Abstract
Binding of the extracellular subunit of human immunodeficiency type 1 (HIV-1) envelope (Env) glycoprotein (gp120) to CD4 triggers the induction or exposure of a highly conserved coreceptor binding site in gp120 that helps mediate membrane fusion. Characterizing the structural features involved in gp120-coreceptor binding and the conditions under which binding occurs is important for understanding the fusion process, the evolution of pathogenic strains in vivo, the identification of novel anti-HIV compounds, and the development of HIV vaccines that utilize triggered structures of Env. Here we use the kinetics of interaction between CCR5 and gp120 to understand temporal and structural changes that occur during viral fusion. Using saturation binding and homologous competition analysis, we estimated the Kd of interaction between CCR5 and gp120 from the macrophage tropic HIV-1 strain JRFL to be 4 nM. Unlike Env-mediated fusion, gp120 binding to CCR5 did not require divalent cations or elevated temperatures. Binding was not significantly affected by the pH of binding, G-protein coupling of CCR5, or partial gp120 deglycosylation. Oligomeric, uncleaved JRFL gp140 failed to bind CCR5 despite its ability to bind CD4 and monoclonal antibody 17b, suggesting that the uncleaved ectodomain of gp41 interferes with full exposure of the chemokine receptor binding site. Exposure of the chemokine receptor binding site on gp120 could be induced rapidly by CD4, but exposure of this site was lost upon CD4 dissociation from gp120, indicating that the conformational changes in gp120 induced by CD4 binding are fully reversible. The functional gp120-soluble CD4 complex was remarkably stable over time and temperature ranges, offering the possibility that complexes in which the highly conserved coreceptor binding site in gp120 is exposed can be used for vaccine development.
The envelope (Env) protein of human immunodeficiency virus type 1 (HIV-1) is responsible for binding virus to the cell surface and for mediating fusion between the viral and cellular membranes. HIV-1 Env is produced as a gp160 precursor that is subsequently cleaved into two noncovalently associated subunits, the gp120 surface subunit responsible for receptor binding and the gp41 transmembrane subunit responsible for mediating membrane fusion (66). The first step in viral fusion is binding of gp120 to its primary receptor, CD4 (7, 30, 38). CD4 binding results in a conformational change in gp120 (53, 55) that enables it to interact with a coreceptor, generally either CCR5 or CXCR4 (34, 60, 63). Coreceptor binding is thought to lead to additional conformational changes that allow the gp41 subunit to mediate lipid bilayer mixing. Simian immunodeficiency virus (SIV) likewise uses CD4 and coreceptors to infect cells (6, 15, 39), though some SIV strains can use CCR5 to infect cells in the absence of CD4 (17).
While the interaction between gp120 and CD4 is now known in atomic detail (32), the interaction between gp120 and the coreceptors is less well understood. Coreceptor usage by a given virus strain is governed largely by the V3 loop and to a lesser extent by the V1/V2 variable loops of gp120 (reviewed in reference 2). However, the recently solved crystal structure of a gp120 core fragment (32) has led to the identification of an extraordinarily well conserved region in gp120 that has been implicated in CCR5 binding (51). This conserved CCR5 binding site, located on the bridging sheet between the inner and outer domains of gp120 and flanked by the bases of the V3-loop and V1/V2 region, is sequestered in the native state but is exposed and/or formed upon CD4 binding (65). Constitutive exposure of this region as a consequence of mutations in HIV-1 gp120 results in virus strains that are much more sensitive to antibody-mediated neutralization (26). In addition, exposure of this region may help explain the recent demonstration that coreceptor-triggered Envs can elicit broadly cross-reactive neutralizing antibodies against primary HIV-1 isolates (33). Thus, characterizing the structural interactions between gp120 and the coreceptors may provide new opportunities for the development of novel HIV immunogens as well as small-molecule inhibitors.
Analysis of the kinetics and affinity of gp120-CD4 binding has helped explain differences between lab-adapted and primary isolate strains of HIV-1 in their resistance to soluble CD4 (27, 42, 43, 46), the effects of temperature on Env inactivation (23, 45), and the adaptation of HIV growth in tissue culture (41, 44). The relationship of gp120-coreceptor affinity and binding kinetics to disease pathogenesis and the immune response has not yet been investigated to the same degree, but the affinity between gp120 and CCR5 has already been implicated in the evolution of disease pathogenesis in at least one nonhuman primate model of viral infection (29).
The use of radiolabeled forms of gp120 for direct chemokine receptor binding assays has been a catalyst for understanding the structural interactions between Env and the coreceptors (40, 51, 63, 64). In this study, we used the kinetics of interaction between CCR5 and gp120 to understand temporal and structural changes that occur during viral fusion. Pharmacological analyses of the interaction between HIV-1 JRFL gp120 and CCR5, including saturation binding, competition analysis, as well as association and dissociation kinetics, revealed a strong interaction between JRFL and CCR5 with a Kd of approximately 4 nM. Binding of JRFL gp120 to CCR5 was CD4 dependent, did not require divalent cations or G-protein coupling, and was minimally dependent on temperature, pH, and the presence of N-linked carbohydrates. Exposure of the chemokine receptor binding site on JRFL gp120 required Env cleavage, was induced rapidly upon binding of CD4, and was reversible upon dissociation of CD4. However, once bound to CD4, gp120 retained the ability to interact with coreceptors for an extended period of time, indicating that exposure of the conserved coreceptor binding region is compatible with a stable, long-lived structure. Consistent with this was the observation that the CD4-gp120 complex was remarkably resistant to heat denaturation, allowing such complexes to be considered for vaccine potential as well as for high-throughput assays to identify gp120-coreceptor antagonists.
MATERIALS AND METHODS
Cells and reagents.
The human kidney cell line 293T was provided by Paul Bates (University of Pennsylvania) and maintained in DMEM (Dulbecco’s modified Eagle medium, high glucose) supplemented with 10% fetal bovine serum, 2 mM glutamine, and 2 mM penicillin-streptomycin. Where indicated, cells were treated overnight with pertussis toxin (250 ng/ml; Sigma). CCR5-DNY was constructed in pcDNA3 by using a QuickChange mutagenesis kit (Stratagene) to mutate the conserved G-protein coupling motif Asp-Arg-Tyr in wild-type CCR5 to Asp-Asn-Tyr.
Vaccinia virus construction and protein production.
A recombinant vaccinia virus producing JRFL gp120 (vBD6) was prepared by introducing a stop codon at the cleavage site of full-length JRFL gp160 under the control of the vaccinia virus synthetic early/late promoter in plasmid pSC59 (pCB28 encoding JRFL gp160 was kindly provided by Chris Broder). A vaccinia virus expressing JRFL gp140 (vBD5) was prepared in a similar manner but by introducing a stop codon at the first transmembrane codon of JRFL Env. Recombinant vaccinia viruses were prepared from parental wild-type strain WR by standard techniques (14) but by performing recombinations in transfected/infected 293T cells. We note that recombination in 293T cells was approximately fivefold more efficient than recombination in the CV-1 cells normally used or in the parental 293 cells without the simian virus 40 large T antigen. For protein production, vaccinia virus was used to infect 293T cells at a multiplicity of infection of 10. Again, 293T cells were capable of producing two- to fivefold more protein than other cell types tested, including HeLa, BHK, and 293 cells. Cells were exposed to virus for 2 h, washed twice with phosphate-buffered saline (PBS), and placed in serum-free DMEM; 24 h postinfection, the supernatant was harvested, clarified by centrifugation at 500 × g, filtered through a 0.45-μm-pore-size filter, and virus inactivated with 0.1% Triton X-100. The recombinant protein was purified by lectin chromatography using Galanthus nivalis-lectin coupled agarose beads (Vector Laboratories) as described elsewhere (13). Protein was determined to be >90% pure by Coomassie blue staining and >90% intact by Western blot analysis. Protein concentrations were determined using a Pierce BCA protein concentration kit (Rockford, Ill.). JRFL gp120 concentration and purity were also assessed by amino acid and high-pressure liquid chromatography analyses for accurate quantification. HXB2 gp120 was produced by using similar techniques (26). Env proteins from the clade E HIV-1 CM235, SIVmac239, and HIV-1 MN, as well as soluble CD4, were obtained from the NIH AIDS Research and Reference Program.
Where indicated, protein was deglycosylated by a method used previously (31) by addition of 0.4 mU of endoglycosidase D (endo D; Calbiochem), 20 mU of endo H, and 20 mU of neuraminidase (Boehringer Mannheim) and incubation at 37°C for 3 h. Additional enzyme was then added, and the mixture was incubated for an additional 3 h at 37°C and then overnight at room temperature (RT). Nondeglycosylated control samples were treated identically but without the addition of enzyme. Fully deglycosylated samples were treated identically except that 0.1 M β-mercaptoethanol, 0.5 M NaCl, and 0.1% Triton X-100 were also included to ensure complete exposure of gp120 glycosylation sites, as recommended by the manufacturer.
