Abstract
Objectives:
Estimate the cost-effectiveness of the use of recombinant zoster vaccine (RZV, Shingrix), which protects against herpes zoster (HZ), among immunocompromised adults aged 19–49 years, as a contribution to deliberations of the Advisory Committee on Immunization Practices (ACIP).
Methods:
Hematopoietic cell transplant (HCT) recipients experience a high incidence of HZ, and the efficacy of RZV in preventing HZ has been studied in clinical trials. The cost-effectiveness model calculated incremental cost-effectiveness ratios that compared vaccination with RZV to a no vaccination strategy among adults aged 19–49 years. Costs and outcomes were calculated until age 50 using the health care sector perspective and summarized as cost per quality-adjusted life-year gained ($/QALY). The base case represents HCT recipients, with scenario analyses representing persons with other immunocompromising conditions, including hematologic malignancies, HIV, and autoimmune and inflammatory conditions. Uncertainty was investigated using univariate, multivariate, and probabilistic sensitivity analyses.
Results:
Base case results indicated vaccination with RZV would avert about 35% of HZ episodes and complications, while saving about 11% of net costs. Compared to no vaccination, vaccination of HCT recipients with RZV generated cost-savings (i.e., lower costs and improved health) in the base case and in 81% of simulations in the probabilistic analysis. In scenario analyses, vaccination cost $9,500/QALY among patients with hematologic malignancies, $79,000/QALY among persons living with HIV, and $208,000/QALY among persons with selected autoimmune and inflammatory conditions.
Conclusions:
Generally favorable economic estimates supported recommendations for vaccination of immunocompromised adults with RZV to prevent episodes of HZ and related complications.
Herpes zoster (HZ, or shingles) is a painful skin condition caused by reactivation of latent varicella-zoster virus. Approximately 1 million episodes of HZ occurred annually in the United States (US) during the pre-vaccine era [1]. In the US, the Centers for Disease Control and Prevention (CDC) sets the immunization schedules for children and adults based on recommendations provided by the Advisory Committee on Immunization Practices (ACIP). In 2017, the ACIP recommended the use of two doses of recombinant zoster vaccine (RZV, Shingrix) for the prevention of HZ and related complications among immunocompetent adults aged 50 years and older [2]. During the October 2017 ACIP deliberations, three economic models were reviewed and considered [3–5], with economic values ranging from $11,000 to $83,000 per QALY gained across the base case estimates.
The risk for HZ among immunocompromised adults can range from 9 to 92 cases/1,000 person-years (PYs) [6]. While HZ incidence is heterogeneous within and across immunocompromised groups [6, 7], it is generally considered to be higher than HZ incidence among immunocompetent adults, which can range from 7 to 20 cases/1,000 PYs with higher incidence occurring at older ages [1, 8]. The manufacturer of RZV submitted an application to the US Food and Drug Administration (FDA) in 2020 to expand the use of RZV to adults who are or will be at increased risk for HZ because of immunodeficiency or immunosuppression caused by known disease or therapy [9]. Anticipating this expanded use application to FDA, the ACIP and ACIP Herpes Zoster Work Group (WG) began deliberations on the expanded use of RZV to potentially include immunocompromised adults.
The immunocompromised adult population under consideration for the expanded use of RZV covers a large and diverse group of patients that have immunodeficiency or immunosuppression caused by disease or therapy, and whose risks for HZ are heterogeneous in several ways. HZ incidence can vary across different patient groups, because each condition may confer a different level of reduced immune system capacity [6, 10]. Within any particular patient group, HZ risk can also vary between patients and across the lifespan of an individual patient, with generally increasing HZ incidence observed in older age groups. Notably, for this study among HCT recipients, patients are typically provided prophylactic antiviral therapy following their HCT procedure [11]. This prophylactic antiviral therapy reduces risks for several important viral conditions, including risk of HZ, for a period of time, but the duration of this therapy can vary across clinical settings and patients [12]. Finally, among adults aged 19–26 years, HZ risk may be lower for those who received varicella vaccination as children [13, 14]. The heterogeneity in HZ risk across and within immunocompromised populations being considered for the recommendation required thoughtful consideration of all aspects of RZV use, including economic analyses, during the ACIP vaccine policy deliberations [15].
The objective of this study was to estimate possible health effects and the economic value for the use of RZV among immunocompromised adults aged 19–49 years, with a primary focus on HCT recipients and with scenario analyses that investigated other patient groups with selected immunocompromising conditions. The 19–49 year age range was the focus of this study because economic assessment of RZV use in this age range had not been conducted and could most meaningfully contribute to the economic research literature on RZV use among immunocompromised populations. Immunocompromised adults ≥50 years were considered likely to have comparable, or more favorable, cost-effectiveness estimates when compared to immunocompetent adults aged ≥50 years, a group which has been previously studied [3–5]. HCT recipients were selected as the base case because these patients generally experience a high incidence of HZ and related complications [6] and because the most rigorous, available data on RZV vaccine efficacy (VE) comes from a clinical trial focused on this patient group [16]. While most groups with immunocompromising conditions do not have VE data available from clinical trials, the heterogeneity in HZ risk across these groups has been documented [6, 10] and motivated the inclusion of the scenario analyses in this study.
Results from this model and another model were presented to ACIP in September 2021 [17] as part of the Evidence to Recommendations (EtR) Framework that informs ACIP decision making [18]. Since 2017, the use of RZV has been recommended for immunocompetent adults who are aged 50 years or older [2]. In October 2021, the ACIP voted to recommend the use of RZV in immunocompromised adults aged ≥19 years [19, 20].
