and/or acute or acute-on-chronic heart failure symptoms. |
Normal JVP Warm and well-perfused
|
Clear lung sounds | Normal lactate | Normal labs
|
Normotensive (SBP ≥100 mmHg or at baseline) | If invasive hemodynamics are assessed:
|
|
B Beginning CS |
A patient who has clinical evidence of hemodynamic instability (including relative hypotension or tachycardia) without hypoperfusion. |
Elevated JVP Warm and well-perfused
|
Rales in lung fields | Normal lactate | Minimal acute renal function impairment Elevated BNP |
Hypotension
|
|
|
C Classic CS |
A patient who manifests with hypoperfusion and who requires one intervention (pharmacological or mechanical) beyond volume resuscitation. These patients typically present with relative hypotension (but hypotension is not required). |
Volume overload | Looks unwell Acute alteration in mental status Feeling of impending doom Cold and clammy Extensive rales Ashen, mottled, dusky, or cool extremities Delayed capillary refill Urine Output <30 mL/h |
Lactate ≥2 mmol/L | Creatinine increase to 1.5 x baseline (or 0.3 mg/dL) or > 50% drop in GFR Increased LFTs Elevated BNP |
If invasive hemodynamics assessed (strongly recommended)
|
|
|
D Deteriorating |
A patient who is similar to category C but is getting worse. Failure of initial support strategy to restore perfusion as evidenced by worsening hemodynamics or rising lactate. | Any of stage C and worsening (or not improving) signs/symptoms of hypoperfusion despite the initial therapy. | Any of stage C and lactate rising and persistently >2 mmol/L | Deteriorating renal function Worsening LFTs Rising BNP |
Any of stage C and requiring escalating doses or increasing numbers of pressors or addition of a mechanical circulatory support device to maintain perfusion | ||
|
E Extremis |
Actual or impending circulatory collapse | Typically unconscious | Near pulselessness Cardiac collapse Multiple defibrillations |
Lactate ≥8 mmol/La | CPR (A-modifier) Severe acidosis
|
Profound hypotension despite maximal hemodynamic support | Need for bolus doses of vasopressors |
BNP, B-type natriuretic peptide; CPR, cardiopulmonary resuscitation; CVP, central venous pressure; GFR, glomerular filtration rate; JVP, jugular venous pressure; LFT, liver function tests; MAP, mean arterial pressure; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; SVP, systolic ventricular pressure.
Stage E prospectively is a patient with cardiovascular collapse or ongoing CPR.
Table 3 has been modified to characterize diagnostic features as those that are typically included and those that may be included when defining the SCAI SHOCK stage. This was carried out to account for variability in patient presentations and in recognition that the data available to the clinician vary between different care settings, both within a given institution and among different institutions. For example, invasive hemodynamic data are obtainable in the catheterization laboratory and potentially in the critical care unit but are not generally available in the emergency department or the rural community hospital. As a patient moves through the health care system from the first medical contact to the more advanced hospital settings, the quantity and quality of data that become available will increase and the SCAI SHOCK stage assignment will be more accurate.
Clarification of SCAI SHOCK stage D, which is defined as failure to stabilize with initial therapy, may be helpful. In general, the need for more than one vasoactive agent or more than one support device, due to failure of appropriate initial therapy to maintain perfusion, defines a patient in stage D. In addition, either escalating doses of medications or need for higher mechanical support settings over time may represent stage D. As such, patients who need more than one vasoactive agent shortly after presentation can be in stage C, but an element of time must pass after initiating therapy to define stage D. Stage D may be the most challenging to define for clinical practice and research because of uncertainty about what constitutes an adequate therapeutic trial in terms of vasoactive drug dosing, device selection, and time.
The CA modifier has been refined to include only those individuals after CA who fail to respond to verbal commands and/or who have a Glasgow Coma Scale of <9 and no longer includes brief CA with normalization of neurologic status.