Iodination and binding.
Soluble JRFL gp120 was iodinated by using Iodogen (Pierce). Specific activities of 500 to 2,000 Ci/mmol were obtained by using 5 μg of protein with 500 μCi of Na125I for 20 min in 5-ml glass tubes precoated with 10 μg of Iodogen by chloroform evaporation of a 100 μl volume of Iodogen under a slow stream of nitrogen gas. Alternatively, proteins were labeled using Iodobead (Pierce) iodination of 10 μg of gp120 for 5 min in a 150-μl volume of PBS, using 250 μCi of Na125I preincubated for 5 min with one Iodobead. Proteins were also labeled successfully by lactoperoxidase and Bolton-Hunter iodination, but Iodobead labeling was used routinely due to its convenience and reliability. Critically, 125I incorporation rates of greater than approximately 50% resulted in oxidative destruction of gp120 that was no longer capable of binding any receptor. Radiolabeled proteins were purified from free Na125I by separation through a 0.3-ml Dowex column prepared in a 1-ml syringe and preequilibrated in a mixture containing 50 mM HEPES (pH 7.4), 5 mM MgCl2, 1 mM CaCl2, 1% bovine serum albumin (BSA), and 150 mM NaCl. Protein fractions were eluted in the void volume of the column, and the fractions containing peaks of labeled protein were combined. Env integrity after radiolabeling was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography (data not shown).
Env binding assays were performed by resuspending cells in 75 μl of HEPES++ binding buffer (50 mM HEPES [pH 7.4], 5 mM MgCl2, 1 mM CaCl2, 5% BSA, 0.1% NaN3). Labeled protein was added to cells in 25 μl of binding buffer for a total volume of 100 μl. Cells were incubated at RT for 1 h unless specified. Unbound radioactivity was removed by filtering cells through 25-mm Whatman GF/C filters presoaked in 0.2% polyethyleneimine (Sigma) and washing them two times with 4 ml of wash buffer (50 mM HEPES [pH 7.4], 500 mM NaCl, 5 mM MgCl2, 1 mM CaCl2). Filters were counted in a Wallac 1470 Wizard gamma counter. Calculations of the data were made with GraphPad Prism.
RESULTS
Binding of gp120 to coreceptors.
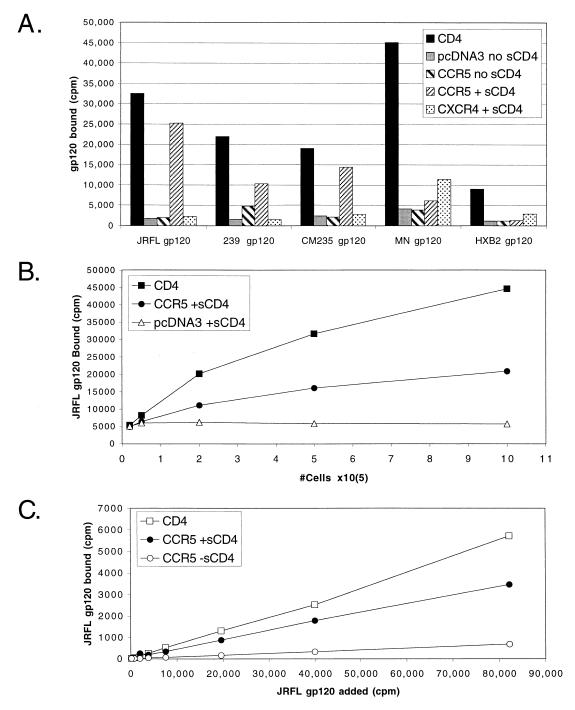
To characterize the interaction between the extracellular Env subunit (gp120) of HIV and SIV Env proteins with coreceptors, we used radiolabeled gp120 in a modified binding assay that has previously been used to detect chemokine (52) and gp120 binding to CCR5 (40, 63, 64). Purified gp120 proteins were obtained or made from the macrophage tropic (M-tropic) HIV-1 strains JRFL and CM235, the T-cell-tropic (T-tropic) HIV-1 strains MN and HXB2, and SIVmac239. All proteins were iodinated and found capable of binding full-length CD4 expressed on the surface of transiently transfected cells (Fig. 1A), thus demonstrating their conformational integrity. Binding of JRFL gp120 to CD4 was also confirmed by SDS-PAGE and autoradiography (see Fig. 7).
FIG. 1.
(A) Binding of gp120 to chemokine receptors. Radioiodinated Env proteins were tested for binding to 2 × 105 to 5 × 105 293T cells transiently transfected with plasmids expressing full-length CD4, CCR5, or CXCR4 or with the parental vector pcDNA3. Binding was performed at RT for 1 h in the presence or absence of 100 nM sCD4 as indicated. Raw values of representative experiments repeated at least three times are shown without background subtraction. The strains used were M-tropic HIV-1 strains JRFL and CM235, T-tropic HIV-1 strains MN and HXB2, and SIVmac239. (B and C) Optimization of JRFL gp120 binding to CCR5. The binding assay was optimized by varying conditions such as cell number (B) or the amount of radiolabeled gp120 added (C). Results are from representative experiments, repeated at least twice, without any background subtraction. Optimization of sCD4 concentrations are shown in Fig. 8B. Optimal specific binding was achieved with 5% BSA, but signal/noise values of greater than 5:1 could also be achieved with 0.1% BSA (data not shown), enabling further modification of this assay for applications such as high-throughput screening. Unless otherwise indicated, all assays were performed with 2 × 105 cells, 100 nM sCD4, and 5% BSA in HEPES++ binding buffer, for 1 h at RT, and with wash buffer containing 500 mM NaCl.
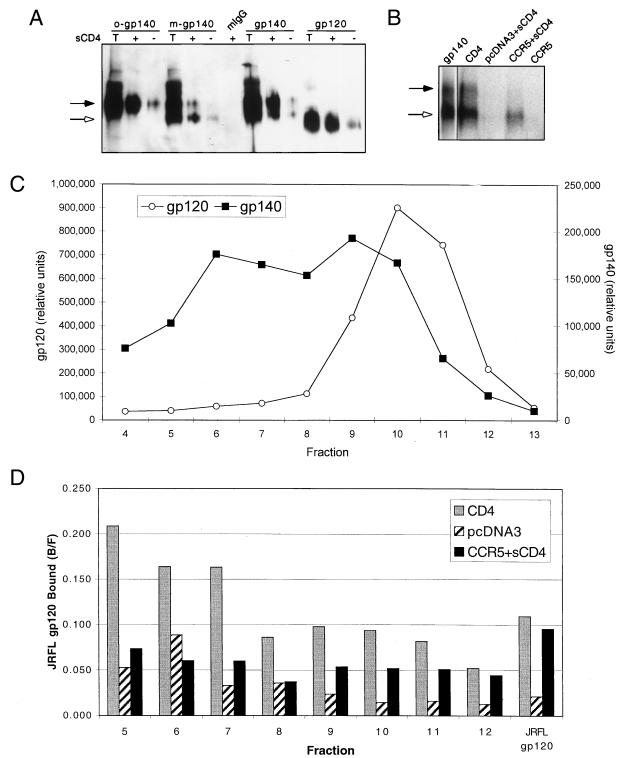
FIG. 7.
(A) Reactivity of JRFL gp140 with MAb 17b. Purified JRFL gp140 protein was fractionated by sucrose gradient sedimentation. Representative fractions containing exclusively oligomeric (o-gp140) or exclusively monomeric (m-gp140) gp140 were immunoprecipitated with the MAb 17b or with mouse IgG (mIgG) in either the presence (+) or the absence (−) of sCD4. Unfractionated gp140 and gp120 samples were also immunoprecipitated. Briefly, 50 μl of sucrose gradient fractions or 100 ng of purified protein was combined with 500 ng of sCD4 (where indicated). After 30 min at room temperature, 20 μl of ProG beads, 1 μg of MAb, 20 μl of 5% BSA, and PBS were added for a total volume of 400 μl, rocked at RT for 2 h, and washed twice with PBS. The samples were run on an 8% SDS-polyacrylamide gel along with a sample of the total input protein (T) and probed with an anti-gp120 rabbit sera by Western analysis. We note that gp120 produced as a gp140 precursor migrated slower than gp120 produced alone, most likely due to differences in glycosylation. (B) Binding of gp140 to CCR5. Radiolabeled JRFL gp140 was bound to 5 × 105 cells expressing the indicated receptors for 2 h as described in Materials and Methods. Instead of being washed through glass fiber filters, however, cells were washed twice with 1 ml of wash buffer in Eppendorf tubes. The protein bound to the cells was detected by lysing the cells in 25 μl of 0.5% Triton X-100, spinning out the cell debris, loading the supernatant onto an 8% SDS-polyacrylamide gel, and visualizing the protein by autoradiography. Approximately 25% of gp140 is binding to CCRS compared to CD4, as measured by PhosphorImager analysis and adjusting for total Env. The gp140 lane is a separate exposure of the initial protein added. The black arrows in panels A and B indicate the location of gp140, and the white arrows indicate the location of gp120. (C) Radiolabeled JRFL gp140 was separated through a 5 to 20% continuous sucrose gradient as previously described (13). A sample of each fraction was loaded onto an SDS-polyacrylamide gel, separated, and visualized by autoradiography. The amount of gp120 and gp140 in each fraction was quantitated by PhosphorImager densitometry. (D) A sample of each fraction from the gp140 sucrose gradient in panel C was bound to cells expressing CD4, CCR5, or pcDNA3 as indicated. The fraction bound (bound/free [B/F]) is indicated for each sample. The results from early fractions appear relatively high in value due to the lower counts of radioactivity in these fractions. All results should therefore be evaluated relative to background (pcDNA3) binding. All results are from representative experiments repeated twice.