Methods
Overview
This cost-effectiveness analysis (CEA) calculated the population-level, health outcomes for the HCT population by comparing costs and outcomes between RZV vaccination vs. no vaccination strategies. Health outcomes included averted HZ episodes, averted hospitalizations, averted deaths, and quality-adjusted life-years (QALYs) gained. Costs and health outcomes were then combined into standard summary measures used in CEAs, including incremental cost-effectiveness ratios (ICERs) and the number needed to vaccinate (NNV) to avert an HZ episode or HZ-associated hospitalization.
Model
A cohort of adults 19–49 years who were HCT recipients were followed using a monthly time-step state transition model (Figure 1). The cohort was separated into three age groups: 19–29 years, 30–39 years, and 40–49 years. In the vaccination strategy, individuals received either one or two doses of RZV following the HCT procedure, with vaccine protection starting immediately after the last month of antiviral prophylaxis therapy. By contrast, in the no vaccination strategy, individuals did not receive RZV vaccination. The starting population of the model was 2,549, which was the average annual number of autologous HCTs over the last three years among adults 19–49 years, based on data from the Center for International Blood and Marrow Transplant Research [21] (Table S1). Costs and outcomes were collected from the health care sector perspective, from the time of the HCT procedure until each age group in the cohort reaches the age of 50 years, with all future costs and benefits discounted by 3% annually.
Figure 1. Diagram of cost-effectiveness model for vaccination with recombinant zoster vaccine (RZV) among hematopoietic cell transplant (HCT) recipients aged 19–49 years.
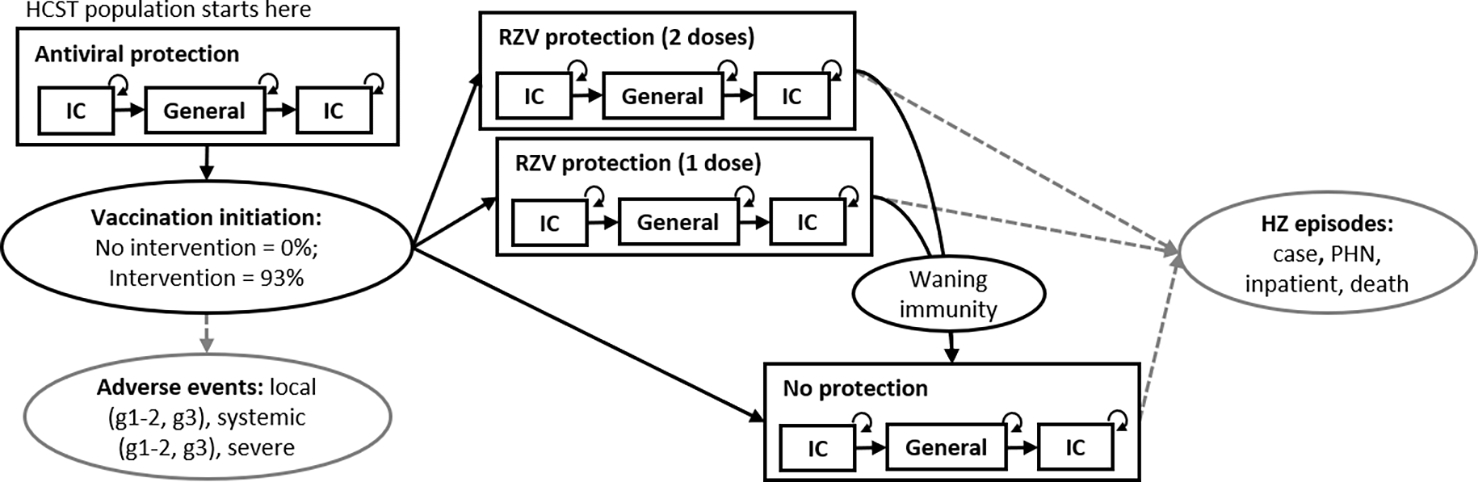
Note(s): HZ = herpes zoster; IC = immunocompromised; PHN = postherpetic neuralgia; g1–2 = grade 1 or grade 2; g3 = grade 3; RZV = recombinant zoster vaccine. General and IC = general and immunocompromised risk status, which affects HZ incidence and background mortality. Not pictured: Background mortality, IC has a higher background mortality rate. Age group transitions. The model starts with 3 age groups, representing adults 19–29, 30–39, and 40–49 years at the beginning of the model. Individuals represented by these age groups all exit the model at age 50. Dashed, grey lines indicate occurrence of HZ episodes (incidence is lower among vaccine-protected individuals) and vaccination adverse events.
The state transition model captured important clinical elements of HZ reactivation risk for immunocompromised patients, HZ disease progression, and vaccination with RZV (Figure 1). While the vaccine had a recommended two-dose schedule, the model allowed some patients to receive one dose, due to imperfect adherence to the two-dose schedule. The model began with all individuals protected by antiviral prophylaxis following the HCT procedure. Under the no vaccination strategy, individuals exited the antiviral period and entered an unprotected state where they experience HZ risk. Under the vaccination strategy, individuals exited the antiviral period and entered states with vaccine protection, with either one or two doses, or enter an unprotected state if they are part of the small portion that did not receive any vaccination. The vaccination strategy applied vaccine coverage levels that were based on available evidence for initiation and completion of the two-dose RZV series [11, 22]. HZ cases could occur while in vaccine protected states due to breakthrough cases, which were documented in the RZV clinical trials among HCT recipients. HZ incidence varied according to immunocompromised or general risk status, which was determined based on length of time after the HCT procedure. The general risk state could be considered as representing a period of remission, where the harmful effects of the initial immunocompromised condition have been successfully suppressed by the HCT. The second immunocompromised state could be considered to represent a relapse of the initial underlying immunocompromising condition. Relapse of underlying disease conditions was observed among patients in the clinical trial for RZV use among HCT recipients [16]. The model and all analyses were conducted using R statistical software (version 4.0.2; The R Foundation) with model replication of the base case and univariate sensitivity analyses conducted using Microsoft Excel®.