SCAI SHOCK stage in the context of acuity of presentation, etiology, phenotype, and other risk modifiers—the 3-axis model
Acute versus acute-on-chronic presentation and shock etiology
The SCAI SHOCK classification was designed for patients presenting acutely, but acute and acute-on-chronic processes can differ in important ways. Patients with decompensation in the context of chronic HF may present with different symptomatology and may also have different hemodynamic profiles in that they may have developed adaptations to allow them to tolerate lower cardiac output and blood pressure.30 Indeed, because of compensatory mechanisms and adaptations, patients with chronic HF may display a lower SCAI SHOCK stage than those without such adaptive mechanisms or may provide a falsely reassuring clinical picture despite high-risk hemodynamics.26 Accordingly, it is critical to interpret physical findings and hemodynamics in this clinical context. That said, these differences are most evident in patients in SCAI SHOCK stages A and B and converge in later stages. SCAI SHOCK stages C, D, and E tend to appear similar regardless of underlying chronicity. Another crucial distinction relates to the etiology of shock which may influence the clinical presentation and outcomes, such as patients with AMICS versus decompensated HF progressing to CS. Although the SCAI SHOCK classification applies equally to both groups of patients, their clinical and hemodynamic findings, prognosis, and optimal treatment strategies may differ markedly.
Shock phenotype
Although hemodynamic measurements are commonly used to make the diagnosis of CS, formal definitions of hemodynamic shock phenotypes may help guide therapy and improve outcomes. The hemodynamic parameters shown in Table 3 generally define the diagnosis of shock with low cardiac output or cardiac power output, high filling pressures, and increased oxygen extraction (ie, reduced venous oxygen saturation) indicative of systemic perfusion failure overall despite adequate volume. Other hemodynamic parameters, such as the ratio of right atrial to pulmonary capillary wedge pressure (right atrial pressure/pulmonary capillary wedge pressure),28 and pulmonary artery pulsatility index31,32 among others are now advised to identify patients with RV failure who may potentially require dedicated RV or biventricular support.28,29 These hemodynamic and echocardiographic measurements reflecting ventricular function may facilitate risk stratification within the SCAI SHOCK stage classification. Recent application of machine learning algorithms supports that distinct phenotypes of CS can be identified and further stratifies mortality risk within each SCAI stage.20 It is essential to differentiate between diagnostic variables that enable assignment of the SCAI SHOCK stage from prognostic variables that help predict mortality risk or assign the structural problem leading to shock, recognizing that many variables serve both purposes.
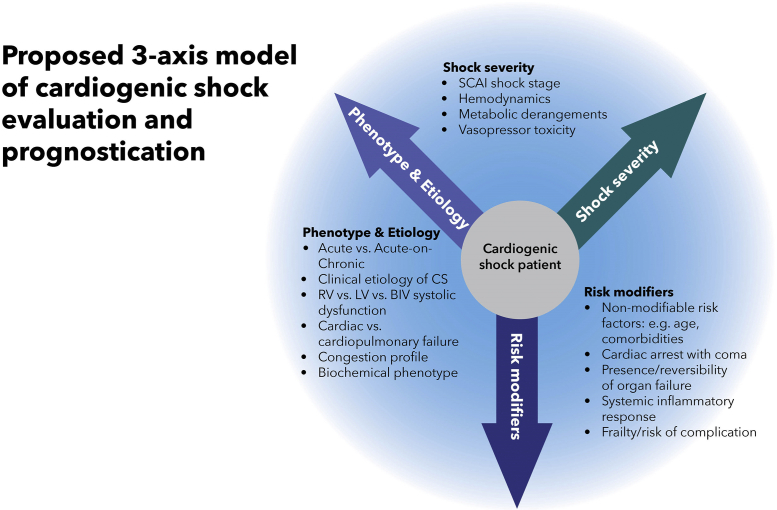
The 3-axis model of predictors of mortality
The outcome of shock is based on a number of factors, including the severity of the shock, risk modifiers such as age, comorbidities, and prior CA with evidence of anoxic encephalopathy, and certain features of the hemodynamic phenotype and clinical presentation. We propose a 3-axis model of CS evaluation and prognostication that integrates shock severity, clinical phenotype, and risk modifiers as distinct constructs that must be considered during clinical decision-making (Fig. 3). Established and emerging biomarkers may further refine risk stratification, and future research will be needed to define how to integrate these into shock severity assessment. Many of these factors are captured in the SCAI SHOCK classification, but others are not, underscoring the importance of evaluating individual parameters in the context of the entire clinical picture. It is essential to differentiate a patient who is “high risk” due to severe shock with poor hemodynamics from a patient who is “high risk” due to nonmodifiable risk factors for mortality.