HIV-1 infection of most cell types is strictly dependent on CD4, and previous direct and indirect measurements of HIV-1 gp120 binding to CCR5 have also demonstrated a strict dependence on CD4 (40, 60, 63). To measure binding of gp120 directly to CCR5, we used a soluble form of CD4 containing the first four immunoglobulin (Ig)-like domains (sCD4). Binding of HIV-1 gp120s to CCR5 was strictly dependent on the presence of sCD4, as expected (Fig. 1A). Binding of SIVmac239 gp120 to CCR5 was maximal in the presence of sCD4 but, with sensitive measurement conditions, was also detected without sCD4, as has been previously demonstrated (40). gp120 preparations from SIVmac239 and M-tropic strains of HIV-1 (JRFL and CM235) that use only CCR5 as a coreceptor (61) bound to CCR5 but not to CXCR4. In contrast, gp120 preparations from X4 strains of HIV-1 (HXB2 and MN) that primarily use CXCR4 as a coreceptor exhibited little if any binding to CCR5 but exhibited a low but highly reproducible degree of binding to CXCR4 in the presence of sCD4 (Fig. 1A). As we have noted previously (12), binding of X4 gp120s to CXCR4 is not as robust as binding of R5 gp120s to CCR5. We have also demonstrated the specificity of this assay by competing JRFL gp120 binding with CCR5-specific monoclonal antibodies (MAbs) and CCR5 chemokine ligands (3, 36).
Optimization of binding conditions.
The kinetics of interaction between gp120, CD4, and the coreceptors, as well as the biochemical relationship of coreceptor binding to viral fusion, has not yet been described. Because a robust gp120 binding assay with high sensitivity is critical for these measurements and for further applications, and also because the conformational complexity of gp120, its sensitivity to denaturation, and extensive glycosylation make iodination binding measurements more difficult to optimize than for many other proteins, we report the details of our binding conditions. Previously published direct binding assays have yielded signal-to-noise values of up to 4:1 (40, 63). By optimizing sCD4 concentrations, cell number, BSA concentration, washing conditions, and incubation times, we obtained signal-to-noise values of greater than 15:1. Because of its wide use in previous coreceptor studies as a prototypic M-tropic HIV-1, gp120 from the JRFL strain of HIV-1 was used to optimize the binding assay. Equimolar amounts of sCD4 and gp120 allowed 40 to 50% of maximal binding, and sCD4 concentrations 5- to 10-fold above that of gp120 concentrations allowed greater than 80% of maximal binding to be achieved (data not shown; see Fig. 8B). To ensure that sCD4 was not limiting, 100 nM sCD4 was used for all other experiments; unless specified, all experiments were performed for 1 h at RT.
FIG. 8.
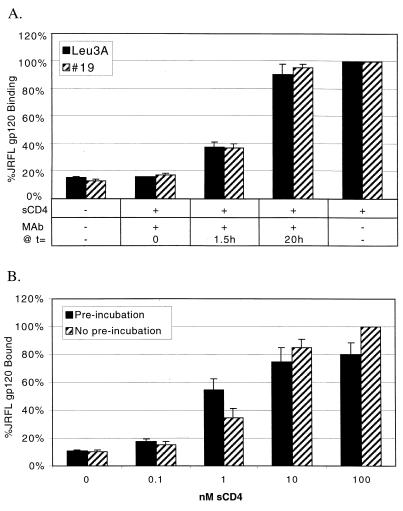
Conformational changes in gp120 are lost upon CD4 dissociation. (A) JRFL gp120 (0.36 nM) was preincubated at RT with sCD4 (5 nM) starting at t = 0 for 1.5 h. Two different CD4 MAbs (63 nM), Leu3A, and #19, were added at t = 0 before addition of sCD4, at t = 1.5 h after incubation of gp120 and sCD4 for 1.5 h, or at t = 20 h after incubation of gp120 and sCD4 for 20 h. At t = 20 h, gp120-sCD4 complexes were added to CCR5-expressing cells, allowed to bind for 1 h, and harvested. −, not added; +, added. (B) JRFL gp120 (1 nM) and increasing amounts of sCD4 were either preincubated at RT for 20 h or not preincubated but treated identically. The complexes were then used for binding to 2 × 105 CCR5-expressing cells for 1 h at RT. When binding was conducted for 2 h, “No pre-incubation” samples reached the same plateau values as preincubation samples (data not shown). Results represent the average and range of two independent experiments without background subtraction.
Signals, but not background values, could be increased substantially by using greater numbers of cells (Fig. 1B), though use of more than 2 × 106 cells resulted in inefficient filtration through glass fiber filters, resulting in decreased signal-to-noise ratios. As few as 105 cells could be used for detection of JRFL gp120 (Fig. 1B and data not shown). Assuming 105 gp120 binding sites per cell (see Fig. 4), we calculate that 1010 total binding sites allowed detection of JRFL gp120 binding. Subsequent assays typically used 2 × 105 to 4 × 105 cells in order to ensure binding of 5 to 10% of free ligand, as required for accurate measurement of pharmacological parameters. At JRFL gp120 concentrations of <4 nM, binding of JRFL gp120 to both CD4 and CCR5 was linearly proportional to the added amount of labeled gp120, even at minimal levels of radioactivity near the detection limits of our gamma counter (Fig. 1C). Signal-to-noise ratios were comparable throughout the range of gp120 concentrations tested. The amount of BSA present during the binding reaction increased specific binding, although total binding counts were reduced (data not shown). Concentrations of BSA greater than 7.5% resulted in inefficient filtration of cells through glass fiber filters. Therefore, 5% BSA was used in all subsequent experiments. Binding was optimal between pH 7 and 8, but significant binding could also be measured at pH as low as 5 (data not shown).
FIG. 4.
(A) Saturation binding of JRFL gp120 to CCR5. An increasing amount of iodinated JRFL gp120 (586 Ci/mmol) was added to 105 cells transiently transfected with CCR5 or pcDNA3 vector alone. Binding was conducted in the presence or absence of 100 nM sCD4. The saturation binding curve shown was fit by nonlinear regression after subtracting nonspecific binding of gp120 to CCR5 in the absence of sCD4 from total binding to CCR5 with sCD4. Identical results were obtained when pcDNA3-transfected cells were used as the reference for nonspecific binding. Results are from a representative experiment repeated three times. Bmax, maximum binding. (B) Competition binding of JRFL gp120 to CCR5. Iodinated JRFL gp120 (0.35 nM) was bound to 105 cells transiently transfected with CCR5 in the presence of increasing amounts of cold JRFL gp120. Binding was conducted in the presence of 100 nM sCD4. Increased levels of sCD4 did not alter the binding profile, and competition with BH8 gp120 (X4) had no effect on JRFL gp120 binding (data not shown). Binding curves were also conducted in the absence of cells (No Cells), with cells transfected with pcDNA3 vector only (data not shown), or without sCD4 (data not shown), with similar negative results.
Wash conditions dramatically influenced binding detection, with 500 mM NaCl being optimal. NaCl concentrations of ≤150 mM dramatically increased background binding levels, while 1 M NaCl was not significantly better than 0.5 M NaCl. GF/C glass fiber filters were blocked with 0.2% polyethylenimine, but GF/B filters and alternative treatment with 0.1% Tween 20–0.5% pyrrolidone–0.5% BSA (56) increased specific binding signals in some cases by 10 to 20%. We have also separated unbound ligand by spinning cells through a mixture of 81% silicone–19% paraffin oil with signal-to-noise values of nearly 20:1 (data not shown). However, this approach does not lend itself as readily to high-throughput analysis. Binding times and temperature conditions are addressed below. The specific activity of iodinated gp120 was an important determinant of signal-to-noise values, with freshly iodinated JRFL gp120 (e.g., 1,100 Ci/mmol) yielding values of >15:1 (Fig. 1A). We would expect that gp120 proteins with higher affinities for CCR5 would yield even higher signal-to-noise values under the conditions used here.
