Abstract
For localized breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), surgical resection is crucial; however, radiation therapy (RT) can be utilized as local-regional therapy if surgery is incomplete or not recommended. We present the case of a woman with BIA-ALCL who received systemic therapy and consolidation RT.
Keywords: Radiation oncology, radiation therapy, breast implant-associated anaplastic large cell lymphoma
Introduction
Breast implant-associated ALCL is an uncommon and emerging entity with unique characteristics that distinguish it from other lymphomas. In contrast to other hematologic malignancies, the role of surgery is critical in the management, and a distinct staging system provides better outcome discrimination amongst early-stage patients [1]. While the role of definitive or consolidative radiation therapy (RT) is well-defined for most lymphoma subtypes, the ideal adjuvant therapy for BIA-ALCL is unknown. Advanced BIA-ALCL follows treatment protocols that include radiation therapy indications as set forth by the National Comprehensive Cancer Network (NCCN), highlighting the network’s guidelines in the management of this rare lymphoma [2,3]. Within this entity, patients with localized disease who are not recommended for surgery are even more rare. We report on one such case where radiation therapy was recommended and delivered in a manner to minimize long-term toxicity risk.
Case report
A woman in her sixties presented to the emergency department of our comprehensive cancer center after she was given an outside diagnosis of breast lymphoma. She had bilateral textured surface breast implants (Allergan Biocell, Irvine, CA) placed at an outside facility approximately ten years before. She began to experience discomfort and swelling of the left breast two months prior to her presentation and returned to that facility for removal of the implants. At the time of the procedure, the surgeon noted abnormal ulcerated tissue in the implant scar capsule of the left breast. During the post-operative period, the right breast healed as expected, but the left breast wound did not completely heal. She then underwent an incision and debridement procedure. At that time, a biopsy was performed which confirmed lymphoma.
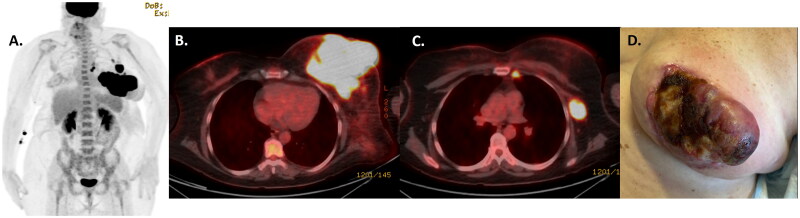
Full pathologic and staging evaluations were performed at our institution. Review of the outside specimen confirmed anaplastic large cell lymphoma, CD30 positive, associated with the breast implant. A new core needle biopsy of the left breast confirmed ALK-negative ALCL, consistent with BIA-ALCL. Bone marrow biopsy was negative for involvement by lymphoma. Positron emission tomography-computed tomography (PET-CT) imaging revealed a hypermetabolic left breast mass measuring 14 × 11 cm with standardized uptake value (SUV) of 27. Fluorodeoxyglucose (FDG) avid lymph nodes were present at the left internal mammary (subcentimeter, SUV 12) and left axillary (2.5 cm, SUV 16) nodal basins, consistent with regional sites of disease (Figure 1). No distant sites of disease were identified.
Figure 1.
PETCT scan confirmed FDG-avid disease involving the left breast and regional lymph nodes (internal mammary and axilla) on both maximal intensity projection (A) and fused axial images at the level of the breast (B) and axilla (C). Clinical photograph (D) of the left breast mass prior to treatment.
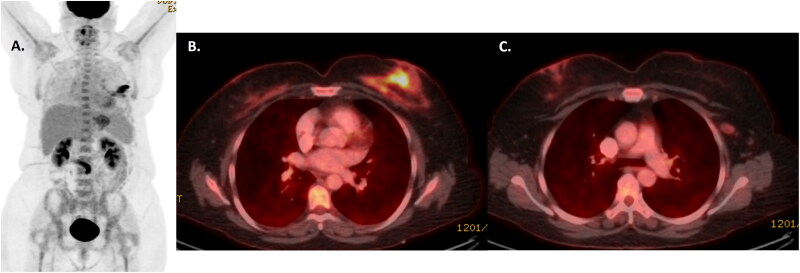
She was evaluated by the multidisciplinary team including plastic surgery, medical oncology, and radiation oncology. Upfront surgical resection was not recommended based on the extent of disease including internal mammary lymphadenopathy. She was started on systemic therapy with brentuximab-vedotin, cyclophosphamide, doxorubicin, and prednisone. After 3 cycles, she had a significant positive response (Figure 2), and after 6 cycles, PET-CT confirmed a complete metabolic response to therapy (Figure 3).
Figure 2.
PETCT scan after 3 cycles of BV-CHP confirming response to treatment within the left breast and lymph nodes on maximal intensity projection (A) and fused axial images at the level of the breast (B) and axilla (C). The lymphoma five point score was assigned as a 4, with uptake above the level of the liver.
