Abstract
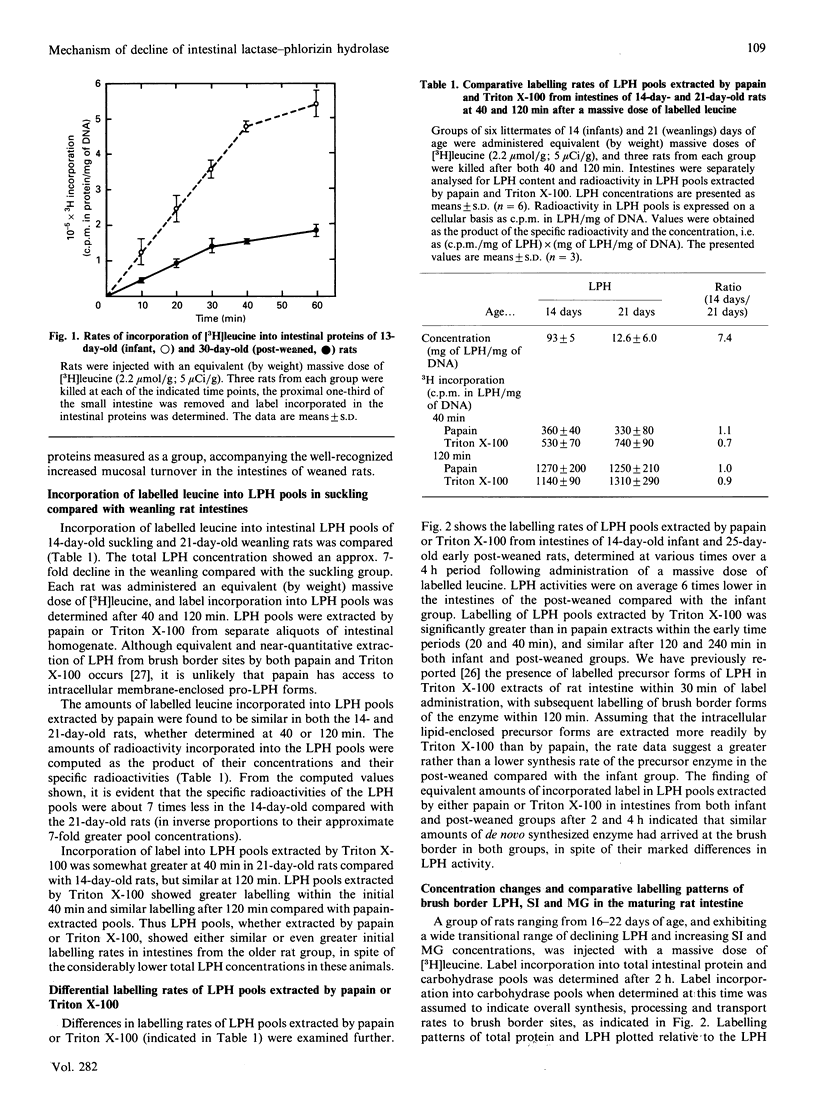
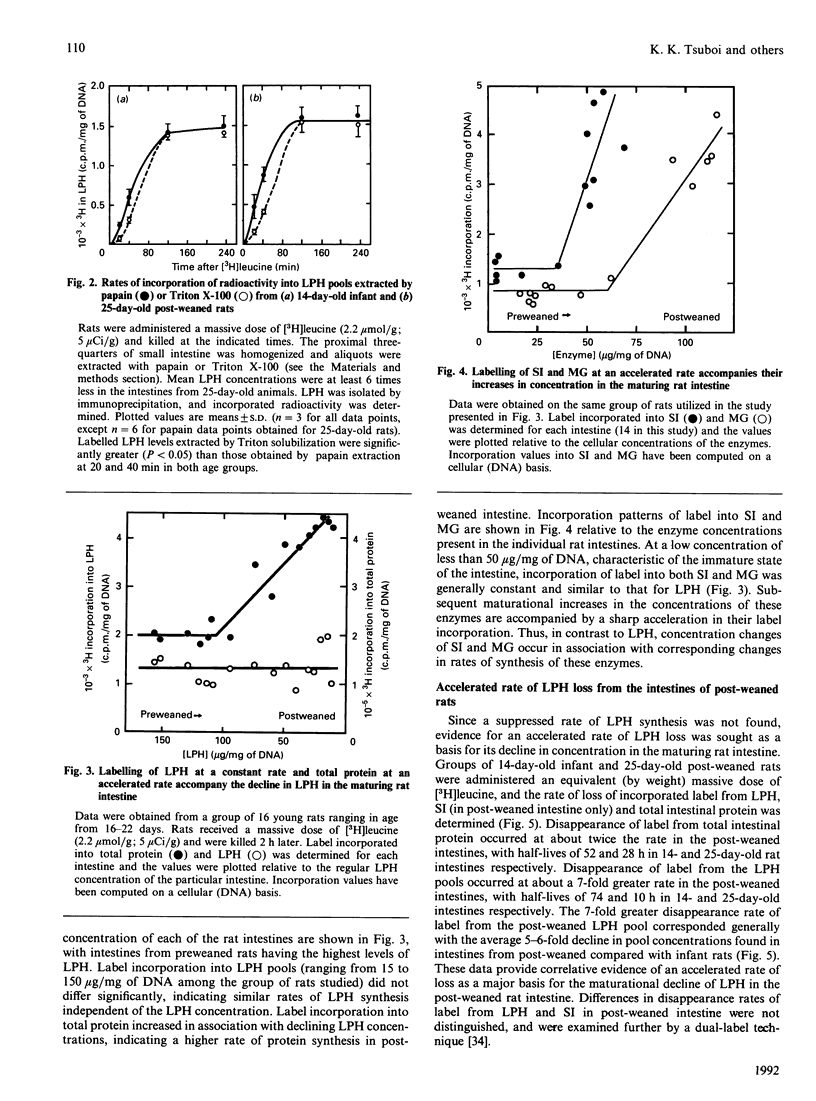
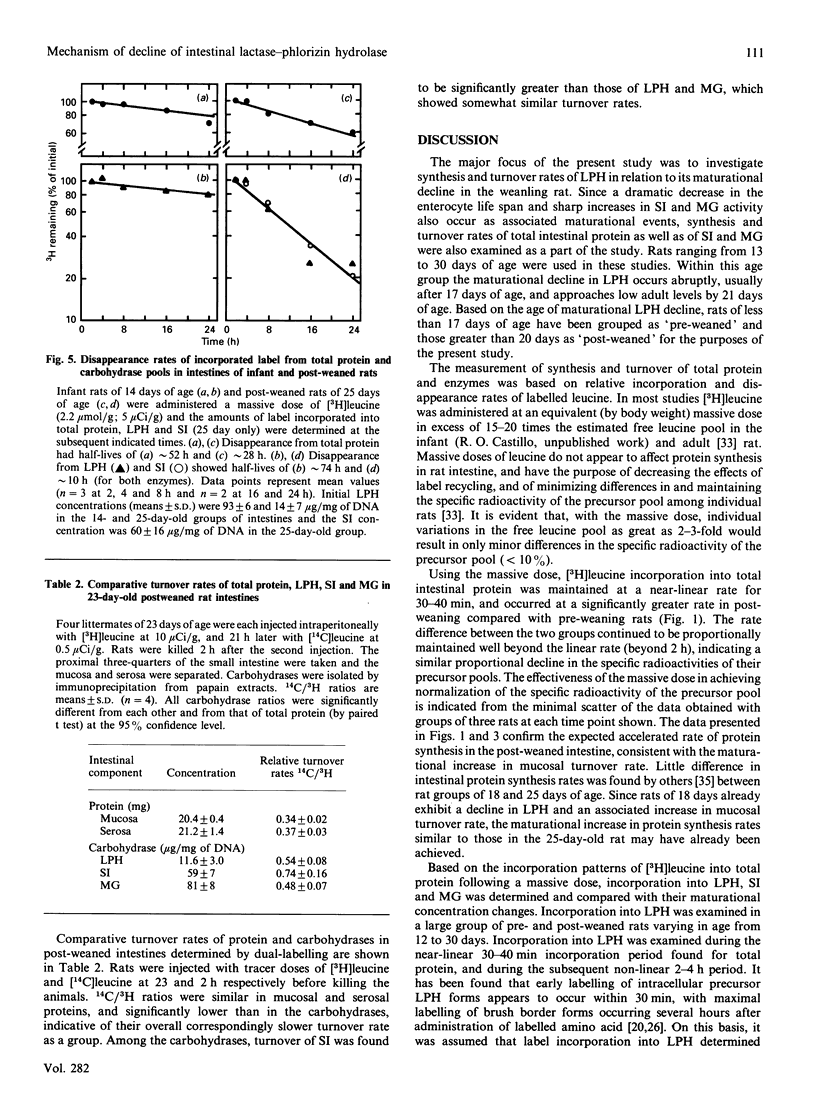
The maturational decline in lactase-phlorizin hydrolase (LPH) activity was studied in groups of young rats ranging from suckling to early post-weaned states. Associated maturational increases in sucrase-isomaltase (SI) and maltase-glucoamylase (MG) activities were also examined as a comparison. Over this time period changes in cellular concentrations of the three enzymes were observed, reflecting corresponding changes in enzyme activities. Synthesis patterns accompanying these maturational changes in concentration were examined using labelled leucine as a marker. Synthesis of LPH was found to be maintained at constant rates independent of the maturation-associated decline in its concentration, whereas the increases in cellular concentrations of SI and MG were due to accelerated synthesis of the enzyme. Turnover of LPH, based on both the fractional synthesis rate and the disappearance rate of labelled leucine from prelabelled LPH pools, was increased in a quantitatively similar way to the decline in LPH concentration. These findings are consistent with our earlier proposal that the maturational decline of LPH occurs because of accelerated turnover, without a decrease in its rate of synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. Separation and isolation of rat and human intestinal beta-galactosidases. J Biol Chem. 1969 Mar 10;244(5):1238–1246. [PubMed] [Google Scholar]
- Altmann G. G., Enesco M. Cell number as a measure of distribution and renewal of epithelial cells in the small intestine of growing and adult rats. Am J Anat. 1967 Sep;121(2):319–336. doi: 10.1002/aja.1001210210. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Birkenmeier E., Alpers D. H. Enzymatic properties of rat lactase-phlorizin hydrolase. Biochim Biophys Acta. 1974 May 20;350(1):100–112. doi: 10.1016/0005-2744(74)90207-1. [DOI] [PubMed] [Google Scholar]