Fig. 3.
The proposed 3-axis conceptual model of cardiogenic shock evaluation and prognostication.
Revised SCAI SHOCK pyramid
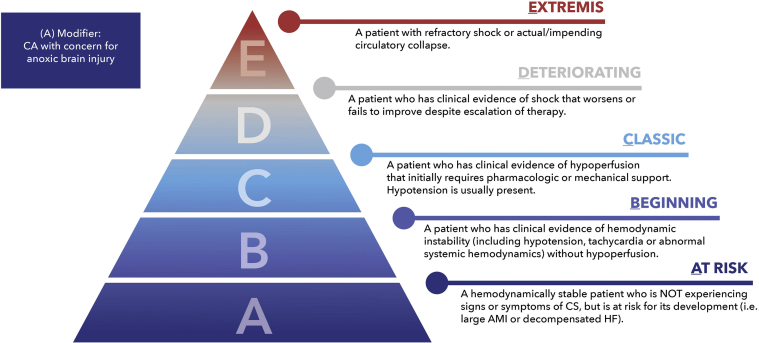
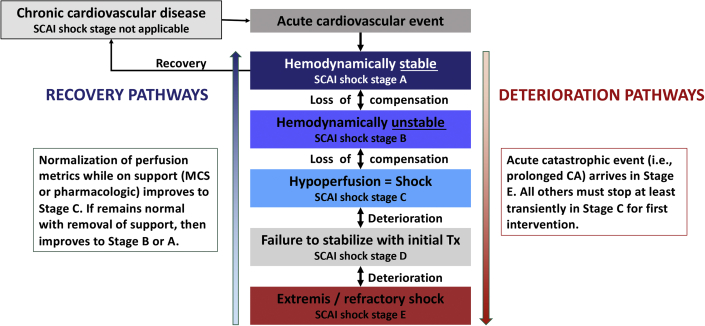
A revision of the SCAI SHOCK pyramid is shown in Figure 4. The underlying structure is the same to prioritize simplicity and widespread applicability. Each of the stages now has gradients of color to denote gradations of shock severity and risk within each stage, as a reminder of the need to individualize patient care based on phenotype, risk modifiers, and comorbidities. We explicitly did not add subcategories within each SCAI SHOCK stage to preserve the simplicity of the classification. The updated CA modifier is incorporated into the pyramid.
Fig. 4.
Updated SCAI SHOCK classification pyramid. AMI, acute myocardial infarction; CS, cardiogenic shock; HF, heart failure; SCAI, Society for Cardiovascular Angiography and Interventions.
Classification and patient trajectories
Figure 5 shows the initial and reclassification process in response to patient response and trajectory. It should be noted that the SCAI SHOCK classification only applies to acute presentations and is not used to stage chronic cardiovascular disease. Patients may respond to therapy, stabilize, and recover, in which case they would move to a progressively lower SCAI SHOCK stage. Alternatively, they may fail to respond to therapy, deteriorate, or experience an acute catastrophic event such as CA or myocardial rupture, in which case they would move to a higher stage. In addition, response failure includes not only patients who are getting worse but also those who are failing to improve with appropriate therapy. Note that decompensation into SCAI SHOCK stage D requires spending some time in SCAI SHOCK stage C because an intervention and an element of time are required, whereas a catastrophic event or decompensation may result in SCAI SHOCK stage E from any of the lower stages.
Fig. 5.
Cardiogenic shock is a dynamic process. CA, cardiac arrest; MCS, mechanical circulatory support; SCAI, Society for Cardiovascular Angiography and Interventions.
