Abstract
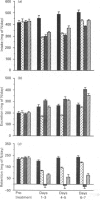
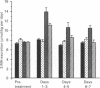
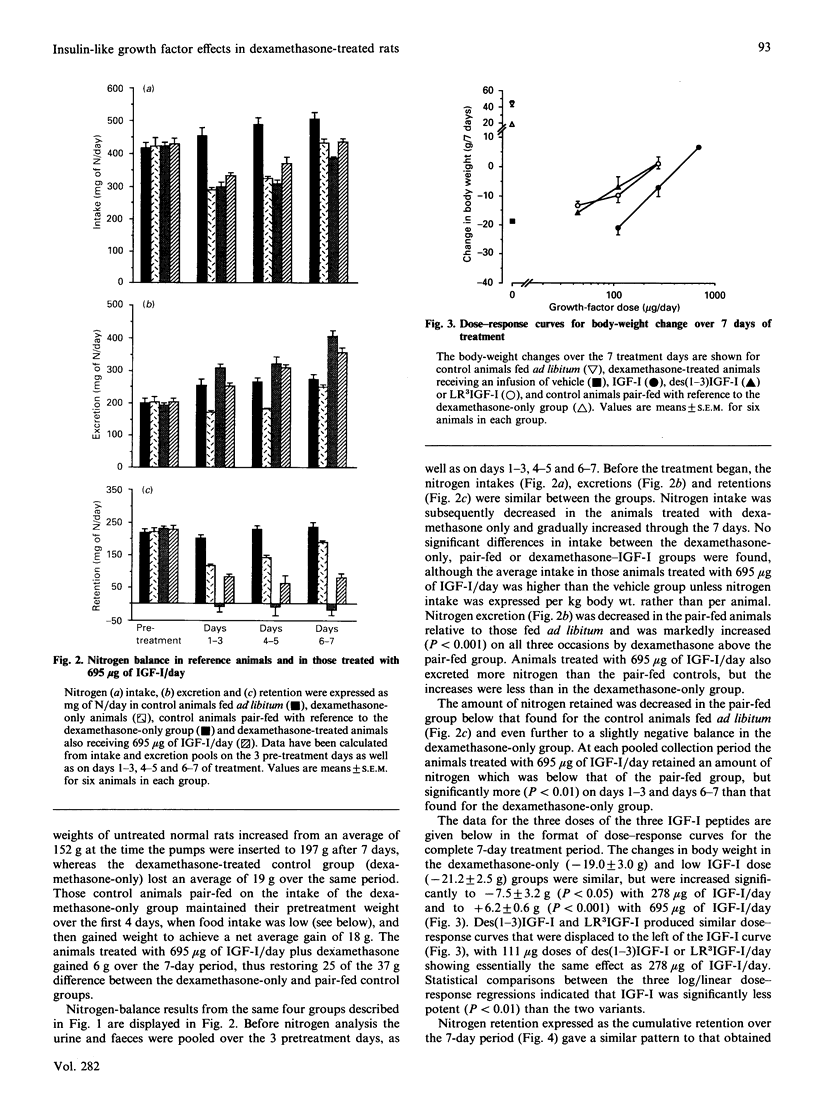
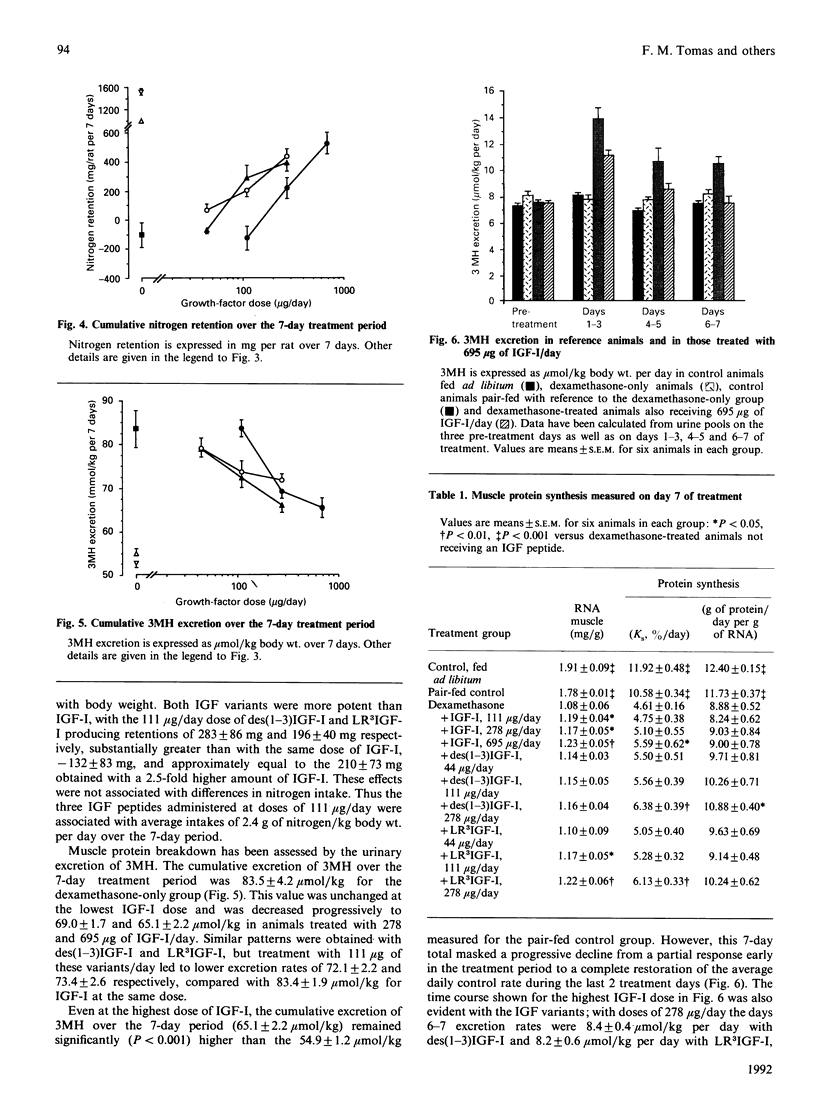
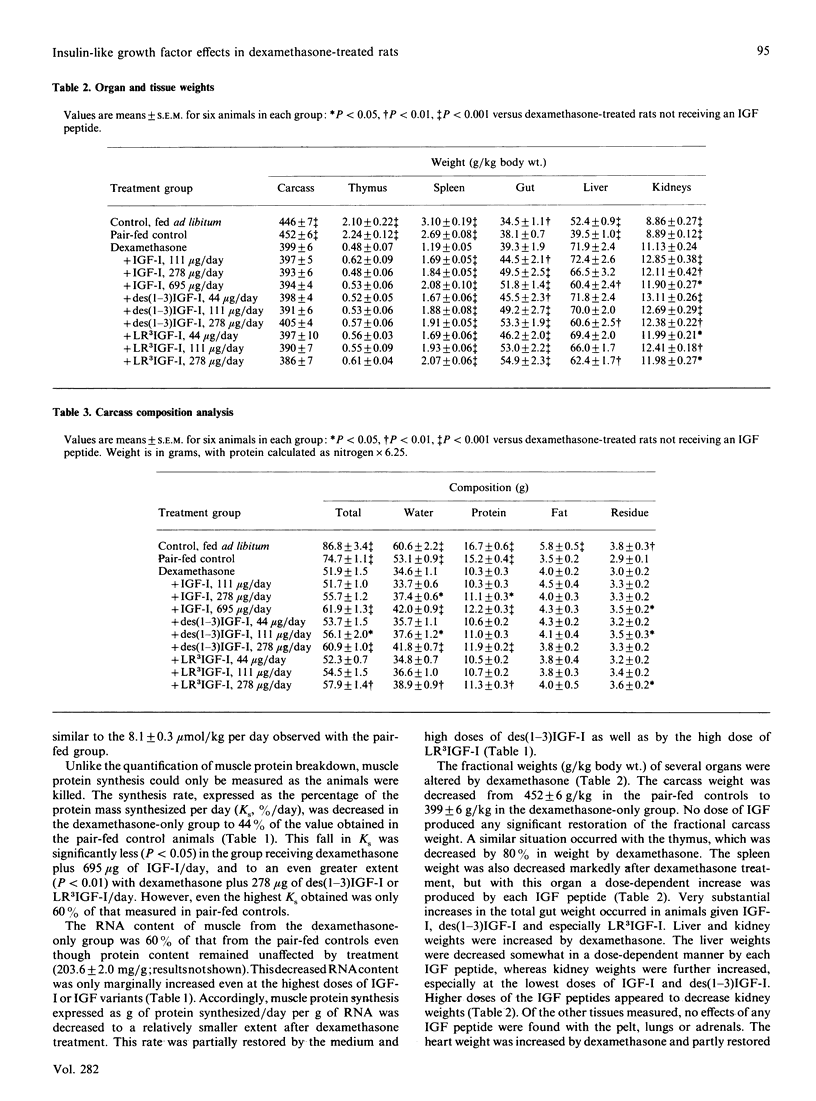
The administration of insulin-like growth factor-I (IGF-I) via subcutaneously implanted osmotic pumps partially reversed a catabolic state produced by the co-administration of 20 micrograms of dexamethasone/day to 150 g male rats. Marked dose-dependent effects on body weight and nitrogen retention were produced, with the highest IGF-I dose, 695 micrograms/day, giving a 6 g increase in body weight over 7 days, compared with a 19 g loss in the dexamethasone-only group and an 18 g gain in pair-fed controls. Two IGF-I analogues that bind poorly to IGF-binding proteins, the truncated form, des(1-3)IGF-I, and a variant with an N-terminal extension as well as arginine at residue 3, LR3IGF-I, were approx. 2.5-fold more potent than IGF-I. The response with LR3IGF-I was particularly striking because this peptide binds 3-fold less well than IGF-I to the type 1 IGF receptor. The increased potencies of the IGF-I variants may relate to the substantially increased plasma levels of IGF-binding proteins, particularly IGFBP-3, produced by the combined treatment of dexamethasone with IGF-I or the variants. These binding proteins would be expected to decrease the transfer of IGF-I, but not that of the variants, from blood to tissue sites of action. Measurements of muscle protein synthesis at the end of the treatment period and muscle protein breakdown by 3-methylhistidine (3MH) excretion throughout the experiment indicated coordinate anabolic effects of the IGF peptides on both processes. Thus 3MH excretion was decreased at the highest IGF-I dose from 83.5 +/- 4.2 (S.E.M.) mumol/kg per 7 days to 65.1 +/- 2.2, compared with 54.9 +/- 1.2 in the pair-fed controls. Part of this response in 3MH excretion may have reflected a decrease in gut protein breakdown, because IGF-I and especially the IGF analogues increased the gut weight by up to 45%. Notwithstanding