- Büller H. A., Montgomery R. K., Sasak W. V., Grand R. J. Biosynthesis, glycosylation, and intracellular transport of intestinal lactase-phlorizin hydrolase in rat. J Biol Chem. 1987 Dec 15;262(35):17206–17211. [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo R. O., Reisenauer A. M., Kwong L. K., Tsuboi K. K., Quan R., Gray G. M. Intestinal lactase in the neonatal rat. Maturational changes in intracellular processing and brush-border degradation. J Biol Chem. 1990 Sep 15;265(26):15889–15893. [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. Studies of small intestine during development. I. Distribution and activity of beta-galactosidase. Biochim Biophys Acta. 1962 Aug 13;62:353–362. doi: 10.1016/0006-3002(62)90097-5. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M., Norén O., Sjöström H. Biosynthesis of microvillar proteins. Biochem J. 1984 Jul 1;221(1):1–14. doi: 10.1042/bj2210001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J. N., Duluc I., Raul F. Discrepancy between the intestinal lactase enzymatic activity and mRNA accumulation in sucklings and adults. Effect of starvation and thyroxine treatment. FEBS Lett. 1989 May 8;248(1-2):39–42. doi: 10.1016/0014-5793(89)80427-2. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning S. J. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981 Sep;241(3):G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Jonas M. M., Montgomery R. K., Grand R. J. Intestinal lactase synthesis during postnatal development in the rat. Pediatr Res. 1985 Sep;19(9):956–962. doi: 10.1203/00006450-198509000-00018. [DOI] [PubMed] [Google Scholar]