Inputs
All input values used in the model along with available sources are presented in Table 1. VE assumptions were primarily obtained from clinical trial data on HCT recipients [16]. VE values used in sensitivity analyses were also informed by reported post hoc clinical trial analyses [23] and observational studies [22]. Breakthrough HZ episodes among vaccinated individuals were allowed to occur and were assumed to present a lower level of disease severity [24]. The rate of breakthrough episodes among vaccine protected states corresponded to the incidence rate ratios implied by the VE inputs. For example, individuals who received two doses of RZV, which has an initial VE of 0.68, experience HZ disease risk corresponding to an incidence rate ratio of 0.32 (i.e., 1 – 0.68) multiplied by the age- and risk-appropriate rates of HZ incidence for unvaccinated individuals. In the base case, breakthrough HZ episodes (i.e., HZ episodes among vaccinated individuals) have a health utility weight of 0.986, which is 0.6% less severe than the health utility weight of 0.977 used for HZ episodes in unvaccinated individuals. Lower severity among breakthrough episodes only impacts uncomplicated HZ episodes, and not the severity of complications such as postherpetic neuralgia (PHN) or episodes requiring inpatient services. Transition rates between immunocompromised risk and general risk status were calibrated based on relapse data available from the clinical trial (additional details available in Figure S1) [16]. The probabilities of experiencing HZ-related complications and all cost inputs, including costs for vaccinations, adverse events, HZ episodes and related sequalae, were estimated from 2012–2019 IBM® MarketScan® [25, 26]. Costs of HZ episodes and related sequalae used a sample of HCT recipients aged 19–49 years (additional details available in Table S2). All costs were adjusted to US$2020, using the medical component of the Consumer Price Index. Quality of life inputs for each disease state associated with an HZ episode were based on available literature [27, 28] and discussions with the WG, with adjustments for age [29], immunocompromised status [30], and breakthrough HZ episodes [24]. In the vaccination strategy, vaccination series initiation was assigned the rate of antiviral therapy among autologous HCT recipients in a recent US study [11], with series completion based on a recent observational study [22]. Additional details on the selection of model inputs were included in the supplemental appendix (Table S1, Table S2).
Table 1.
Base case values and ranges for inputs in the cost-effectiveness model for vaccination with recombinant zoster vaccine (RZV) among hematopoietic cell transplant (HCT) recipients, adults aged 19–49 years.
| Input | Base | Low | High | Source(s) |
|---|---|---|---|---|
| HCT population size | 2,549 | [21] | ||
| Age groups a | ||||
| Portion aged 19 to 29 years | 0.220 | 0.198 | 0.228 | [21] |
| Portion aged 30 to 39 years | 0.250 | 0.249 | 0.257 | |
| Portion aged 40 to 49 years | 0.530 | 0.554 | 0.515 | |
| Vaccination coverage | ||||
| Probability of receiving at least 1 dose in vaccination strategy | 0.93 | 0.86 | 1.0 | [11] |
| Probability of series completion, given 1 dose | 0.86 | 0.82 | 0.9 | [22] |
| RZV Vaccine efficacy (VE) b | ||||
| Initial VE 1 dose | 0.39 | 0.22 | 0.65 | [16, 22] |
| Initial VE 2 dose | 0.68 | 0.56 | 0.78 | [16] |
| Years until no vaccine protection from 1 dose (waning immunity) | 11 | 5 | 30 | [4] |
| Years until no vaccine protection from 2 dose (waning immunity) | 19.4 | 5 | 30 | |
| Probabilities of RZV-associated adverse events | ||||
| Local, mild | 0.716 | 0.666 | 0.760 | [16] |
| Local, grade 3 | 0.142 | 0.12 | 0.167 | |
| Systemic, mild | 0.171 | 0.071 | 0.296 | |
| Systemic, grade 3 | 0.072 | 0.008 | 0.110 | |
| Severe | 0.0001 | 0 | 0.0002 | [4] |
| Transitions between health risk states | ||||
| Duration of antiviral therapy following HCT procedure (months) | 6 | 1 | 24 | Assumptionc |
| Median years to general risk from initial immunocompromised state | 0.67 | 0.50 | 5.00 | Calibrationd based on [16] |
| Median years to final immunocompromised state from general risk | 1.75 | 1.25 | 20.00 | |
| Herpes zoster (HZ) incidence, immunocompromised states (cases per 1,000 person-years) | ||||
| 19 to 49 years | 40.20 | 35.60 | 45.12 | [10] |
| 50 to 59 years | 43.22 | 38.20 | 48.65 | |
| 60 to 64 years | 50.71 | 41.80 | 60.92 | |
| 65+ years | 44.73 | 33.20 | 58.97 | |
| HZ incidence, general risk states (cases per 1,000 person-years) | ||||
| 19 to 29 years | 1.4 | 1.3 | 1.5 | [1] |
| 30 to 39 years | 2.0 | 1.8 | 2.2 | |
| 40 to 49 years | 2.9 | 2.7 | 3.0 | |
| 50 to 59 years | 4.6 | 4.4 | 4.8 | |