Association and dissociation kinetics.
To measure the rate at which gp120-sCD4 complexes bound CCR5, we harvested identical binding experiments at various time points after mixing of JRFL gp120-sCD4 complexes with CCR5-expressing cells (Fig. 2A). Since HIV-1 gp120 requires CD4 for binding to CCR5, our kinetic analyses measure, by definition, a three-component interaction involving a series of conformational changes that are not directly amenable to traditional kinetic analysis. To test if the conformational changes in gp120 induced by sCD4 are rate limiting, we preincubated JRFL gp120 and sCD4 for either 1 h or overnight prior to the addition of CCR5-positive cells. We used saturating amounts of sCD4 (100 nM sCD4 and 0.37 nM gp120) to ensure that the binding of gp120 to sCD4 was not rate limiting.
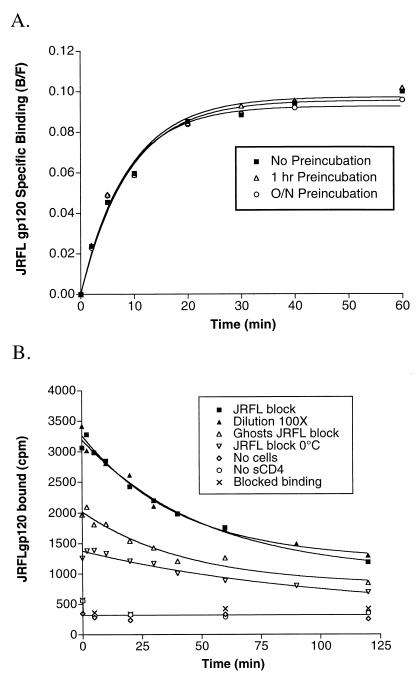
FIG. 2.
(A) Association binding of JRFL gp120 to CCR5. gp120 (0.37 nM, final concentration) and sCD4 (100 nM, final concentration) were incubated at RT either overnight, for 1 h, or not at all before addition to cells expressing CCR5 at t = 0 in a total volume of 100 μl. Binding was stopped by harvesting samples at the indicated time points. Results are from a representative experiment repeated twice and analyzed by fitting data to a single-phase exponential association curve (GraphPad Prism). (B) Dissociation binding of JRFL gp120 to CCR5. Iodinated JRFL gp120 (0.28 nM) was bound to 2 × 105 cells expressing CCR5 for 1 h. After 1 h, binding was stopped by adding an excess of cold JRFL gp120 (200 nM, final concentration; JRFL block) or by diluting the reaction volume 100-fold (Dilution 100×), with identical results. Binding was also conducted on ice (JRFL block 0°C) and with osmotically lysed cell ghosts (Ghosts JRFL block). No binding was observed in the absence of cells (No cells), without sCD4 (No sCD4), or when 200 nM JRFL gp120 was added prior to addition of radiolabeled gp120 (Blocked binding). Unless otherwise indicated, binding was performed at RT in the presence of 100 nM sCD4 and was stopped at t = 0 by adding excess cold JRFL gp120. Results are from representative experiments repeated at least twice and analyzed by fitting data to a single-phase exponential dissociation curve (GraphPad Prism).
Our results indicate that our experimental conditions permitted full binding equilibrium to be reached, with a half-life (t1/2) of 5.8 ± 1.1 min. Preincubation of gp120 with sCD4 did not affect binding rates, indicating that the conformational changes induced in gp120 by CD4 occur rapidly beyond the time resolution of these experiments. In addition, the ability of the complex to bind CCR5 after 16 h of incubation with sCD4 indicates that the conformational changes in gp120 induced by CD4 are stable. This conclusion is important given the potential use of triggered-Env complexes for vaccine development (33).
To determine the rate of JRFL gp120-CCR5 dissociation, JRFL gp120-sCD4 complexes were first bound to cells expressing CCR5 for 1 h in the presence of 0.1% NaN3 to inhibit receptor internalization. Dissociation rates were measured either by the addition of a 100-fold excess of cold JRFL gp120 or by diluting cells 100-fold in binding buffer. These approaches gave t½ values of 35.9 and 28.5 min, respectively (Fig. 2B). Dissociation kinetics were best fit to a one-site dissociation model rather than a two-site model (data not shown). More than 95% of bound gp120 could be removed by acid washing with buffer at pH 3, indicating that we were measuring reversible binding to cell surface receptors (data not shown). We have also successfully used membranes from cells expressing CCR5 that were osmotically lysed (and are thus incapable of receptor internalization) for binding and dissociation measurements (Fig. 2B, Ghosts JRFL block), indicating that such membranes are capable of gp120 binding and may be considered for use in large-scale screening applications.
Temperature dependence of CCR5 binding.
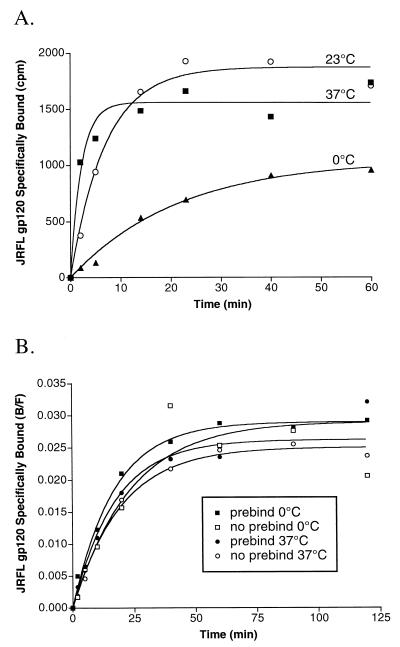
HIV Env binding to CD4 can occur at 0°C (21, 53), but Env-mediated membrane fusion requires temperatures above 25°C (21). To determine if CCR5 binding is a temperature-dependent event that restricts Env-mediated fusion, we conducted ligand association experiments at 37°C, at RT, and on ice (Fig. 3A). JRFL gp120-sCD4 complexes were generated by incubation on ice for 15 min prior to addition to CCR5-positive cells at the indicated temperature. Binding of gp120-sCD4 complexes to CCR5 at 37°C (t½ = 1.6 min) occurred threefold faster than at room temperature (t½ = 4.9 min) and ninefold faster than at 0°C (t½ = 14.8 min). While binding of gp120-sCD4 to CCR5 on ice was slow, over a 1-h binding period the amount of bound gp120-sCD4 approached plateau values >50% of the values obtained at higher temperatures. These results indicate that binding of gp120 to CCR5, as well as the conformational changes induced in gp120 by binding of CD4, do not exhibit a strict temperature-dependent threshold, as does membrane fusion.
FIG. 3.
(A) Temperature requirements for association binding of JRFL gp120. Association binding of JRFL gp120 to cells expressing CCR5 was conducted at either 37°C, 23°C (RT) or 0°C (on ice). The slightly decreased binding maximum at 37°C is likely due to slight denaturation of gp120 (Fig. 9). (B) Pretriggering of gp120 with sCD4. JRFL gp120 was preincubated at 37°C or kept on ice (0°C) for 15 min in the presence (prebind) or absence (no prebind) of sCD4 before performance of association binding experiments at 0°C with CCR5-expressing cells. This experiment was performed on ice to maximize the detection of potential differences in association kinetics. The final concentration of sCD4 for all experiments was 100 nM. All results are from a representative experiment repeated twice and analyzed by fitting data to a single-phase exponential association curve (GraphPad Prism).
While binding of gp120 to CCR5 occurs faster at 37°C than at lower temperatures, it is not clear if the increased rate is due to faster association of the gp120-sCD4 complex with CCR5 or possibly due to an increased rate of gp120 structural changes induced by CD4. We reasoned that if such a CD4-induced structural change is a limiting step for CCR5 binding, a brief pulse of the gp120-sCD4 complex at an elevated temperature might subsequently enable it to bind CCR5 at a faster rate even at reduced temperatures. To address this, JRFL gp120-sCD4 complexes were incubated at 37°C for 15 min prior to addition to CCR5-positive cells on ice (Fig. 3B). We found that the rate of binding was unaffected. Thus, we conclude that the CD4-induced conformational changes in gp120 that enable CCR5 binding occur rapidly, beyond the time resolution of our experiments, and are not temperature dependent.
Affinity of JRFL gp120 for CCR5.
