Abstract
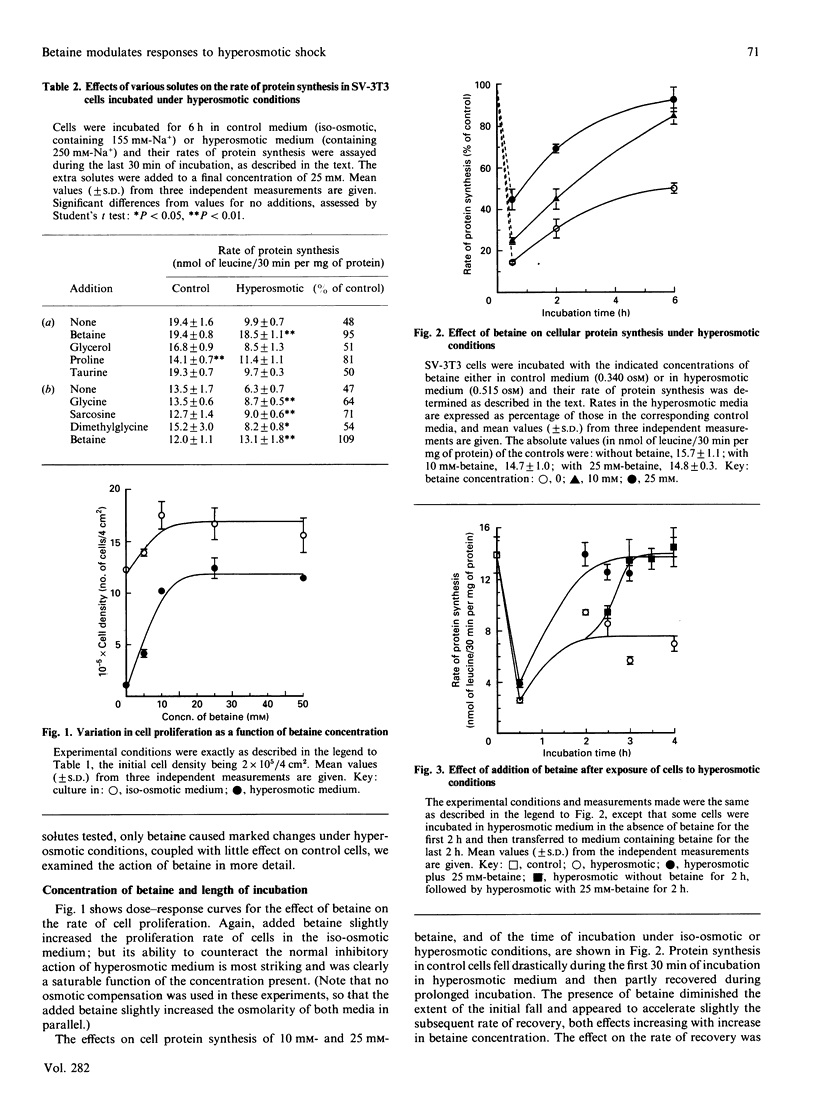
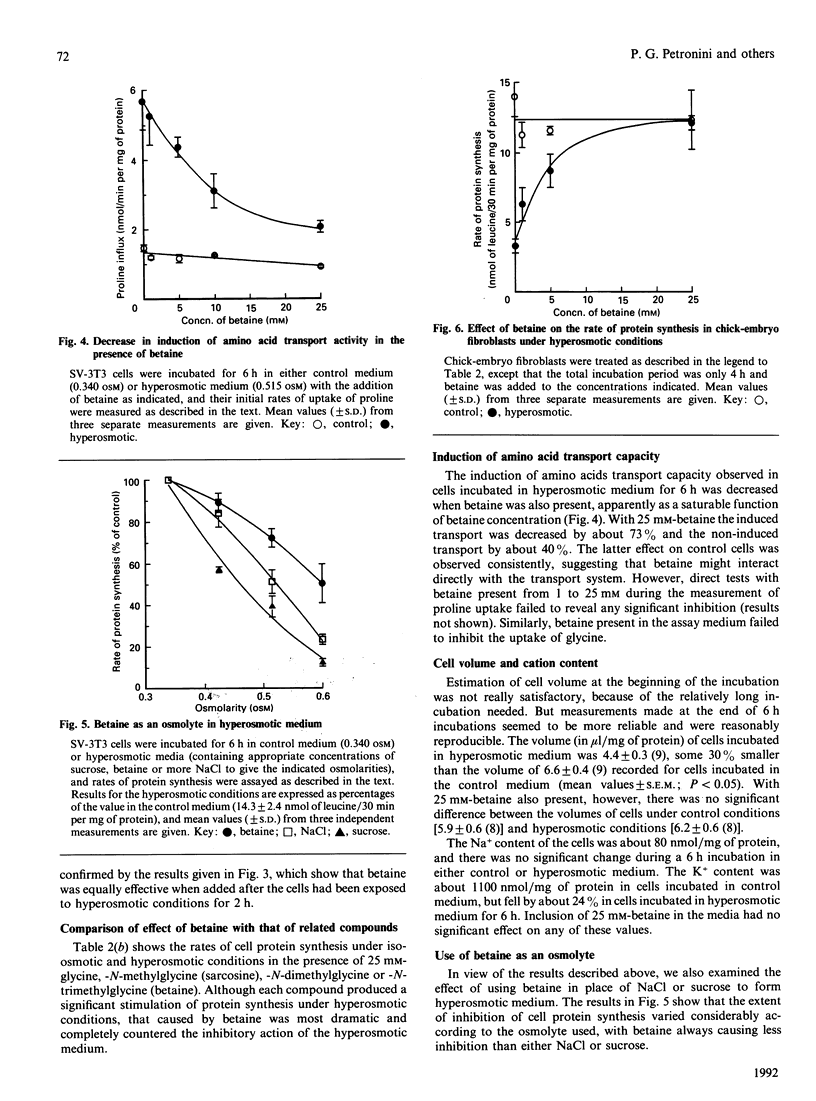
Various solutes were tested to see if they could modify the responses of SV-3T3 cells to hyperosmotic (0.5 osM) conditions, which cause an inhibition of general cell protein synthesis and of the rate of cell proliferation, coupled with an induction of amino acid transport activity. The added solutes were glycerol, proline, taurine, betaine, dimethylglycine and sarcosine. Of these, betaine produced the most dramatic and consistent effects. Addition of 10-25 mM-betaine to the hyperosmotic medium largely prevented the 90% inhibition of cell proliferation that occurred in its absence. Whether it was added initially or after the cells were exposed to hyperosmotic medium, 25 mM-betaine also converted a 50% recovery of the rate of protein synthesis into 100%. Similarly, the same concentrations of betaine prevented a 30% decrease in cell volume and decreased the induction of amino acid transport via system A by 73%. Lower concentrations of betaine produced smaller but still significant changes in these functional responses. With chick-embryo fibroblasts, under identical hyperosmotic conditions, 25 mM-betaine completely counteracted a 75% inhibition of the rate of protein synthesis. At present it is not clear how betaine modulates these effects of hyperosmolarity on cell functions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borghetti A. F., Piedimonte G., Tramacere M., Severini A., Ghiringhelli P., Guidotti G. G. Cell density and amino acid transport in 3T3, SV3T3, and SV3T3 revertant cells. J Cell Physiol. 1980 Oct;105(1):39–49. doi: 10.1002/jcp.1041050107. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Erlinger S. U., Saier M. H., Jr Decrease in protein content and cell volume of cultured dog kidney epithelial cells during growth. In Vitro. 1982 Mar;18(3 Pt 1):196–202. doi: 10.1007/BF02618571. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Burg M. B. Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membr Biol. 1991 Jan;119(1):1–13. doi: 10.1007/BF01868535. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Turner R. J., Burg M. B. Osmoregulation of betaine transport in mammalian renal medullary cells. Am J Physiol. 1990 Apr;258(4 Pt 2):F1061–F1067. doi: 10.1152/ajprenal.1990.258.4.F1061. [DOI] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Na+ influx and cell growth in cultured human fibroblasts. Effect of indomethacin. Exp Cell Res. 1983 Jan;143(1):37–46. doi: 10.1016/0014-4827(83)90106-4. [DOI] [PubMed] [Google Scholar]