| 60 to 69 years | 6.9 | 6.6 | 7.2 | |
| 70 to 79 years | 9.5 | 9.0 | 9.9 | |
| 80+ years | 10.9 | 10.2 | 11.6 | |
| Probabilities of HZ complications, given HZ episode | ||||
| PHN | 0.091 | 0.060 | 0.410 | [6, 10, 16, 26] |
| Inpatient visit | 0.106 | 0.064 | 0.160 | [16, 26] |
| Death from HZ, in immunocompromised state | 0.0010 | 0.0006 | 0.0015 | [37] |
| Death from HZ, in general risk state | 0.0006 | 0.0003 | 0.0010 | |
| Background mortality, annual probability | ||||
| 19 to 29 years | 0.001 | [38] | ||
| 30 to 39 years | 0.002 | |||
| 40 to 49 years | 0.003 | |||
| 50 to 59 years | 0.006 | |||
| 60 to 69 years | 0.013 | |||
| 70 to 79 years | 0.029 | |||
| 80 to 89 years | 0.082 | |||
| 90+ years | 0.224 | |||
| Multiplier for immunocompromised status | 10.307 | 9.614 | 15.010 | [39, 40] |
| Background health utility weights, by age | ||||
| 19 to 29 years | 0.9205 | 0.9135 | 0.9270 | [29] |
| 30 to 39 years | 0.9055 | 0.8990 | 0.9125 | |
| 40 to 49 years | 0.8750 | 0.8675 | 0.8825 | |
| 50 to 59 years | 0.8490 | 0.8410 | 0.8580 | |
| 60 to 69 years | 0.8255 | 0.8135 | 0.8370 | |
| 70 to 79 years | 0.7865 | 0.7730 | 0.8000 | |
| 80+ years | 0.7530 | 0.7290 | 0.7770 | |
| Health utility weight for immunocompromised status | 0.8675 | 0.7807 | 0.9542 | [30] |
| Health utility weights associated with HZ episodes | ||||
| HZ | 0.9770 | 0.9671 | 0.9891 | [27, 28] |
| Adjustment for breakthrough HZ episodee | 1.0063 | 1.0000 | 1.0121 | [24] |
| PHN | 0.4730 | 0.2720 | 0.6318 | [27, 28] |
| Inpatient service | 0.9860 | 0.9612 | 1.0000 | |
| Health utility weights associated vaccination adverse events | ||||
| Local, mild | 1.000 | 0.995 | 1.000 | [4, 41, 42] |
| Local, grade 3 | 0.998 | 0.995 | 1.000 | |
| Systemic, mild | 1.000 | 0.980 | 1.000 | |
| Systemic, grade 3 | 0.993 | 0.980 | 1.000 | |
| Severe | 0.990 | 0.346 | 0.993 | [41–43] |
| Costs associated with HZ episodes | ||||
| HZ | 1,549 | 889 | 7,418 | [26, 44, 45] |
| PHN | 3,357 | 3,171 | 21,563 | |
| Inpatient service | 36,303 | 4,296 | 57,111 | |
| Costs associated with vaccinations | ||||
| Vaccination, 1 dose | 191 | 165 | 219 | [25, 46] |
| Local, mild adverse event | 0.00 | 0.00 | 0.00 | Assumption |
| Local, grade 3 adverse event | 26.00 | 17.93 | 56.99 | [26] |
| Systemic, mild adverse event | 0.00 | 0.00 | 0.00 | Assumptionc |
| Systemic, grade 3 adverse event | 39.29 | 24.34 | 56.64 | [26] |
| Severe adverse event | 7,822 | 7,306 | 8,339 | [47] |
Note(s): RZV = recombinant zoster vaccine; HZ = herpes zoster; PHN = postherpetic neuralgia; QALY = quality-adjusted life-year; VE = vaccine efficacy. All costs are reported in US$2020.
Portions of individuals within each age group are treated as a group of inputs to ensure a sum across all groups would equal 1.0. The low values for these portions correspond to an age distribution that contains the fewest individuals in the youngest age group (19–29 years) and the high values correspond to an age distribution that contains the most individuals in the youngest age group.
Individuals in the vaccine protected states experience a risk of breakthrough HZ episodes that is equal to the incidence rate ratios (IRR) implied by the VE inputs (base case two dose IRR = 1 – 0.68; base case one dose IRR = 1 – 0.39) multiplied by the age- and risk-group appropriate HZ incidence rate among unvaccinated individuals.
Antiviral therapy duration inputs were identified during discussions with subject matter experts on the ACIP WG for HZ vaccines. In addition, the base case input of 6 months duration was considered to be reasonably close to values reported in a recent observational study of privately insured patients [11].
More details on the calibration process are available in the supplemental appendix (Figure S1).
Health utility weights for breakthrough HZ episodes are less severe, so they are increased by 1.006 (or by 0.6%) relative to the health utility of an HZ episode among unvaccinated individuals.
Summary measures
The ICER was used to summarize estimates of economic value, with a calculation that follows the standard formula [31], defined in this equation:
| (1) |
The strategies were identified in the subscripts, either Vaccination or NoVaccination. The total costs for a given strategy was represented by the term Costs. includes health care costs for HZ episodes, costs of adverse events, and costs of vaccination; includes only the health care costs for HZ episodes. The term Outcomes represented the outcome of interest for a given ICER calculation, which included QALYs for most results but also included the number of averted cases, hospitalizations, or deaths as alternative and/or intermediate outcomes for consideration.