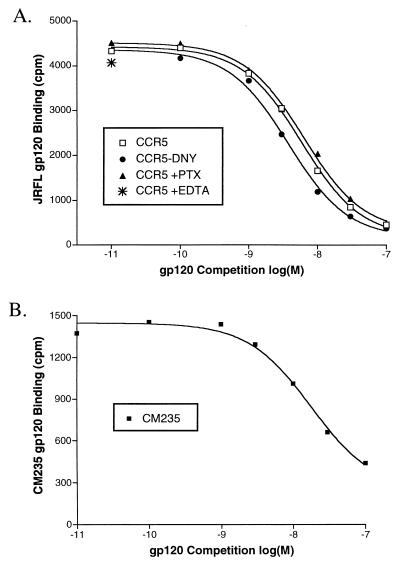
Previous characterizations of the interaction between gp120s and CCR5 have obtained Kd affinity values of 5 to 10 nM by homologous competition (40, 63). To assess the affinity between JRFL gp120 and CCR5 by using a separate quantitative technique, we took two approaches. In Fig. 4A, we added increasing amounts of radiolabeled JRFL gp120 to cells expressing CCR5. Background was defined as binding of gp120 to cells expressing pcDNA3 alone or as binding to cells expressing CCR5 but in the absence of sCD4, with identical results. Results of saturation binding were fitted with nonlinear regression to obtain a Kd of 4.35 ± 0.75 nM. In Fig. 4B, homologous competition was performed by using 0.35 nM radiolabeled gp120 and increasing levels of cold gp120. Results were fit by nonlinear regression to obtain values of 3.62 ± 1.20 nM (Fig. 4B), in excellent agreement with saturation binding results. The number of JRFL gp120 binding sites in transiently transfected cells, as measured both by saturation binding and by competition binding, varied between 50,000 and 150,000 sites per cell, depending on transfection conditions, in relative agreement with our measurement of the number of CCR5 sites per cell by using other methods and ligands (reference 37 and unpublished results). Thus, as few as 1010 total gp120 binding sites (105 cells with 105 sites/cell) could support detectable gp120 binding. To ensure that sCD4 was not limiting in these assays, a similar homologous competition experiment was performed with larger amounts of sCD4 (200 nM), with identical results (data not shown). In addition, no competition was observed when cold BH8 gp120 (an X4 gp120 that binds CD4 and uses CXCR4 but not CCR5) was used instead of JRFL gp120 (data not shown). Somewhat lower affinities were obtained for the clade E M-tropic strain CM235 (Kd, 12 nM) and SIVmac239 (Kd, 15 nM) when measured by homologous competition (Fig. 5B and data not shown), consistent with their generally lower signal-to-noise ratios (Fig. 1A). As we have noted elsewhere (1), the assay conditions described here are capable of measuring gp120 affinities of interaction as low as 30 nM.
FIG. 5.
(A) Conditions for JRFL gp120 binding to CCR5. Radioiodinated JRFL gp120 was competed with cold JRFL gp120 for binding to CCR5-expressing cells, CCR5 cells treated with pertussis toxin (PTX), or CCR5-DNY-expressing cells that are incapable of G-protein coupling and signaling. Binding curves are representative of a single experiment repeated at least twice that gave Kds of 5.7 nM (CCR5), 6.1 nM (PTX), and 3.6 nM (DNY). Also shown is binding to CCR5-expressing cells without divalent cations and in the presence of 10 mM EDTA. (B) Competition binding of CM235 gp120 to CCR5. Iodinated CM235 gp120 (3 nM) was bound to 5 × 105 cells transiently transfected with CCR5 in the presence of increasing amounts of cold CM235 gp120. Binding was conducted in the presence of 100 nM sCD4. Results are from a representative experiment repeated twice.
Conditions for gp120 binding.
Calcium ions are required for Env-mediated fusion in a post-CD4 binding step (11). To test whether this requirement is at the level of coreceptor binding, we conducted Env binding assays in a modified binding buffer containing no divalent cations and including 10 mM EDTA. These conditions had no apparent effect on JRFL gp120 binding to CCR5 (Fig. 5A), indicating that the requirement of divalent cations for HIV fusion is not at the level of coreceptor binding.
Since G-protein coupling can affect the extracellular structure of G-protein-coupled receptors (22), we also tested whether eliminating G-protein coupling would affect gp120 binding. We found no effect on the ability of JRFL gp120 to bind CCR5 in the presence of pertussis toxin (Fig. 5A), a condition that eliminates >95% of detectable signaling of CCR5 in these cells (data not shown). JRFL gp120 was also able to bind to CCR5-DNY, a form of CCR5 that is incapable of signaling and that cannot couple to G proteins due to mutation of the G-protein-coupling motif Asp-Arg-Tyr to Asp-Asn-Tyr (Fig. 5A) (19).
Effects of glycosylation on CCR5 binding.
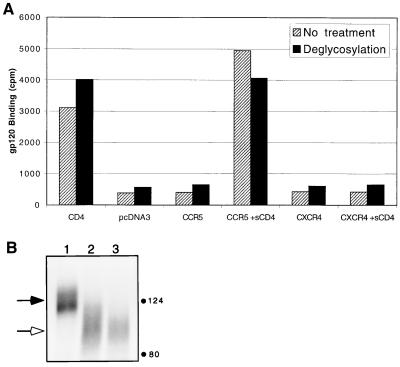
The native form of gp120 is highly glycosylated with the N-linked carbohydrates believed to shield conserved regions of the gp120 protein core, including the coreceptor binding site, from immune recognition (66). Mutation of select glycosylation sites can alter the immune recognition of gp120 and result in more potent neutralization of these viruses (26, 49), and recent reports implicate glycosylation sites as influencing CD4 independence (26). To test whether enzymatic deglycosylation of gp120 is sufficient to expose the chemokine receptor binding site in a native gp120 molecule, we enzymatically treated JRFL gp120 similarly to previously described treatments used in the crystallization of gp120 (31). Deglycosylation of gp120 had little or no effect on the ability of Env to bind CD4 or CCR5 (Fig. 6A). Of particular note, the deglycosylated form of JRFL gp120 still required sCD4 for CCR5 interaction, suggesting that deglycosylation alone does not expose the chemokine receptor binding site. We note, however, that our enzymatic treatment did not completely remove all carbohydrates (Fig. 6B). Complete deglycosylation as described elsewhere (31) could not be achieved, perhaps because the JRFL gp120 that we used possesses all variable loops and was produced in a vaccinia virus-based eukaryotic expression system. Complete deglycosylation could be achieved with endo F treatment (data not shown), but use of this gp120 in binding assays resulted in high background binding, consistent with the aggregation caused by endo F treatment noted previously (31).
FIG. 6.
(A) Binding of deglycosylated JRFL gp120. Iodinated JRFL gp120 protein was enzymatically treated to remove N-linked carbohydrates and then used for binding to cells expressing the indicated receptors. Results of representative experiment repeated three times are shown. (B) A sample of the deglycosylated gp120 used in panel A was run on an 8% SDS-polyacrylamide gel, autoradiographed, and then quantified by PhosphorImager analysis for extent of deglycosylation. Black arrow, undigested gp120; white arrow, deglycosylated gp120. Lane 1, control sample treated identically but without enzymes; lane 2, deglycosylated gp120 used in panel A; lane 3, sample deglycosylated in an identical manner but also in the presence of 0.1 M β-mercaptoethanol, 0.5 M NaCl, and 0.1% Triton X-100 to ensure optimal exposure of carbohydrate sites in gp120 to enzymes, as recommended by the manufacturer. Digestion of gp120 in lane 2 achieved 87% of the maximal deglycosylation under ideal conditions as achieved in lane 3. The predicted mass of the amino acids of JRFL gp120 without any deglycosylation is 57 kDa. No other gp120-specific bands were detected below 80 kDa.
JRFL gp140 oligomers do not bind CCR5.
Binding of Env on a virus to CD4 and CCR5 is fundamentally different than binding of gp120 due to the fact that Env on the virion surface is oligomeric and associated with the gp41 transmembrane domain of Env. To estimate what these effects might have on the interaction of gp120 with CCR5, we constructed JRFL gp140, a form of Env with a stop codon at the transmembrane domain and that is therefore composed of gp120 and the ectodomain of gp41. This molecule is secreted from expressing cells as a mixture of monomer and oligomer that is most easily visualized on a sucrose gradient (Fig. 7C). The secreted form of gp140 consists of two different cleaved species: a form that has been cleaved normally into the gp120 and gp41 components, and a form that has not been cleaved. Importantly, the oligomeric fraction of JRFL gp140 contained no detectable cleaved species, suggesting that cleaved gp140 is not stable enough to maintain oligomerization, consistent with the previous characterization of gp140 from the X4 BH8 strain (13). JRFL gp140 is conformationally intact, as judged by reactivity with several conformation-dependent MAbs, including D25 to the CD4 binding site, T4 that reacts with oligomeric but not monomeric forms of Env, and D61 to the gp41 ectodomain (4, 13) (data not shown). JRFL gp140 is also capable of reacting with MAb 17b, whose CD4-induced epitope overlaps the coreceptor binding site and is often used as a surrogate for exposure of the coreceptor binding site (32, 59, 65) (Fig. 7A).