Summary of the new classification
The revision of the SCAI SHOCK stage classification moves toward eliminating variables that are either redundant or that have been shown not to add additional prognostic value in the interest of making the classification simpler to use and more data-driven. This process is ongoing and will be refined as high-quality data accumulate. Some of the elements are defined with greater precision, including lactate levels and also the CA modifier, which now excludes very brief episodes with rapid response to defibrillation and comprises only those patients who have impaired mental status with unknown neurologic recovery status after CPR.28,29,33
The classification tends to err on the side of being practical and simple over being comprehensive and is most applicable to acute presentations with CS. However, we have now outlined a 3-axis model for evaluation and prognostication that takes into account shock severity, risk modifiers, etiology, and phenotypes that should be applied to individualize patient management. The revised pyramid has gradations of color to represent gradations of risk within each stage.
Finally, additional emphasis has been placed on patient trajectories, to help recognize patients who are responding to therapy but more importantly to identify those who are failing to respond or deteriorating and who should be considered for more intensive therapy (or interhospital transfer) or conversely considered for palliation based on patient and family wishes or futility.
Our hope is that the revised criteria will allow for more uniform classification to help clinicians choose patients for advanced therapies, but also define criteria for entry into clinical trials to better understand the value of potential therapies. A crucial next step in this field will be to compare the outcomes associated with drug and device therapies, systems of care, and treatment protocols for patients at different stages or trajectories, phenotypes, and modifiers of shock.
Future considerations and research
The clinical uptake and scientific confirmation of the SCAI SHOCK stage classification framework, as outlined in section 1 of this document, have been rapid; however, ongoing validation, refinement, knowledge translation, and implementation are required. The staging system has yet to be evaluated in all clinical environments throughout the CS spectrum of care including in the prehospital setting, in the emergency department, or among patients treated with durable MCS or postcardiotomy shock. Moreover, the SCAI SHOCK model was designed to be applied dynamically throughout all phases of care, and more work is required to understand the optimal reassessment intervals and the association between mortality risk and temporal changes in SCAI SHOCK stages from presentation through deterioration and recovery, destination therapy, or palliation.1
A major limitation of the current system is that multiple elements within the staging remain subject to variable interpretation including differential threshold for MCS deployment between institutions, necessitating unified definitions of each SCAI SHOCK stage that are less dependent on local practice patterns. The CA modifier continues to include a heterogeneous population with variable risk of neurologic injury; thus, improved collection of intra-arrest information such as arrest duration, rhythm, and treatment could facilitate hypoxic-ischemic neurologic injury discrimination and could refine or improve this “A” modifier.34 Similarly, the development of uniform definitions of hypoperfusion, hypotension, and LV, RV, or biventricular failure has the potential to improve interuser reliability of shock staging. It remains unclear how best to utilize invasive hemodynamic parameters, laboratory measures of hypoperfusion, biomarkers, or a combination thereof to discriminate the risk of morbidity and mortality. The framework for defining the SCAI SHOCK stages described in this document may be inadequate to directly use without modification for clinical trial enrollment, and precise individualized research definitions of the SCAI SHOCK stages will be required if stratification by stage is desired based on the target population.
Strategies to improve clinical dissemination of this model and uptake among frontline health care workers potentially include incorporation into international societal clinical practice guidelines, embedding the score within institutional electronic health records, and increasing education though traditional scientific (eg, congress presentation, journal clubs) and emerging educational streams (eg, social media awareness, podcasts, and SCAI SHOCK stage calculators). We believe that CS registries and clinical trials could be improved by including the SCAI SHOCK stage classification system as a risk marker of acuity, as a study inclusion/exclusion criterion, and/or by stratifying therapeutic interventions across SCAI SHOCK stages. This could potentially allow for a better understanding of the baseline risk of each population, facilitate interstudy comparisons of CS populations which traditionally pooled this group of patients, and allow for the evaluation of the efficacy and safety of treatments across the severity spectrum. We acknowledge, however, that these prospective strategies require uniform definitions of all variables to allow for accurate SCAI SHOCK staging and good interuser reliability.
Summary and conclusion
In summary, since 2019, the SCAI SHOCK stage classification has been widely adopted and subsequently validated by multiple groups across the spectrum of CS. The SCAI SHOCK consensus workgroup reviewed the validation studies in detail to identify potential areas of refinement for the classification scheme. In particular, we clarified the precise role of the SCAI SHOCK classification within a more comprehensive 3-axis model incorporating predictors of mortality, provided more granularity to the CA modifier and the constituent domains of the classification, including physical examination, biochemical, and hemodynamic criteria, and allowed for gradations of risk within each SCAI SHOCK stage. More emphasis is placed on the trajectory of the patient with CS through hospitalization, including as patients are transferred to higher levels of care (hubs and spokes), as well as potential future directions. It is our desire and belief that the revised SCAI SHOCK stage classification will enhance both clinical care and CS research trial design.