The other summary measure used was the NNV, which is calculated using a model-based formula [32, 33]:
| (2) |
The numerator was the number of vaccinations performed within the vaccination strategy. For the purposes of this summary measure, partial series vaccinations (one dose only) were counted as 0.5 vaccinations and completed series were counted as 1.0 vaccinations.
Sensitivity analyses
Uncertainty among input values was investigated using univariate, multivariate, and probabilistic sensitivity analyses. The univariate sensitivity analyses included an evaluation of the impact that a single input can have on the estimated ICER. This is done by calculating the ICER of vaccination vs. no vaccination when an input is set to either the high or low value in the uncertainty range presented in Table 1. For example, in the univariate sensitivity analysis that evaluated the impact of the duration of antiviral prophylaxis, the duration of antiviral prophylaxis was varied from 1 month to 24 months, which were the low and high values that encompassed the base case value of 6 months (Table 1). Probabilistic sensitivity analyses were conducted using input values randomly drawn from beta distributions for 1,000 iterations (additional details available in Table S4). Additional scenarios were also developed to address the potential impact of vaccination in patients with other selected immunocompromising conditions, including hematologic malignancies, persons living with HIV, and persons with autoimmune and inflammatory conditions (Table 2). When available, condition-specific input data were used to set up these scenarios. The autoimmune and inflammatory conditions scenario represented several different patient groups, each with different levels of HZ risk [7] and each with uncertain VE relative to other patient groups, such as HCT recipients and hematologic malignancy patients which had estimates based on available clinical trial data. To investigate this diverse group of patients, additional sensitivity analyses were conducted where HZ incidence was varied from 0 to 50 cases per 1,000 PYs and initial VE was set either 68%, 87%, or 94%, with all other inputs set to the autoimmune and inflammatory conditions scenario settings. Another scenario investigated the possible impact that pediatric varicella vaccination might have on the economic value of the use of RZV among adults 19–49 years who are immunocompromised, a portion of whom may have received the varicella vaccine as children. This scenario used a set of assumptions based on historical vaccination coverage data [34] that indicated approximately 40.7% of adults aged 19–29 years had received varicella vaccinations as children. This rate of varicella vaccination could potentially reduce the estimated incidence of HZ among 19–49 year olds (i.e., a broader age range than 19–29) from 40.2 to 36.6 cases per 1,000 PYs while an individual is in immunocompromised status, and from 1.4 to 0.8 cases per 1,000 PYs while an individual is in general risk status. Additional details on the inputs used in these scenarios were described in the supplemental appendix.
Table 2.
Inputs used in scenario analyses in the cost-effectiveness model for vaccination with recombinant zoster vaccine (RZV) among hematopoietic cell transplant (HCT) recipients and patients with selected immunocompromising conditions, adults aged 19–49 years.
| Input | Base case | Myeloma | Non-Hodgkin Lymphoma | Hematologic Malignancies | HIV | Autoimmune / Inflammatory Conditions | Varicella Vaccination |
|---|---|---|---|---|---|---|---|
| Age group 19–29 years | 0.22 | 0.20 | 0.23 | a | 0.43 | a | a |
| Age group 30–39 years | 0.25 | 0.25 | 0.26 | a | 0.35 | a | a |
| Age group 40–49 years | 0.53 | 0.55 | 0.52 | a | 0.21 | a | a |
| Source(s) | b | [21] c | [21] c | [48] | |||
| Antiviral therapy duration (months) | 6 | a | a | None | Noned | Noned | a |
| Source(s) | b | Assumption | Assumption | Assumption | |||
| Time to general risk status (years) | 0.58 | a | a | a | 100d | 100d | a |
| Time to final immunocompromised status (years) | 2.00 | a | 20 | a | NA | NA | a |
| Source(s) | b | Assumption | Assumption | Assumption | |||
| VE 1 dose only | 0.39 | 0.42 | 0.37 | 0.61 | 0.61 | 0.61 | a |
| VE 2 doses | 0.68 | 0.72 | 0.64 | 0.87 | 0.87 | 0.87 | a |
| Source(s) | b | [16, 22] | [16, 22] | [22, 23] | [22, 23] | [23] | a |
| Background mortality, IC multiplier | 10.31 | 9.61 | 11.00 | a | 11.52 | 1.0 | |
| Source(s) | b | [40] | [39] | [49] | Assumption | ||
| HZ incidence, 19–49 years, IC status | 40.2 | a | a | 28.6 | 17.83 | 11.57 | 36.6 |
| Source(s) | b | [50] | [10] | [10] | [10, 51] | ||
| HZ incidence, 19–29 years, general risk status | 1.4 | a | a | a | a | a | 0.8 |
| Source(s) | b | [1, 51] | |||||
| Probability of PHN given HZ episode | 0.075 | a | a | 0.060 | 0.061 | 0.063 | a |
| Source(s) | b | [6, 50] | [10] | [10] | |||
| Probability of inpatient services given HZ episode | 0.106 | a | a | 0.101 | 0.080 | 0.099 | a |
| Source(s) | b | [52] | [52] | [52] | |||
| HZ cost adjustment | None | a | a | a | 0.23 | Lower bounds | a |
| Source(s) | [53] | Assumption |
Note(s): HCT = hematopoietic cell transplant recipients; RZV = recombinant zoster vaccine; HZ = herpes zoster; IC = immunocompromised; PHN = postherpetic neuralgia; VE = vaccine efficacy.