the effects on protein synthesis and breakdown, the fractional carcass weights remained low in the IGF-treated groups, although the increase in total carcass weight reflected nitrogen rather than fat gain. The dexamethasone-induced changes in liver, spleen and heart weight were restored towards normal by the IGF treatment. The experiment demonstrates the potential of IGF-I treatment of catabolic states and especially the value of modified forms of growth factors that bind weakly to IGF-binding proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Knowles S. E., Walton P. E., Edson K., Owens P. C., Mohler M. A., Ferraiolo B. L. Plasma clearance and tissue distribution of labelled insulin-like growth factor-I (IGF-I), IGF-II and des(1-3)IGF-I in rats. J Endocrinol. 1991 Feb;128(2):197–204. doi: 10.1677/joe.0.1280197. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Read L. C., Francis G. L., Bagley C. J., Wallace J. C. Binding properties and biological potencies of insulin-like growth factors in L6 myoblasts. Biochem J. 1986 Jan 1;233(1):223–230. doi: 10.1042/bj2330223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R. C., Axiak S., Raison R. L. Monoclonal antibody against human somatomedin-C/insulin-like growth factor-I. J Clin Endocrinol Metab. 1982 Feb;54(2):474–476. doi: 10.1210/jcem-54-2-474. [DOI] [PubMed] [Google Scholar]
- Binz K., Joller P., Froesch P., Binz H., Zapf J., Froesch E. R. Repopulation of the atrophied thymus in diabetic rats by insulin-like growth factor I. Proc Natl Acad Sci U S A. 1990 May;87(10):3690–3694. doi: 10.1073/pnas.87.10.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Dickerson R. N., Brown R. O., Hak L. J., MacPhee R. D., Heizer W. D. Use of plasma somatomedin-C/insulin-like growth factor I measurements to monitor the response to nutritional repletion in malnourished patients. Am J Clin Nutr. 1985 Feb;41(2):191–198. doi: 10.1093/ajcn/41.2.191. [DOI] [PubMed] [Google Scholar]
