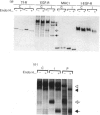
Abstract
The epidermal growth factor (EGF) receptor is down-regulated during early infection with adenovirus, and this has been attributed to accelerated internalization and degradation of the receptor in the absence of ligand (Carlin, Tollefson, Brady, Hoffman & Wold (1989) Cell 57, 135-144]. Using pulse-chase analysis, we show that loss of functional EGF receptors after infection of human KB and A431 cells with adenovirus type 5 is accompanied by accumulation of a receptor precursor that remains fully sensitive to endoglycosidase H, indicative of retention in the endoplasmic reticulum. A truncated receptor, normally secreted by A431 cells, also accumulates intracellularly as an endoglycosidase H-sensitive precursor. In no case is the block in intracellular transport of EGF receptors complete. We conclude that both stimulation of EGF receptor internalization and degradation and inhibition of intracellular transport of newly synthesized EGF receptors from the endoplasmic reticulum towards the cell surface contribute to EGF receptor down-regulation in adenovirus-infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Bellot F., Moolenaar W., Kris R., Mirakhur B., Verlaan I., Ullrich A., Schlessinger J., Felder S. High-affinity epidermal growth factor binding is specifically reduced by a monoclonal antibody, and appears necessary for early responses. J Cell Biol. 1990 Feb;110(2):491–502. doi: 10.1083/jcb.110.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. IL-1 and TNF transmodulate epidermal growth factor receptors by a protein kinase C-independent mechanism. J Immunol. 1989 Jan 1;142(1):126–133. [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987 Jul;6(7):2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Knowles B. B. Biosynthesis and glycosylation of the epidermal growth factor receptor in human tumor-derived cell lines A431 and Hep 3B. Mol Cell Biol. 1986 Jan;6(1):257–264. doi: 10.1128/mcb.6.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Tollefson A. E., Brady H. A., Hoffman B. L., Wold W. S. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989 Apr 7;57(1):135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Collins M. K., Sinnett-Smith J. W., Rozengurt E. Platelet-derived growth factor treatment decreases the affinity of the epidermal growth factor receptors of Swiss 3T3 cells. J Biol Chem. 1983 Oct 10;258(19):11689–11693. [PubMed] [Google Scholar]
- Cummings R. D., Soderquist A. M., Carpenter G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J Biol Chem. 1985 Oct 5;260(22):11944–11952. [PubMed] [Google Scholar]
- Donato N. J., Gallick G. E., Steck P. A., Rosenblum M. G. Tumor necrosis factor modulates epidermal growth factor receptor phosphorylation and kinase activity in human tumor cells. Correlation with cytotoxicity. J Biol Chem. 1989 Dec 5;264(34):20474–20481. [PubMed] [Google Scholar]
- Gabathuler R., Kvist S. The endoplasmic reticulum retention signal of the E3/19K protein of adenovirus type 2 consists of three separate amino acid segments at the carboxy terminus. J Cell Biol. 1990 Nov;111(5 Pt 1):1803–1810. doi: 10.1083/jcb.111.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. L., Ullrich A., Wold W. S., Carlin C. R. Retrovirus-mediated transfer of an adenovirus gene encoding an integral membrane protein is sufficient to down regulate the receptor for epidermal growth factor. Mol Cell Biol. 1990 Oct;10(10):5521–5524. doi: 10.1128/mcb.10.10.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Hudson L. G., Santon J. B., Glass C. K., Gill G. N. Ligand-activated thyroid hormone and retinoic acid receptors inhibit growth factor receptor promoter expression. Cell. 1990 Sep 21;62(6):1165–1175. doi: 10.1016/0092-8674(90)90393-s. [DOI] [PubMed] [Google Scholar]