Acknowledgments
Declaration of competing interest
Disclosure information for all authors is available in Supplemental Table S1.
Peer review statement
Given her role as Associate Editor, Cindy Grines had no involvement in the peer review of this article and has no access to information regarding its peer review.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at https://doi.org/10.1016/j.jscai.2021.100008.
Supplementary material
References
- 1.Baran D.A., Grines C.L., Bailey S., et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94(1):29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 2.Szerlip M., Feldman D.N., Aronow H.D., et al. SCAI publications committee manual of standard operating procedures. Catheter Cardiovasc Interv. 2020;96(1):145–155. doi: 10.1002/ccd.28754. [DOI] [PubMed] [Google Scholar]
- 3.Schrage B., Dabboura S., Yan I., et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96(3):E213–E219. doi: 10.1002/ccd.28707. [DOI] [PubMed] [Google Scholar]
- 4.Baran D.A., Long A., Badiye A.P., Stelling K. Prospective validation of the SCAI shock classification: single center analysis. Catheter Cardiovasc Interv. 2020;96(7):1339–1347. doi: 10.1002/ccd.29319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thayer K.L., Zweck E., Ayouty M., et al. Invasive hemodynamic assessment and classification of in-hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13(9):e007099. doi: 10.1161/CIRCHEARTFAILURE.120.007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanson I.D., Tagami T., Mando R., et al. SCAI shock classification in acute myocardial infarction: insights from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2020;96(6):1137–1142. doi: 10.1002/ccd.29139. [DOI] [PubMed] [Google Scholar]
- 7.Jentzer J.C., Schrage B., Holmes D.R., et al. Influence of age and shock severity on short-term survival in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2021;10(6):604–612. doi: 10.1093/ehjacc/zuaa035. [DOI] [PubMed] [Google Scholar]
- 8.Jentzer J.C., van Diepen S., Barsness G.W., et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74(17):2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 9.Jentzer J.C., Baran D.A., van Diepen S., et al. Admission Society for Cardiovascular Angiography and Intervention shock stage stratifies post-discharge mortality risk in cardiac intensive care unit patients. Am Heart J. 2020;219:37–46. doi: 10.1016/j.ahj.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Lawler P.R., Berg D.D., Park J.-G., et al. The range of cardiogenic shock survival by clinical stage: data from the Critical Care Cardiology Trials Network registry. Crit Care Med. 2021;49(8):1293–1302. doi: 10.1097/CCM.0000000000004948. [DOI] [PubMed] [Google Scholar]
- 11.Pareek N., Dworakowski R., Webb I., et al. SCAI cardiogenic shock classification after out of hospital cardiac arrest and association with outcome. Catheter Cardiovasc Interv. 2021;97(3):E288–E297. doi: 10.1002/ccd.28984. [DOI] [PubMed] [Google Scholar]
- 12.Jentzer J.C., Burstein B., Van Diepen S., et al. Defining shock and preshock for mortality risk stratification in cardiac intensive care unit patients. Circ Heart Fail. 2021;14(1):e007678. doi: 10.1161/CIRCHEARTFAILURE.120.007678. [DOI] [PubMed] [Google Scholar]
- 13.Jentzer J.C., Henry T.D., Barsness G.W., Menon V., Baran D.A., Diepen S.V. Influence of cardiac arrest and SCAI shock stage on cardiac intensive care unit mortality. Catheter Cardiovasc Interv. 2020;96(7):1350–1359. doi: 10.1002/ccd.28854. [DOI] [PubMed] [Google Scholar]
- 14.Jentzer J.C., Lawler P.R., van Diepen S., et al. Systemic inflammatory response syndrome is associated with increased mortality across the spectrum of shock severity in cardiac intensive care patients. Circ Cardiovasc Qual Outcomes. 2020;13(12):e006956. doi: 10.1161/CIRCOUTCOMES.120.006956. [DOI] [PubMed] [Google Scholar]