- Petronini P. G., Tramacere M., Kay J. E., Borghetti A. F. Adaptive response of cultured fibroblasts to hyperosmolarity. Exp Cell Res. 1986 Jul;165(1):180–190. doi: 10.1016/0014-4827(86)90542-2. [DOI] [PubMed] [Google Scholar]
- Petronini P. G., Tramacere M., Mazzini A., Kay J. E., Borghetti A. F. Control of protein synthesis by extracellular Na+ in cultured fibroblasts. J Cell Physiol. 1989 Aug;140(2):202–211. doi: 10.1002/jcp.1041400203. [DOI] [PubMed] [Google Scholar]
- Petronini P. G., Tramacere M., Mazzini A., Piedimonte G., Silvotti L., Borghetti A. F. Hyperosmolarity-induced stress proteins in chick embryo fibroblasts. Exp Cell Res. 1987 Oct;172(2):450–462. doi: 10.1016/0014-4827(87)90403-4. [DOI] [PubMed] [Google Scholar]
- Petronini P. G., Tramacere M., Wheeler K. P., Borghetti A. F. Induction of amino acid transport activity in chick embryo fibroblasts by replacement of extracellular sodium chloride with disaccharide. Biochim Biophys Acta. 1990 Jul 12;1053(2-3):144–150. doi: 10.1016/0167-4889(90)90006-y. [DOI] [PubMed] [Google Scholar]
- Piedimonte G., Borghetti A. F., Guidotti G. G. Effect of cell density on growth rate and amino acid transport in simian virus 40-transformed 3T3 cells. Cancer Res. 1982 Nov;42(11):4690–4693. [PubMed] [Google Scholar]
- Silvotti L., Petronini P. G., Mazzini A., Piedimonte G., Borghetti A. F. Differential adaptive response to hyperosmolarity of 3T3 and transformed SV3T3 cells. Exp Cell Res. 1991 Apr;193(2):253–261. doi: 10.1016/0014-4827(91)90094-b. [DOI] [PubMed] [Google Scholar]
- Tramacere M., Petronini P. G., Severini A., Borghetti A. F. Osmoregulation of amino acid transport activity in cultured fibroblasts. Exp Cell Res. 1984 Mar;151(1):70–79. doi: 10.1016/0014-4827(84)90356-2. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Burg M. B. Counteracting effects of urea and betaine in mammalian cells in culture. Am J Physiol. 1990 Jan;258(1 Pt 2):R198–R204. doi: 10.1152/ajpregu.1990.258.1.R198. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]