Empty cells represent values that did not change from the base case inputs.
Sources for the base case inputs are in Table 1.
Age distributions based on data from the Center for International Blood and Marrow Transplant Research were identified for use in the model as a base case, a low (or younger) age distribution, and a high (or older) age distribution; and were not specific to the underlying cause of the HCT. The myeloma scenario was assigned the older age distribution. Non-hodgkin lymphoma was assigned the younger age distribution.
For the purposes of the model’s base case focused on HCT recipients, the antiviral therapy period is characterized as a short-term clinical treatment and is associated with a minimal risk of HZ. To modify the model assumptions to better represent other selected IC groups, this antiviral period was reduced to zero. In the scenarios for HIV and autoimmune/inflammatory (AI/INF) conditions, the immunocompromised risk period was assumed to occur for the full duration of a patient’s lifetime (i.e., 100 years duration before a transition to general risk). This long-lasting immunocompromised risk period represents the HIV or AI/INF patient average level of risk and standard of care for most of their life, following their diagnosis.
Results
Among HCT recipients, the starting population was 2,549 persons. In the vaccination strategy, 79% of these individuals completed the two-dose series, 13% received one dose only, and 8% remained unvaccinated, with 4,368 doses of RZV administered in total. These vaccinations averted 35% of HZ episodes, 35% of HZ-associated inpatient care stays, and 34% of HZ-related deaths; and 11 discounted QALYs were gained until the cohort reached age 50 years. The total costs of the vaccination strategy were 11% lower than the total costs of the no vaccination strategy (additional details available in Table S3). The ICER calculations indicated cost-savings (i.e., negative incremental costs and positive incremental health outcomes, with an incremental cost-effectiveness ratio < $0/QALY). In addition, the model estimated 10 vaccinations would avert an episode of HZ and 95 vaccinations would avert an HZ-associated hospitalization.
The base case findings were largely supported by the sensitivity analyses, where 93% of univariate and 75% of multivariate analyses yielded economic values that were cost-saving (Table 3). Univariate analyses that yielded ICERs with higher costs in the vaccination strategy than in the no vaccination strategy (i.e., no longer cost-saving) included faster waning vaccine protection for two-dose vaccine recipients with an ICER of $133,000/QALY, more years until final immunocompromised status (or increased duration of general risk period) with an ICER of $133,000/QALY, higher utility weights on adverse events with an ICER having lower costs and higher QALYs, and lower inpatient costs with an ICER of $32,000/QALY. Lower costs & lower QALYs indicates negative incremental costs and negative incremental health outcomes, which would be a less favorable outcome than a cost-saving result, which is characterized by negative incremental costs and positive incremental health outcomes (i.e., higher QALYs). In probabilistic sensitivity analyses, 81% of iterations were cost-saving for the vaccination strategy, with 99% of iterations exhibiting positive incremental health outcomes and 82% of iterations exhibiting negative incremental costs (Figure 2).
Table 3.
Main results and selected sensitivity analyses (univariate, multivariate, and scenario analyses) results reported as incremental cost-effectiveness ratios ($/QALY), comparing vaccination with recombinant zoster vaccine (RZV) to no vaccination, from a cost-effectiveness model of herpes zoster vaccination among HCT recipients and patients with selected immunocompromising conditions, adults aged 19—49 years.
| Main result | Incremental cost-effectiveness ratio ($/QALY) |
|---|---|
| HCT recipients (i.e., base case) | Cost-savinga |
| Selected univariate analyses b | |
| Low rate of waning protection among 2 dose recipients | 133,000 |
| 20 years to final IC state from general risk state (i.e., longer duration of general risk state) | 133,000 |
| More severe (i.e., low value) QALY weight for local, mild AEs | Lower costs & lower QALYsc |
| More severe (i.e., low value) QALY weight for systemic, mild AEs | Lower costs & lower QALYsc |
| Low costs for inpatient HZ episodes | 32,000 |
| Selected multivariate analyses d | |
| All VE inputs (i.e., low initial VE and high rates of waning protection for 1 and 2 doses) | 353,000 |
| More severe (i.e., low values) QALY weights for all AE types | Lower costs & lower QALYs |
| Low costs for all HZ outcomes (i.e., uncomplicated, inpatient, PHN episodes) | 45,000 |
| Low costs for all HZ outcomes and high costs for vaccine doses and all AE types | 58,000 |
| Scenario analyses e | |
| Multiple myeloma | Cost-saving |
| Non-Hodgkin lymphoma | 165,000 |
| Hematologic malignancies | 9,500 |
| HIV | 79,000 |
| Autoimmune/inflammatory conditions | 208,000 |
| Varicella vaccination | Cost-saving |
Note(s): HZ = herpes zoster; IC = immunocompromised; QALY = quality-adjusted life-year; RZV = recombinant zoster vaccine. Numeric values were rounded to nearest 1,000 or 100 to improve readability. All costs are reported in US$2020.
Base case results were cost-saving, with negative incremental costs and positive incremental health outcomes.