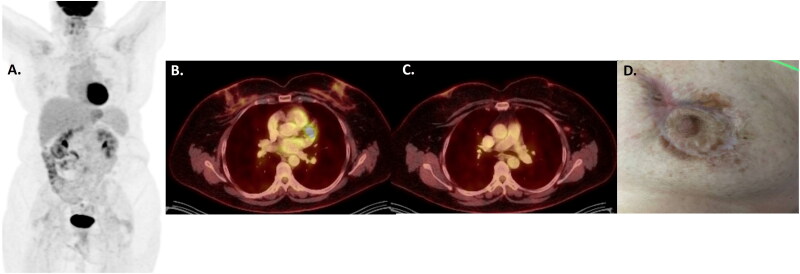
Figure 3.
PETCT scan after 6 cycles of BV-CHP confirming complete response to systemic therapy within the left breast and regional lymph nodes on maximal intensity projection (A) and fused axial images at the level of the breast (B) and axilla (C). the lymphoma five point score was assigned as a 3, indicating a complete metabolic response. Photograph (D) confirming an excellent clinical response to treatment with only hyperpigmentation and scar remaining.
As this patient did not undergo surgical resection, had bulky disease involving the skin of the breast, and had unresected nodal disease, consolidation radiation therapy was recommended to improve local control.
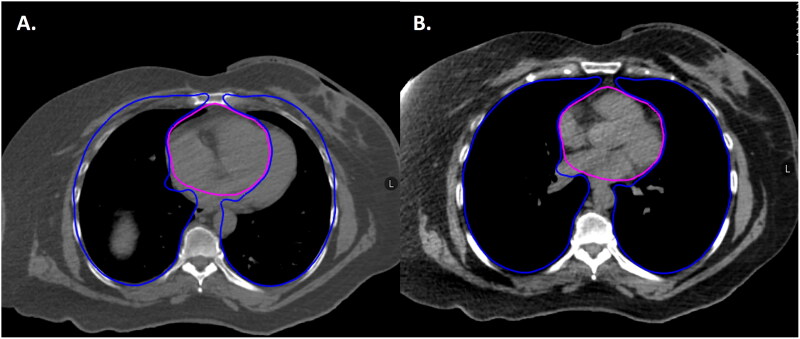
During RT simulation, she was positioned on an angled board to help with a reproducible breast setup. Bolus material was placed over the skin of the left breast to increase surface dose given her skin involvement at presentation. A deep inspiration breath hold technique was used to increase her lung volume and the distance between the left breast and her heart (Figure 4).
Figure 4.
Axial representation of the patient’s RT simulation scan in the free breathing (A) and deep inspiration breath hold (DIBH) (B) positions showing the benefit of increased distance between heart (pink) and breast as well as increased lung volume (blue) during DIBH.
She was treated using volumetric arc therapy (VMAT), which is an advanced RT planning technique that achieves highly conformal dose distributions with improved target coverage and sparing of normal tissues compared with conventional techniques. During the planning process, avoidance of the heart, lungs, and contralateral breast were prioritized, including optimization of the 5 Gy line to limit low doses to normal tissues. Due to time constraints, she was treated with a slightly hypofractionated radiation therapy course with 25 Gy given over ten fractions (Figure 5). With this prescription, we were able to achieve doses to normal structures far below those recommended by national guidelines, including mean heart and lung doses of 2.3 and 3.3 Gy, respectively [4]. She tolerated therapy well with no significant toxicities.
Figure 5.
Axial representation of the patient’s RT simulation scan with RT treatment plan at the level of the internal mammary and axillaryl lymph nodes (A) and left breast mass (B). the prescription radiation isodose line (2500 cGY) encompasses the intended targets while avoiding critical structures such as the heart and lungs.
Discussion
For patients presenting with localized BIA-ALCL, surgical resection provides the best outcome regardless of pathologic stage [1]. However, some patients with more locally advanced disease are not recommended surgery due to the expected morbidity and others have residual disease remaining after maximally safe surgical resection. For these patients, alternative methods to improve local-regional control are necessary.
The role of radiation therapy in the management of other lymphoma subtypes is well established. For patients with diffuse large B cell lymphoma, studies support improved outcomes with the addition of radiation therapy after systemic therapy for patients with localized early-stage disease or for patients with bulky or extranodal disease [5]. For patients with early-stage primary systemic ALCL, favorable outcomes have been reported when RT is given after doxorubicin-based chemotherapy [6]. For cutaneous ALCL, focal RT is the preferred management for patients with localized disease [7]. Although moderate RT doses ≥30 Gy have historically been given with excellent outcomes, studies support the success of treatment even with 10 Gy or less [7]. Given the indolent and chronic nature of the disease, the importance of toxicity minimization is underscored. Due to the success of RT in the local-regional management of lymphomas that share features of BIA-ALCL, this modality is often considered to improve local control when surgery alone is not sufficient.
The optimal nature and timing of adjuvant therapy for BIA-ALCL is unknown [8], and some practitioners might argue that RT is unnecessary if effective systemic therapy is given with complete response. However, if RT is given thoughtfully with incorporation of modern RT techniques, the expected short and long-term toxicity is minimal. Therefore, patients with disease factors known to increase the risk of local-regional recurrence should be evaluated by a radiation oncologist with experience in the treatment of hematologic malignancies.