- Forbes B., Szabo L., Baxter R. C., Ballard F. J., Wallace J. C. Classification of the insulin-like growth factor binding proteins into three distinct categories according to their binding specificities. Biochem Biophys Res Commun. 1988 Nov 30;157(1):196–202. doi: 10.1016/s0006-291x(88)80032-9. [DOI] [PubMed] [Google Scholar]
- Francis G. L., McNeil K. A., Wallace J. C., Ballard F. J., Owens P. C. Sheep insulin-like growth factors I and II: sequences, activities and assays. Endocrinology. 1989 Mar;124(3):1173–1183. doi: 10.1210/endo-124-3-1173. [DOI] [PubMed] [Google Scholar]
- Francis G. L., Upton F. M., Ballard F. J., McNeil K. A., Wallace J. C. Insulin-like growth factors 1 and 2 in bovine colostrum. Sequences and biological activities compared with those of a potent truncated form. Biochem J. 1988 Apr 1;251(1):95–103. doi: 10.1042/bj2510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N., Price D. A., Maycock P. F., Carroll S. M. Plasma somatomedin activity after injury in man and its relationship to other hormonal and metabolic changes. Clin Endocrinol (Oxf) 1984 Feb;20(2):179–187. doi: 10.1111/j.1365-2265.1984.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem. 1969 Jun 25;244(12):3223–3229. [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Scheiwiller E., Froesch E. R. Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizuka N., Takano K., Shizume K., Asakawa K., Miyakawa M., Tanaka I., Horikawa R. Insulin-like growth factor I stimulates growth in normal growing rats. Eur J Pharmacol. 1986 Jun 5;125(1):143–146. doi: 10.1016/0014-2999(86)90093-2. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Seurin D., Segovia-Quinson B., Hardouin S., Binoux M. Analysis of serum insulin-like growth factor binding proteins using western blotting: use of the method for titration of the binding proteins and competitive binding studies. Anal Biochem. 1986 Apr;154(1):138–143. doi: 10.1016/0003-2697(86)90507-5. [DOI] [PubMed] [Google Scholar]
- Lemmey A. B., Martin A. A., Read L. C., Tomas F. M., Owens P. C., Ballard F. J. IGF-I and the truncated analogue des-(1-3)IGF-I enhance growth in rats after gut resection. Am J Physiol. 1991 Feb;260(2 Pt 1):E213–E219. doi: 10.1152/ajpendo.1991.260.2.E213. [DOI] [PubMed] [Google Scholar]