- 15.Jentzer J.C., Wiley B.M., Anavekar N.S., et al. Noninvasive hemodynamic assessment of shock severity and mortality risk prediction in the cardiac intensive care unit. JACC Cardiovasc Imaging. 2021;14(2):321–332. doi: 10.1016/j.jcmg.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Padkins M., Breen T., Anavekar N., et al. Age and shock severity predict mortality in cardiac intensive care unit patients with and without heart failure. ESC Heart Fail. 2020;7(6):3971–3982. doi: 10.1002/ehf2.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padkins M., Breen T., Van Diepen S., Barsness G., Kashani K., Jentzer J.C. Incidence and outcomes of acute kidney injury stratified by cardiogenic shock severity. Catheter Cardiovasc Interv. 2021;98(2):330–340. doi: 10.1002/ccd.29692. [DOI] [PubMed] [Google Scholar]
- 18.Jentzer J.C., Kashani K.B., Wiley B.M., et al. Laboratory markers of acidosis and mortality in cardiogenic shock: developing a definition of hemometabolic shock. Shock. 2022;57(1):31–40. doi: 10.1097/SHK.0000000000001812. [DOI] [PubMed] [Google Scholar]
- 19.Garan A.R., Kanwar M., Thayer K.L., et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail. 2020;8(11):903–913. doi: 10.1016/j.jchf.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Zweck E., Thayer K.L., Helgestad O.K.L., et al. Phenotyping cardiogenic shock. J Am Heart Assoc. 2021;10(14):e020085. doi: 10.1161/JAHA.120.020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jentzer J.C., van Diepen S., Henry T.D., Baran D.A., Barsness G.W., Holmes D.R. Influence of intra-aortic balloon pump on mortality as a function of cardiogenic shock severity. Catheter Cardiovasc Interv. 2021 doi: 10.1002/ccd.29800. [DOI] [PubMed] [Google Scholar]
- 22.Jentzer J.C. Understanding cardiogenic shock severity and mortality risk assessment. Circ Heart Fail. 2020;13(9):e007568. doi: 10.1161/CIRCHEARTFAILURE.120.007568. [DOI] [PubMed] [Google Scholar]
- 23.Harjola V.-P., Lassus J., Sionis A., et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 24.Pöss J., Köster J., Fuernau G., et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(15):1913–1920. doi: 10.1016/j.jacc.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Ceglarek U., Schellong P., Rosolowski M., et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur Heart J. 2021;42(24):2344–2352. doi: 10.1093/eurheartj/ehab110. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Montfort J., Sinha S.S., Thayer K.L., et al. Clinical outcomes associated with acute mechanical circulatory support utilization in heart failure related cardiogenic shock. Circ Heart Fail. 2021;14(5):e007924. doi: 10.1161/CIRCHEARTFAILURE.120.007924. [DOI] [PubMed] [Google Scholar]
- 27.Hochman J.S., Sleeper L.A., Webb J.G., et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 28.van Diepen S., Katz J.N., Albert N.M., et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 29.Henry T.D., Tomey M.I., Tamis-Holland J.E., et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143(15):e815–e829. doi: 10.1161/CIR.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 30.Hollenberg S.M., Warner Stevenson L., Ahmad T., et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2019;74(15):1966–2011. doi: 10.1016/j.jacc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Korabathina R., Heffernan K.S., Paruchuri V., et al. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80(4):593–600. doi: 10.1002/ccd.23309. [DOI] [PubMed] [Google Scholar]
- 32.Morine K.J., Kiernan M.S., Pham D.T., Paruchuri V., Denofrio D., Kapur N.K. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail. 2016;22(2):110–116. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen N., Wetterslev J., Cronberg T., et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 34.Jentzer J.C., van Diepen S., Henry T.D. Understanding how cardiac arrest complicates the analysis of clinical trials of cardiogenic shock. Circ Cardiovasc Qual Outcomes. 2020;13(9):e006692. doi: 10.1161/CIRCOUTCOMES.120.006692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.