When purified and iodinated, JRFL gp140 appeared to be capable of binding to both CD4 and CCR5 (data not shown). However, this experiment cannot distinguish which species (gp120, gp140, cleaved, or uncleaved) is binding. To address this question, the bound protein was separated by SDS-PAGE and visualized to distinguish gp120 and gp140. As shown in Fig. 7B, both the gp140 and gp120 species were able to bind CD4, but the gp140 species could not bind CCR5. The oligomeric form of JRFL gp140 would be predicted to have an increased avidity for CCR5 compared to monomeric gp140 or gp120 and would thus be the species most likely to bind to CCR5 if any gp140 was capable of binding. To measure the ability of oligomeric gp140 to bind CCR5, radiolabeled JRFL gp140 was separated through a sucrose gradient (Fig. 7C), and fractions containing oligomeric or monomeric Env were used for binding to cells expressing CD4 or CCR5. As demonstrated in Fig. 7D, oligomeric gp140 containing exclusively oligomeric, noncleaved gp140 (e.g., fractions 5 to 7) could bind CD4 but not CCR5. In contrast, monomeric gp140 and gp120 (e.g., fractions 9 to 12) were capable of binding both CD4 and CCR5. Our data thus suggest that cleavage of gp140 must occur for complete exposure of the coreceptor binding site on gp120.
Conformational changes in gp120 induced by CD4 are reversible.
While binding of CD4 to Env is required to induce the conformational changes in Env that expose the coreceptor binding site, it is not clear if these conformational changes are reversible. To test this, we preincubated JRFL gp120 (0.36 nM) with sCD4 (5 nM) for 1.5 h, allowing equilibrium binding to be achieved, and then added an excess of MAb (63 nM) directed to sCD4 for 20 h before binding to CCR5. In this experiment, the antibodies to CD4, Leu3A, and 19, are present in large molar excess over gp120 and cannot bind sCD4 that is already complexed with gp120. Thus, the antibodies should serve as a sink, binding sCD4 that dissociates from gp120 and preventing it from rebinding. With a dissociation t½ as fast as 15 min as measured by BIACore (67) (the actual dissociation rate for this experiment may vary due to high BSA, RT, and bulk volume effects), nearly all sCD4-gp120 complexes should dissociate multiple times over a 20-h time period. If the CD4-induced changes in gp120 are irreversible, gp120 would still be able to bind CCR5. If the changes are reversible, binding activity would be lost. We found that incubation of sCD4-gp120 complexes with anti-CD4 MAbs for an extended period of time resulted in the loss of CCR5 binding (Fig. 8A, added at t = 1.5 h). When the antibodies were present only during the binding reaction after gp120-sCD4 complexes had formed (added at t = 20 h), CCR5 binding occurred normally. Thus, we conclude that the sCD4-induced changes in gp120 that render it competent to bind CCR5 are fully reversible.
To confirm these results with a second independent approach, we incubated 1 nM JRFL gp120 with suboptimal levels of sCD4 (Fig. 8B). Exposure of gp120 to sCD4 for an extended period of time would allow binding of gp120 and sCD4 and then subsequent dissociation of the complex multiple times. If binding of the two molecules results in an irreversible conformational change in gp120, sCD4 should act as a catalyst in activating gp120 for CCR5 binding. Our results indicated that this is not the case: preincubation of gp120 with suboptimal amounts of sCD4 for 20 h had little or no effect on CCR5 binding compared to no-preincubation controls, and preincubation of a suboptimal amount of sCD4 did not permit binding at levels achieved by increased amounts of sCD4. We found that the small increase in gp120 binding by preincubation with 1 nM sCD4 was due to the greater amount of time needed to reach equilibrium during the binding reaction. When binding was carried out for 2 h or more, this difference was not observed (data not shown).
Stability of the gp120-sCD4 complex.
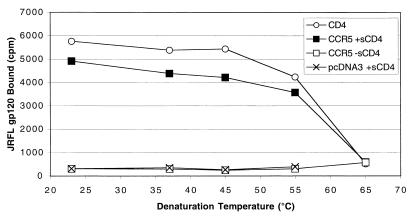
A recent study has suggested that incubation of cells expressing HIV-1 Env with cells expressing CD4 and an appropriate coreceptor results in generation of an Env conformation that induces highly potent, broadly cross-reactive neutralizing antibodies (33). Thus, characterization of the gp120-sCD4 complex for its structural integrity and stability may have implications for vaccine development. We characterized the stability of this complex by pulsing gp120-sCD4 complexes at increasingly elevated temperatures prior to addition to CCR5-expressing cells at room temperature (Fig. 9). We found that even at temperatures as high as 55°C, the CCR5 binding capability of the complex remained largely intact. At temperatures above 65°C, binding activity was lost. Binding of JRFL gp120 to CD4 and CCR5 could also be measured when the entire reaction mixture was incubated at higher temperatures up to 55°C (data not shown). We also note that binding of gp120 to CCR5 remained strictly CD4 dependent at all temperatures. Thus, incubation of gp120 at elevated temperatures did not lead to the conformational changes normally induced by CD4 binding.
FIG. 9.
Denaturation of the gp120-sCD4 complex. In 25 μl of binding buffer containing 5% BSA, JRFL gp120 with or without sCD4 was incubated at the indicated temperature for 15 min. The protein was then cooled at room temperature (or on ice, with similar results) and added to cells expressing CD4, CCR5, or pcDNA3. Binding was conducted for 1 h at RT. Binding to CD4 on the surface of cells was conducted in the absence of sCD4. Denaturation temperatures above 65°C resulted in protein aggregation that caused high nonspecific binding. Results shown are from a representative experiment repeated twice.
DISCUSSION
The ability of HIV and SIV gp120 subunits to interact with CCR5 and CXCR4 was initially demonstrated both by direct binding assays (34, 63) and indirectly by competition with labeled chemokines and antibodies (25, 60). The ability to measure the direct interaction of gp120 with the chemokine receptors is required to identify structural elements of the chemokine receptors that are involved in binding Env, but this necessitates the development of sensitive, quantitative direct binding assays. In addition, direct binding assays can be used to identify antibodies and small molecule inhibitors that block this important interaction as well as to study virus strain-dependent differences in coreceptor binding. For example, while some viral Envs interact with coreceptors only in the presence of CD4, a number of CD4-independent HIV-1, HIV-2, and SIV strains that can utilize coreceptors in its absence have been described (16–18, 26, 48). Direct binding assays can be used to determine if CD4-independent Envs interact with higher affinity or by using fundamentally different structures on Env and/or the coreceptors (16, 26, 40). By modifying existing direct binding assays, we were able to detect the direct interaction of several HIV-1 gp120 subunits, as well as an SIV gp120 subunit, to the coreceptors CCR5 and CXCR4. Binding was consistent with the tropism of the virus strain used and, except for SIVmac239, was strictly CD4 dependent. We obtained affinity values for this interaction of approximately 4 nM for JRFL, consistent with previous reports of YU2 affinities using competition binding (40, 63).
The ability to detect direct Env-coreceptor interactions allowed us to examine whether the environmental conditions that are required for HIV-1 Env-mediated membrane fusion are at the level of gp120-CCR5 interaction (summarized in Fig. 10). HIV-1 Env requires both a temperature above 25°C (21) and the presence of Ca+2 ions (11) in order to mediate membrane fusion. However, we found that neither Ca+2 nor elevated temperatures are required for binding of gp120-sCD4 complexes to CCR5, suggesting that events following chemokine receptor binding, most likely involving conformational changes in gp41 and lipid bilayer mixing, are the basis for these fusion requirements.
FIG. 10.