- Koldovsky O., Sunshine P., Kretchmer N. Cellular migration of intestinal epithelia in suckling and weaned rats. Nature. 1966 Dec 17;212(5068):1389–1390. doi: 10.1038/2121389a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- McAllister G., Bailey D. S. Cell-free synthesis of high-molecular-weight small intestinal polypeptides. Biochim Biophys Acta. 1986 Sep 25;861(1):197–200. doi: 10.1016/0005-2736(86)90580-8. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim H. Y., Sterchi E. E., Lentze M. J. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem J. 1987 Jan 15;241(2):427–434. doi: 10.1042/bj2410427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsi-Emvo E., Launay J. F., Raul F. Is adult-type hypolactasia in the intestine of mammals related to changes in the intracellular processing of lactase? Cell Mol Biol. 1987;33(3):335–344. [PubMed] [Google Scholar]
- Quan R., Santiago N. A., Tsuboi K. K., Gray G. M. Intestinal lactase. Shift in intracellular processing to altered, inactive species in the adult rat. J Biol Chem. 1990 Sep 15;265(26):15882–15888. [PubMed] [Google Scholar]
- RUBINO A., ZIMBALATTI F., AURICCHIO S. INTESTINAL DISACCHARIDASE ACTIVITIES IN ADULT AND SUCKLING RATS. Biochim Biophys Acta. 1964 Nov 22;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Haggarty P., Wahle K. W., Fletcher J. M. Tissue and whole-body protein synthesis in immature Zucker rats and their relationship to protein deposition. Biochem J. 1982 May 15;204(2):393–398. doi: 10.1042/bj2040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Sahi T. Dietary lactose and the aetiology of human small-intestinal hypolactasia. Gut. 1978 Nov;19(11):1074–1086. doi: 10.1136/gut.19.11.