During the simulation process, the anatomy can be optimized to create a reproducible setup that allows for treatment of the target with avoidance of surrounding normal structures. When treating the thorax, an incline board allows for a reliable and favorable setup of the breast which is displaced from the infraclavicular area and arm by gravity. Deep inspiration breath hold is a technique used during simulation and treatment to increase the volume of the patient’s lungs [9]. This technique, when performed successfully, decreases the amount of lung treated with radiation and also increases the distance between the heart and the chest wall, allowing for improved cardiac avoidance [10]. Finally, advanced RT planning techniques such as intensity modulated radiation therapy (IMRT) or volumetric arc therapy (VMAT) accomplish critical structure sparing that is not possible with simpler 3D conformal radiation techniques. Using these simulation and planning techniques with the moderate RT prescription doses typically recommended for BIA-ALCL, doses to adjacent organs at risk such as the heart, lungs, and contralateral breast can be kept very low. When we offer consolidation radiation therapy in this setting, we typically treat to a dose of 20-30 Gy in 2-2.5 Gy fractions. Currently published RT dose constraints for Hodgkin lymphoma provide more conservative goals than those used for solid tumor RT2; with the exception of the breast constraint (as the breast is a target for BIA-ALCL), these constraints should be easily achieved.
Conclusions
Optimal outcomes for patients with BIA-ALCL require maximal surgical resection. However, for those patients with disease risk factors necessitating additional or alternative local-regional management, radiation therapy should be considered. This therapy can be given safely with low risk of long-term toxicities in a patient population with an overall favorable prognosis.
Funding Statement
Supported in part by National Institutes of Health (NIH), National Cancer Institute Cancer Center Support (Core) Grant P30 CA016672 to The University of Texas MD Anderson Cancer Center (PI: PW Pisters).
Author contributions statement
JRG, CCP contributed to the clinical care, study design, writing and editing of the manuscript. SPI, KKH, HF, SAW, MWC contributed to the clinical care and editing of the manuscript.
Disclosure statement
Dr. Pinnix has research support from Merck. Otherwise, none of the authors report any relevant conflict of interest.
Dr. Hunt has the following disclosures: Medical Advisory Board – ArmadaHealth and AstraZeneca; Research funding to my institution – Cairn Surgical, Eli Lilly & Co., Lumicell.
Informed consent
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Data availability statement
Deidentified individual participant data that form the basis for the reported results will be made available 3 months after publication for a period of 5 years after the publication data after initiation and execution of a materials transfer agreement between MD Anderson Cancer Center and the party requesting the data.
References
- 1.Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34(2):160–168. doi: 10.1200/JCO.2015.63.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens MW, , Jacobsen ED, , Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Supplement_1):S3–S13. doi: 10.1093/asj/sjy331. [DOI] [PubMed] [Google Scholar]
- 3.Clemens MW, Myckatyn T, Di Napoli A, et al. . Breast Implant Associated Anaplastic Large Cell Lymphoma: Evidence-Based Consensus Conference Statement From The American Association of Plastic Surgeons. Plast Reconstr Surg. 2024. [cited 2024 February 27]. doi: 10.1097/PRS.0000000000011370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . Hodgkin Lymphoma (Version 2.2024); 2024. [cited 2024 Feb 2]. https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf
- 5.Imber BS, Yahalom J.. Radiotherapy for non-Hodgkin lymphomas. Cancer J. 2020;26(3):217–230. doi: 10.1097/PPO.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang XM, Li YX, Wang WH, et al. Favorable outcome with doxorubicin-based chemotherapy and radiotherapy for adult patients with early stage primary systemic anaplastic large-cell lymphoma. Eur J Haematol. 2013;90(3):195–201. doi: 10.1111/ejh.12060. [DOI] [PubMed] [Google Scholar]
- 7.Smith GL, Duvic M, Yehia ZA, et al. Effectiveness of low-dose radiation for primary cutaneous anaplastic large cell lymphoma. Adv Radiat Oncol. 2017;2(3):363–369. doi: 10.1016/j.adro.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta-Shah N, Clemens MW, Horwitz SM.. How I treat breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132(18):1889–1898. doi: 10.1182/blood-2018-03-785972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voong KR, McSpadden K, Pinnix CC, et al. Dosimetric advantages of a "butterfly" technique for intensity-modulated radiation therapy for young female patients with mediastinal Hodgkin’s lymphoma. Radiat Oncol. 2014;9(1):94. doi: 10.1186/1748-717X-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stowe HB, Andruska ND, Reynoso F, et al. Heart sparing radiotherapy techniques in breast cancer: a focus on deep inspiration breath hold. Breast Cancer (Dove Med Press). 2022;14:175–186. doi: 10.2147/BCTT.S282799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified individual participant data that form the basis for the reported results will be made available 3 months after publication for a period of 5 years after the publication data after initiation and execution of a materials transfer agreement between MD Anderson Cancer Center and the party requesting the data.