- Luo J. M., Murphy L. J. Regulation of insulin-like growth factor binding protein-3 expression by dexamethasone. Mol Cell Endocrinol. 1990 Dec 21;74(3):213–219. doi: 10.1016/0303-7207(90)90226-x. [DOI] [PubMed] [Google Scholar]
- Luo J., Reid R. E., Murphy L. J. Dexamethasone increases hepatic insulin-like growth factor binding protein-1 (IGFBP-1) mRNA and serum IGFBP-1 concentrations in the rat. Endocrinology. 1990 Sep;127(3):1456–1462. doi: 10.1210/endo-127-3-1456. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Bates P. C. 3-Methylhistidine turnover in the whole body, and the contribution of skeletal muscle and intestine to urinary 3-methylhistidine excretion in the adult rat. Biochem J. 1983 Aug 15;214(2):607–615. doi: 10.1042/bj2140607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier H. D., Jr, Spencer E. M., Dearden L. C., Jansons R. A. The effect of glucocorticoids on plasma insulin-like growth factor I concentration in the rat fetus. Pediatr Res. 1987 Jul;22(1):92–95. doi: 10.1203/00006450-198707000-00021. [DOI] [PubMed] [Google Scholar]
- Odedra B. R., Millward D. J. Effect of corticosterone treatment on muscle protein turnover in adrenalectomized rats and diabetic rats maintained on insulin. Biochem J. 1982 Jun 15;204(3):663–672. doi: 10.1042/bj2040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiwiller E., Guler H. P., Merryweather J., Scandella C., Maerki W., Zapf J., Froesch E. R. Growth restoration of insulin-deficient diabetic rats by recombinant human insulin-like growth factor I. Nature. 1986 Sep 11;323(6084):169–171. doi: 10.1038/323169a0. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Hauri C., Steiner T., Froesch E. R. Comparison of in vivo effects of insulin-like growth factors I and II and of growth hormone in hypophysectomized rats. Acta Endocrinol (Copenh) 1985 Feb;108(2):167–174. doi: 10.1530/acta.0.1080167. [DOI] [PubMed] [Google Scholar]