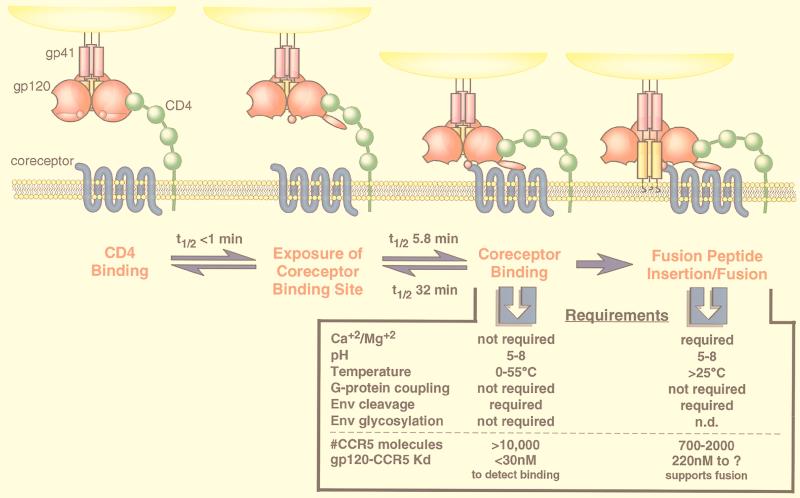
Requirements and kinetics of HIV fusion events. HIV entry begins when the gp120 subunit of Env contacts CD4 on the surface of a cell (far left). CD4 binding induces a rapid and reversible conformational change in gp120 that exposes a previously hidden coreceptor binding site. Binding to a coreceptor brings the virus in close proximity to the cellular membrane and induces a conformational change in the gp41 subunit of Env. The change in gp41 exposes the fusion peptide of Env that mediates mixing of the cellular and viral membranes for viral entry (far right). The kinetics of gp120 binding and conformational change, as measured in this study, are indicated. The kinetics of JRFL gp120 binding to sCD4 are described in detail elsewhere (67). The requirements of gp120-CCR5 binding and Env-membrane fusion are compared. We note that the parameter “#CCR5 molecules” is a comparison of the sensitivity of different assays (binding versus infection). The parameter “gp120-CCR5 Kd” indicates what gp120-CCR5 affinity is needed to detect binding and what gp120-CCR5 affinity is still consistent with Env-coreceptor activity. Both parameters are a function of the form of Env used in each assay (e.g., monomeric gp120 and oligomeric Env) and may change with subsequent improvements in assay sensitivity and methodology. The requirement of Env glycosylation for membrane fusion has not been conclusively demonstrated by using enzymatic deglycosylation. n.d., not determined. The requirements of gp120 binding are described in this report and by Baik et al. (1). The requirements of Env-mediated fusion have been described elsewhere (2, 11, 21, 35, 47, 57).
The extracellular structure of many G-protein-coupled receptors can change depending on intracellular G-protein coupling (22), though receptor signaling is not required for HIV infection of cell lines (2). However, some Env proteins, including JRFL, have been reported to be functional agonists of chemokine receptors (8, 62) that can trigger apoptosis in CD8+ T cells (24). Thus, Env-receptor interactions may influence cellular activation and viability even in the absence of virus infection. We found that G-protein coupling of CCR5 had no apparent effect on gp120 binding in our studies, as measured by treatment of CCR5-expressing cells with pertussis toxin and by binding to a nonsignaling mutant, CCR5-DNY. It will be important to compare Env proteins that differ in their ability to induce receptor signaling or apoptosis to determine why some Env-coreceptor interactions lead to a functional response while others do not.
In addition to studying receptor determinants required for Env-coreceptor interactions, a direct binding assay readily lends itself to studying structural determinants in Env that mediate receptor binding (51), as well as the conditions under which conformational changes needed for binding and membrane fusion occur. The HA2 (transmembrane) subunit of the influenza virus hemagglutinin (HA) Env protein exists in a metastable “spring-loaded” state that is normally triggered into a fusogenic conformation by lowered pH but can also be irreversibly triggered by an elevated temperature or mild urea treatment (5). We were able to test the parallels of the HA model to the triggering of HIV-1 gp120 by sCD4, using CCR5 binding as a functional measure of gp120 conformational changes. We were unable to induce gp120 to bind CCR5 by heat treatment, suggesting that the sCD4-induced conformational change in gp120 involves specific structural changes and not simply conversion of gp120 into a more stable, low-energy state.
Consistent with these results, we found that the gp120 conformational changes induced by CD4 were lost upon CD4 dissociation. Thus, while CD4 binding induces a stable and long-lived conformational change in gp120 that makes it competent to bind CCR5, these changes are reversible upon CD4 dissociation. While we were able to conclude that the conformational changes induced in gp120 by CD4 are nearly instantaneous, we could not measure the rate at which this conformational change reverses. The reversibility of CD4-induced conformations has implications for vaccine development, as gp120-sCD4 complexes used for immunization (9, 10, 28) would quickly revert to native gp120, following dissociation of sCD4. A more permanent exposure of the conserved coreceptor binding site will require fixation as has recently been used successfully (33) or genetic triggering as has recently been reported (26).
Despite the utility of a direct gp120-coreceptor binding assay, our interpretation of the kinetics of gp120 interaction with CCR5 is fundamentally limited. Most importantly, we are measuring a three-part interaction involving a series of conformational changes and binding reactions between gp120, sCD4, and CCR5, rather than the simple two-component binding reaction that the laws of mass action dictate. In addition, because of the technical limitations in performing this assay, we are using small amounts of radioligand and buffer conditions that may not be optimal for such kinetic measurements (e.g., 5% BSA and 100-μl volume). Nevertheless, we have compensated for these limitations, where possible, by using an excess of sCD4, by preincubating gp120 with sCD4, and by using conditions that might minimize diffusion limited processes (e.g., shaking and temperature). We have noted elsewhere that the failure to detect binding of gp120 to a coreceptor does not necessarily predict the ability of that coreceptor to function for viral entry (1, 12). Thus, our inability to detect binding in an experiment does not necessarily mean that the coreceptor cannot function for fusion under the same conditions. By consistently measuring the high-affinity interaction between wild-type CCR5 and JRFL gp120 in our experiments, we have been able to minimize such negative conclusions, but it should be clear that the biochemical parameters and conditions that we have measured, by definition, describe the interaction between gp120 and CCR5 and that the results may be different for full-length Env and CCR5.
In an effort to better mimic the interaction between full-length Env and CCR5, we used a soluble, oligomeric form of Env, gp140, that was, at least in part, antigenically and functionally intact. An oligomeric gp140 could have a profound affect on the development of Env subunit vaccines and the study of Env triggering during the fusion process. While JRFL gp140 appeared antigenically intact and was capable of binding CD4, we found no evidence that monomeric or oligomeric uncleaved JRFL gp140 could bind CCR5, similar to what we have observed previously for SIV (16), despite the fact that an oligomeric form would be predicted to have an increased avidity for the coreceptor. We believe that the most likely explanation for this lack of binding is that the conformational changes in gp120 that are induced upon CD4 binding require a cleaved gp120 C terminus in order to fully expose the hidden coreceptor binding site. We cannot rule out other possibilities, however, such as the truncated gp41 ectodomain interfering with CCR5 binding or differential glycosylation of gp140 affecting its ability to bind CCR5. Nevertheless, these results also suggest that a change in the interaction of gp120 and gp41 may occur at the time of CD4 binding. These results have implications for vaccine development since oligomeric forms of Env may act as better immunogens than monomeric Env (4, 13, 20, 50, 54, 58). Our results suggest that an immune response to an uncleaved gp140 protein may not yield effective antibodies directed to the conserved coreceptor binding site. Development of a soluble form of Env that is successfully cleaved and that remains oligomeric is a hurdle worthy of further pursuit but that has yet to be achieved.
The utility of a direct binding assay for measuring gp120-chemokine receptor interactions also includes the applied aspect of using this interaction to identify and design better HIV inhibitors. In particular, a direct binding assay with high sensitivity and specificity will be particularly useful for high-throughput screening of potential inhibitors of HIV entry. An assay that exploits the direct gp120-chemokine receptor interaction for discovering such compounds may have a significant advantage over assays that only indirectly mimic this interaction using chemokine competition. For example, our results elsewhere suggest that chemokines and HIV Env utilize quite different structural elements of a coreceptor such as CCR5 (36), and we predict that screening with a chemokine ligand may yield very different results than screening with gp120 (1). The binding assay conditions that we report here provide some of the details necessary for designing such a high-throughput screen.
ACKNOWLEDGMENTS
We thank Jane Sung for excellent technical assistance. We thank Joe Rucker, Richard Horuk, Joe Hesselgesser, Meina Liang, Mike Orsini, Irwin Chaiken, Wentau Zhang, Geoff Mills, Chris Broder, Julie DeMartino, Rolf Windh, and members of the Doms lab for helpful discussions, technical advice, and critical review. We gratefully thank Larry Arthur for amino acid analysis. A number of important reagents were provided by the NIH AIDS Research and Reference Program.
This work was supported by NIH grant AI-40880, a Burroughs Wellcome Fund Award for Translational Research, and an Elizabeth Glaser Scientist Award to R.W.D. B.J.D. was supported, in part, by a Howard Hughes Medical Institute predoctoral fellowship.