Additional univariate sensitivity analyses were conducted but not presented here. These additional analyses all found cost-savings using high and low input values, and included: age distribution, antiviral duration, initial VE (1 dose), initial VE (2 dose), waning protection (1 dose), probability AE (local, grade 3), probability AE (systemic, grade 3), probability AE (severe), years to general risk, incidence (given IC status), incidence (given general status), probability PHN (given HZ), probability inpatient (given HZ), probability death (given HZ and IC status), probability death (given HZ and general status), background mortality (IC multiplier), background utilities (age-based), background utilities (IC multiplier), utility weight (HZ episode), utility weight (HZ with PHN episode), utility weight (inpatient HZ episode), utility adjustment for breakthrough HZ episode, utility weight (AE local, mild), utility weight (AE systemic, grade 3), utility weight (AE severe), cost vaccine dose, cost HZ episode, cost PHN, cost AE (local, mild), cost AE (local, grade 3), cost AE (systemic, mild), cost AE (systemic, grade 3), cost (AE severe).
Lower costs & lower QALYs indicates negative incremental costs and negative incremental health outcomes, which would be a less preferred outcome than a cost-saving result, such as was found in the base case which is characterized by negative incremental costs and positive incremental health outcomes (i.e., higher QALYs).
Additional multivariate sensitivity analyses were conducted but not presented here. These additional analyses all found cost-savings using high and low input values, and included: probability all AE events, probability all severe HZ outcomes, utility loss (all HZ episode types), cost (all HZ outcomes), cost (all cost inputs). An analysis where the upper age limit was changed from 50 to 100 years was also conducted, which found cost-savings.
Input values used in the scenario analyses are presented in Table 2.
Figure 2. Results from the probabilistic sensitivity analyses from a cost-effectiveness model for vaccination with recombinant zoster vaccine (RZV) among hematopoietic cell transplant (HCT) recipients aged 19–49 years.

Note(s): HZ = herpes zoster; QALY = quality-adjusted life-year; RZV = recombinant zoster vaccine; VE = vaccine efficacy. All costs are reported in US$2020. Each point in the graph represents one of 1,000 iterations of the probabilistic sensitivity analyses. The majority of points (81.0%) fall in the lower right quadrant, which indicates cost-savings. Smaller percentages of points fall into the other quadrants: top left quadrant, where vaccination is dominated by no vaccination, contains 0.3%; the lower left quadrant, where negative costs and negative health outcomes occur, contains 1.1%; the top right quadrant, where costs are positive and health outcomes are positive, contains 17.6%.
In the scenario analyses that explored other selected immunocompromising conditions, the ICERs were $9,500/QALY for the hematologic malignancies scenario, $79,000/QALY for the HIV scenario, and $208,000/QALY for the autoimmune and inflammatory conditions scenario (Table 3). The additional analyses of autoimmune and inflammatory conditions that varied HZ incidence and VE resulted in ICER estimates for a vaccination strategy ranging from cost-savings to being dominated in this patient group (Figure 3). In the varicella vaccination scenario, which assumed a reduction in HZ incidence among HCT patients aged 19–29 years in proportion to estimated varicella vaccination coverage rates, the ICER remained cost-saving (Table 3).
Figure 3. Results from a threshold analysis of vaccination with recombinant zoster vaccine (RZV) vs. no vaccination, looking at herpes zoster (HZ) incidence in a scenario representing patients with autoimmune and inflammatory conditions aged 19—49 years.
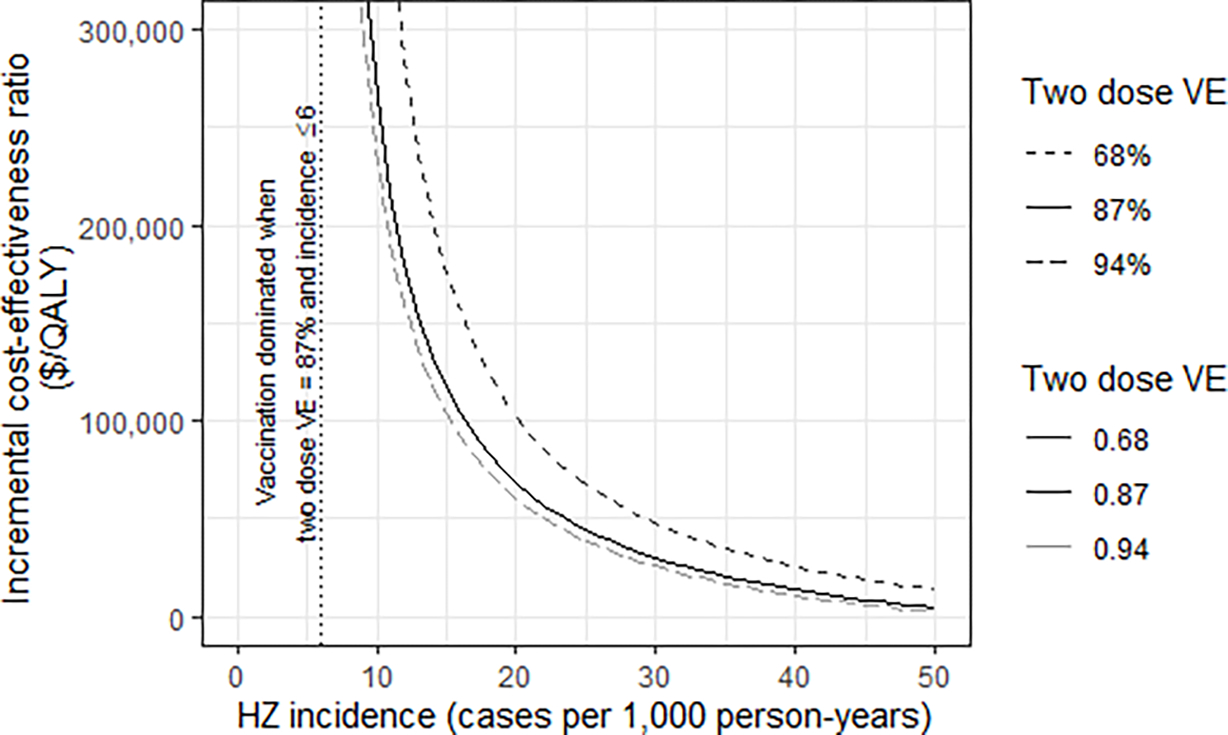
Note(s): HZ = herpes zoster; QALY = quality-adjusted life-year; RZV = recombinant zoster vaccine. VE = vaccine efficacy. All costs are reported in US$2020. The vertical dotted line indicates the highest HZ incidence that yielded a dominated ICER (higher costs and lower health) while assuming VE of 87%. The incidence input used in the autoimmune and inflammatory conditions scenario was 11.57 [10]. For additional reference, the HZ incidence ranges were 3.7–6.4 for patients with psoriasis, 6.6–10.0 for patients with rheumatoid arthritis, and 15.2–24.6 for patients with systemic lupus erythematosus [7]. The set of VE inputs were based on estimates from clinical trials [23, 54] and observational data [22]. Additional details on this analysis are available in the supplemental appendix.