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel-Haueter S., Hore P., Kerry K. R., Semenza G. The preparation of lactase and glucoamylase of rat small intestine. Biochim Biophys Acta. 1972 Feb 28;258(2):506–519. doi: 10.1016/0005-2744(72)90242-2. [DOI] [PubMed] [Google Scholar]
- Smith M. W., James P. S. Cellular origin of lactase decline in postweaned rats. Biochim Biophys Acta. 1987 Dec 11;905(2):503–506. doi: 10.1016/0005-2736(87)90481-0. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Kwong L. K., Burrill P. H., Sunshine P. Sugar hydrolases and their arrangement on the rat intestinal microvillus membrane. J Membr Biol. 1979 Oct 15;50(2):101–122. doi: 10.1007/BF01868943. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Kwong L. K., D'Harlingue A. E., Stevenson D. K., Kerner J. A., Jr, Sunshine P. The nature of maturational decline of intestinal lactase activity. Biochim Biophys Acta. 1985 May 29;840(1):69–78. doi: 10.1016/0304-4165(85)90163-1. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Kwong L. K., Ford W. D., Colby T., Sunshine P. Delayed ontogenic development in the bypassed ileum of the infant rat. Gastroenterology. 1981 Jun;80(6):1550–1556. [PubMed] [Google Scholar]
- Tsuboi K. K., Kwong L. K., Neu J., Sunshine P. A proposed mechanism of normal intestinal lactase decline in the postweaned mammal. Biochem Biophys Res Commun. 1981 Jul 30;101(2):645–652. doi: 10.1016/0006-291x(81)91307-3. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Schwartz S. M., Burrill P. H., Kwong L. K., Sunshine P. Sugar hydrolases of the infant rat intestine and their arrangement of the brush border membrane. Biochim Biophys Acta. 1979 Jun 13;554(1):234–248. doi: 10.1016/0005-2736(79)90021-x. [DOI] [PubMed] [Google Scholar]