REFERENCES
- 1.Baik S S W, Doms R W, Doranz B J. HIV and SIV gp120 binding does not predict coreceptor function. Virology. 1999;259:267–273. doi: 10.1006/viro.1999.9779. [DOI] [PubMed] [Google Scholar]
- 2.Berson J F, Doms R W. Structure-function studies of the HIV-1 coreceptors. Semin Immunol. 1998;10:237–248. doi: 10.1006/smim.1998.0130. [DOI] [PubMed] [Google Scholar]
- 3.Blanpain C, Migeotte I, Lee B, Vakili J, Doranz B J, Govaerts C, Vassart G, Doms R W, Parmentier M. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999;94:1899–1905. [PubMed] [Google Scholar]
- 4.Broder C C, Earl P L, Long D, Abedon S T, Moss B, Doms R W. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr C M, Chaudhry C, Kim P S. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc Natl Acad Sci USA. 1997;94:14306–14313. doi: 10.1073/pnas.94.26.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greeves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 8.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denisova G, Stern B, Raviv D, Zwickel J, Smorodinsky N I, Gershoni J M. Humoral immune response to immunocomplexed HIV envelope glycoprotein 120. AIDS Res Hum Retroviruses. 1996;12:901–909. doi: 10.1089/aid.1996.12.901. [DOI] [PubMed] [Google Scholar]
- 10.DeVico A L, Rahman R, Welch J, Crowley R, Lusso P, Sarngadharan M G, Pal R. Monoclonal antibodies raised against convalently crosslinked complexes of human immunodeficiency virus type 1 gp120 and CD4 receptor identify a novel complex-dependent epitope on gp120. Virology. 1995;211:583–588. doi: 10.1006/viro.1995.1441. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrov D S, Broder C C, Berger E A, Blumenthal R. Calcium ions are required for cell fusion mediated by the CD4-human immunodeficiency virus type 1 envelope glycoprotein interaction. J Virol. 1993;67:1647–1652. doi: 10.1128/jvi.67.3.1647-1652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doranz B J, Orsini M J, Turner J D, Hoffman T L, Berson J F, Hoxie J A, Peiper S C, Brass L F, Doms R W. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl P L, Moss B. Expression of proteins in mammalian cells using vaccinia. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons; 1991. [Google Scholar]
- 15.Edinger A L, Amedee A, Miller K, Doranz B J, Endres M, Sharron M, Samson M, Lu Z, Clements J E, Murphey-Corb M, Peiper S C, Parmentier M, Broder C C, Doms R W. Differential utilization of CCR5 by macrophage and T-cell tropic SIV strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edinger A L, Blanpain C, Kunstman K J, Wolinsky S M, Parmentier M, Doms R W. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J Virol. 1999;73:4062–4073. doi: 10.1128/jvi.73.5.4062-4073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edinger A L, Mankowski J L, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Berson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 19.Farzan M, Choe H, Martin K A, Sun Y, Sidelko M, Mackay C R, Gerard N P, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein-1β-mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 20.Fouts T R, Trkola A, Fung M S, Moore J P. Interactions of polyclonal and monoclonal anti-glycoprotein 120 antibodies with oligomeric glycoprotein 120-glycoprotein 41 complexes of a primary HIV type 1 isolate: relationship to neutralization. AIDS Res Hum Retroviruses. 1998;14:591–597. doi: 10.1089/aid.1998.14.591. [DOI] [PubMed] [Google Scholar]
- 21.Frey S, Marsh M, Gunther S, Pelchen-Matthews A, Stephens P, Ortlepp S, Stegmann T. Temperature dependence of cell-cell fusion induced by the envelope glycoprotein of human immunodeficiency virus type 1. J Virol. 1995;69:1462–1472. doi: 10.1128/jvi.69.3.1462-1472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gether U, Kobilka B K. G protein-coupled receptors II. Mechanism of agonist activation. J Biol Chem. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 23.Hart T K, Kirsh R, Ellens H, Sweet R W, Lambert D M, Petteway S R, Leary J, Bugelski P J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1991;88:2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O’Brien W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 25.Hill C M, Deng H, Unutmaz D, Kewalramani V N, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman T L, LaBranche C C, Zhang W, Canziani G, Robinson J, Chaiken I, Hoxie J A, Doms R W. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc Natl Acad Sci USA. 1999;96:6359–6364. doi: 10.1073/pnas.96.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivey-Hoyle M, Culp J S, Chaikin M A, Hellmig B D, Matthews T J, Sweet R W, Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci USA. 1991;88:512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang C Y, Hariharan K, Nara P L, Sodroski J, Moore J P. Immunization with a soluble CD4-gp120 complex preferentially induces neutralizing anti-human immunodeficiency virus type 1 antibodies directed to conformation-dependent epitopes of gp120. J Virol. 1994;68:5854–5862. doi: 10.1128/jvi.68.9.5854-5862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Eternad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 31.Kwong P D, Wyatt R, Desjardins E, Robinson J, Culp J S, Hellmig B D, Sweet R W, Sodroski J, Hendrickson W A. Probability analysis of variational crystallization and its application to gp120, the exterior envelope glycoprotein of type 1 human immunodeficiency virus (HIV-1) J Biol Chem. 1999;274:4115–4123. doi: 10.1074/jbc.274.7.4115. [DOI] [PubMed] [Google Scholar]
- 32.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaCasse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 34.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 35.Larsen C, Ellens H, Bentz J. Membrane fusion induced by the HIV env glycoprotein. In: Aloia R C, Curtain C C, Gordon L M, editors. Membrane fluidity. Vol. 6. New York, N.Y: Wiley-Liss, Inc.; 1992. [Google Scholar]
- 36.Lee B, Sharron M, Blanpain C, Doranz B J, Vakili J, Setoh P, Berg E, Liu G, Guy H R, Durell S R, Parmentier M, Chang C N, Price K, Tsang M, Doms R W. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 37.Lee B, Sharron M, Montaner L J, Weissman D, Doms R W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 39.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus, SIVmac239. J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin K A, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard N P. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 41.Moore J P, Burkly L C, Connor R I, Cao Y, Tizard R, Ho D D, Fisher R A. Adaptation of two primary human immunodeficiency virus type 1 isolates to growth in transformed T cell lines correlates with alterations in the responses of their envelope glycoproteins to soluble CD4. AIDS Res Hum Retroviruses. 1993;9:529–539. doi: 10.1089/aid.1993.9.529. [DOI] [PubMed] [Google Scholar]
- 42.Moore J P, McKeating J A, Huang Y, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore J P, McKeating J A, Norton W A, Sattentau Q J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore J P, Sweet R W. The HIV gp120-CD4 interaction: a target for pharmacological or immunological intervention? Perspect Drug Discov Des. 1993;1:235–250. [Google Scholar]
- 45.O’Brien W A, Mao S-H, Cao Y, Moore J P. Macrophage-tropic and T-cell line-adapted chimeric strains of human immunodeficiency virus type 1 differ in their susceptibilities to neutralization by soluble CD4 at different temperatures. J Virol. 1994;68:5264–5269. doi: 10.1128/jvi.68.8.5264-5269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orloff S L, Kennedy M S, Belperron A A, Maddon P J, McDougal J S. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J Virol. 1993;67:1461–1471. doi: 10.1128/jvi.67.3.1461-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves J D, McKnight A, Potempa S, Simmons G, Gray P W, Power C A, Wells T, Weiss R A, Talbot S J. CD4-independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR-4, CCR-3, and V28 for entry. Virology. 1997;231:130–134. doi: 10.1006/viro.1997.8508. [DOI] [PubMed] [Google Scholar]
- 49.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 50.Richardson T M, Stryjewski B L, Broder C C, Hoxie J A, Mascola J R, Earl P L, Doms R W. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J Virol. 1996;70:753–762. doi: 10.1128/jvi.70.2.753-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzuto C D, Wyatt R, Hernández-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 52.Samson M, LaRosa G, Libert F, Paindavoine P, Detheux M, Vassart G, Parmentier M. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J Biol Chem. 1997;272:24934–24941. doi: 10.1074/jbc.272.40.24934. [DOI] [PubMed] [Google Scholar]
- 53.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott C W, Gomes B C, Hubbs S J, Koenigbauer H C. A filtration-based assay to quantitate granulocyte-macrophage colony-stimulating factor binding. Anal Biochem. 1995;228:150–154. doi: 10.1006/abio.1995.1326. [DOI] [PubMed] [Google Scholar]
- 57.Sinangil M A, Loyter A, Volsky D J. Quantitative measurement of fusion between human immunodeficiency virus and cultured cells using membrane fluorescence dequenching. FEBS Lett. 1988;239:88–92. doi: 10.1016/0014-5793(88)80551-9. [DOI] [PubMed] [Google Scholar]
- 58.Stamatos N M, Mascola J R, Kalyanaraman V S, Louder M K, Frampton L M, Birx D L, VanCott T C. Neutralizing antibodies from the sera of human immunodeficiency virus type 1-infected individuals bind to monomeric gp120 and oligomeric. J Virol. 1998;72:9656–9667. doi: 10.1128/jvi.72.12.9656-9667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 61.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weissman D, Rabin R L, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber J M, Fauci A S. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- 63.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 64.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J P, Mackay C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 66.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 67.Zhang W, Canziani G, Plugariu C, Wyatt R, Sodroski J, Sweet R, Kwong P, Hendrickson W, Chaiken I. Conformational changes of gp120 in epitopes near the CCR5 binding site are induced by CD4 and a CD4 miniprotein mimetic. Biochemistry. 1999;38:9405–9416. doi: 10.1021/bi990654o. [DOI] [PubMed] [Google Scholar]