- Tomas F. M. Effect of corticosterone on myofibrillar protein turnover in diabetic rats as assessed by Ntau-methylhistidine excretion. Biochem J. 1982 Dec 15;208(3):593–601. doi: 10.1042/bj2080593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas F. M., Knowles S. E., Owens P. C., Read L. C., Chandler C. S., Gargosky S. E., Ballard F. J. Effects of full-length and truncated insulin-like growth factor-I on nitrogen balance and muscle protein metabolism in nitrogen-restricted rats. J Endocrinol. 1991 Jan;128(1):97–105. doi: 10.1677/joe.0.1280097. [DOI] [PubMed] [Google Scholar]
- Tomas F. M., Knowles S. E., Owens P. C., Read L. C., Chandler C. S., Gargosky S. E., Ballard F. J. Increased weight gain, nitrogen retention and muscle protein synthesis following treatment of diabetic rats with insulin-like growth factor (IGF)-I and des(1-3)IGF-I. Biochem J. 1991 Jun 1;276(Pt 2):547–554. doi: 10.1042/bj2760547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas F. M., Murray A. J., Jones L. M. Interactive effects of insulin and corticosterone on myofibrillar protein turnover in rats as determined by N tau-methylhistidine excretion. Biochem J. 1984 Jun 1;220(2):469–479. doi: 10.1042/bj2200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas F. M., Murray A. J., Jones L. M. Modification of glucocorticoid-induced changes in myofibrillar protein turnover in rats by protein and energy deficiency as assessed by urinary excretion of Ntau-methylhistidine. Br J Nutr. 1984 May;51(3):323–337. doi: 10.1079/bjn19840039. [DOI] [PubMed] [Google Scholar]
- Unterman T. G., Phillips L. S. Glucocorticoid effects on somatomedins and somatomedin inhibitors. J Clin Endocrinol Metab. 1985 Oct;61(4):618–626. doi: 10.1210/jcem-61-4-618. [DOI] [PubMed] [Google Scholar]
- Zapf J., Hauri C., Waldvogel M., Futo E., Häsler H., Binz K., Guler H. P., Schmid C., Froesch E. R. Recombinant human insulin-like growth factor I induces its own specific carrier protein in hypophysectomized and diabetic rats. Proc Natl Acad Sci U S A. 1989 May;86(10):3813–3817. doi: 10.1073/pnas.86.10.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]