- Iwashita S., Mitsui K., Shoji-Kasai Y., Senshu-Miyaike M. cAMP-mediated modulation of signal transduction of epidermal growth factor (EGF) receptor systems in human epidermoid carcinoma A431 cells. Depression of EGF-dependent diacylglycerol production and EGF receptor phosphorylation. J Biol Chem. 1990 Jun 25;265(18):10702–10708. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Lax I., Bellot F., Honegger A. M., Schmidt A., Ullrich A., Givol D., Schlessinger J. Domain deletion in the extracellular portion of the EGF-receptor reduces ligand binding and impairs cell surface expression. Cell Regul. 1990 Jan;1(2):173–188. doi: 10.1091/mbc.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Vass W. C., Schiller J. T., Lowy D. R., Velu T. J. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989 Oct 6;59(1):21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- Mayes E. L., Waterfield M. D. Biosynthesis of the epidermal growth factor receptor in A431 cells. EMBO J. 1984 Mar;3(3):531–537. doi: 10.1002/j.1460-2075.1984.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Petti L., Nilson L. A., DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991 Apr;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Nilsson T., Peterson P. A. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9665–9669. doi: 10.1073/pnas.83.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slieker L. J., Lane M. D. Post-translational processing of the epidermal growth factor receptor. Glycosylation-dependent acquisition of ligand-binding capacity. J Biol Chem. 1985 Jan 25;260(2):687–690. [PubMed] [Google Scholar]
- Slieker L. J., Martensen T. M., Lane M. D. Synthesis of epidermal growth factor receptor in human A431 cells. Glycosylation-dependent acquisition of ligand binding activity occurs post-translationally in the endoplasmic reticulum. J Biol Chem. 1986 Nov 15;261(32):15233–15241. [PubMed] [Google Scholar]
- Soderquist A. M., Carpenter G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J Biol Chem. 1984 Oct 25;259(20):12586–12594. [PubMed] [Google Scholar]
- Stroobant P., Rice A. P., Gullick W. J., Cheng D. J., Kerr I. M., Waterfield M. D. Purification and characterization of vaccinia virus growth factor. Cell. 1985 Aug;42(1):383–393. doi: 10.1016/s0092-8674(85)80133-1. [DOI] [PubMed] [Google Scholar]
- Thompson K. L., Assoian R., Rosner M. R. Transforming growth factor-beta increases transcription of the genes encoding the epidermal growth factor receptor and fibronectin in normal rat kidney fibroblasts. J Biol Chem. 1988 Dec 25;263(36):19519–19524. [PubMed] [Google Scholar]
- Tollefson A. E., Krajcsi P., Yei S. P., Carlin C. R., Wold W. S. A 10,400-molecular-weight membrane protein is coded by region E3 of adenovirus. J Virol. 1990 Feb;64(2):794–801. doi: 10.1128/jvi.64.2.794-801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Verheijden G. F., Verlaan I., van Iersel M. J., Moolenaar W. H. Second messenger modulation of epidermal growth factor receptor function does not occur at the level of receptor dimerization. Biochem J. 1990 Oct 1;271(1):215–221. doi: 10.1042/bj2710215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W., Gill G. N., Spiess J. Production of an epidermal growth factor receptor-related protein. Science. 1984 Apr 20;224(4646):294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- Yan D. H., Chang L. S., Hung M. C. Repressed expression of the HER-2/c-erbB-2 proto-oncogene by the adenovirus E1a gene products. Oncogene. 1991 Feb;6(2):343–345. [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. H., Scorsone K., Hung M. C. Adenovirus type 5 E1A gene products act as transformation suppressors of the neu oncogene. Mol Cell Biol. 1991 Mar;11(3):1745–1750. doi: 10.1128/mcb.11.3.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Suen T. C., Yan D. H., Chang L. S., Hung M. C. Transcriptional repression of the neu protooncogene by the adenovirus 5 E1A gene products. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4499–4503. doi: 10.1073/pnas.87.12.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn M., Geurts van Kessel A. H., Kroezen V., van Agthoven A. J., Verstijnen K., Terhorst C., Hilgers J. Localization of a gene controlling the expression of the human transferrin receptor to the region q12 leads to qter of chromosome 3. Cytogenet Cell Genet. 1983;36(3):525–531. doi: 10.1159/000131967. [DOI] [PubMed] [Google Scholar]