Discussion
In this study of the cost-effectiveness of RZV use among HCT recipients and persons with other selected immunocompromising conditions aged 19–49 years, economic estimates were generally favorable. In particular, the base case analysis and a majority of sensitivity analyses among HCT recipients found cost-savings, which indicate the use of RZV likely improves health in a way that also saves costs for the health system by averting future costly expenses related to HZ episodes and HZ-associated disease. Relative to an immunocompetent population, HCT recipients would likely experience lower VE but have higher HZ incidence and would experience relatively higher averted health care costs. These factors contributed to the economic favorability of cost-effectiveness estimates among the base case HCT recipient group. Other selected immunocompromised patient groups, which used different inputs than the base case, appeared to have economic values for RZV use that were slightly less favorable than the base case. The ICERs for these groups ranged from cost-saving to $208,000, depending on the immunocompromising condition. Across the scenarios looking at different immunocompromised patient groups and within the additional analyses of autoimmune and inflammatory conditions, higher ICERs appeared to be associated with lower HZ incidence inputs. In addition to having different estimates of economic value, the different groups of patients also have heterogeneous population sizes with a wide range of numbers of individuals who are now recommended to receive RZV across the patient groups. There are approximately 3 million individuals in the US who are HCT recipients or patients who have experienced hematologic or solid tumor malignancy, renal or other solid organ transplants, or living with HIV, with an additional 22 million individuals considered as having autoimmune and inflammatory conditions.
Given that vaccine efficacy data was only available for HCT recipients, estimates from the other selected immunocompromised groups should be considered as having greater uncertainty than estimates using the base case inputs. Overall, considering the entire immunocompromised population that would be impacted by a recommendation for RZV use among immunocompromised adults and the high health care costs typically associated with immunocompromising conditions, the use of RZV was considered a reasonable allocation of resources [15].
A number of recent studies have estimated the economic value of HZ vaccines, both RZV and zoster vaccine live [4, 5, 35]. In a systematic review that looked at the economic value of the two HZ vaccines across many different countries, the majority of findings supported that vaccination against HZ could be considered economically favorable in many circumstances [35]. Recent studies that looked at use of RZV among the older adult immunocompetent population in the US found cost-effectiveness estimates of using RZV ranged from $10,000 to $47,000/QALY [4, 5]. These estimates are slightly higher than estimates found in our base case assessment of RZV use among HCT recipients, which is reasonable given that HCT recipients face greater risks of HZ disease risk as well as higher health care costs.
Three additional factors warrant further discussion because they relate to HZ risk and, by extension, also have an impact on the economic value estimated for RZV use in these immunocompromised groups: 1) prophylactic antiviral therapy among HCT recipients, 2) the heterogeneity of HZ risk among immunocompromised patients, particularly those with autoimmune and inflammatory conditions, and 3) the changing epidemiology of HZ due to pediatric varicella vaccination. This study assumed the use of prophylactic antiviral therapy following HCT for 6 months, with a range from 1 month to 2 years used in sensitivity analyses, and vaccine protection that begins as the antiviral period concludes. While the use of antiviral therapies are represented in the model, this study does not estimate the economic value of changes to antiviral regimens and does not provide any direct assessment on the use or duration of antivirals following HCT. The prophylactic use of antivirals reduces risk of HZ and thereby could reduce the economic value of RZV use, especially if the time that antivirals provide HZ protection overlaps to a large extent with the protection provided by vaccination with RZV. Clinical guidance states that for patients on antiviral therapy, vaccination with RZV should be administered two months prior to the discontinuation of antiviral therapy [20]. For patients with different autoimmune and inflammatory conditions, the HZ risk (in terms of cases per 1,000) can range widely, from 3.7–6.4 for patients with psoriasis, to 6.6–10.0 for patients with rheumatoid arthritis, to 15.2–24.6 for patients with systemic lupus erythematosus [7]. The different levels of HZ risk associated with different autoimmune and inflammatory conditions result in a wide range of economic values for vaccination with RZV and so the economic value of vaccination for these patients would also likely be characterized as having substantial uncertainty. Finally, the impact of pediatric varicella vaccination on the incidence of HZ is an area of ongoing research. If the reduction of HZ risk following varicella vaccination persists into adulthood, including among patients with immunocompromising conditions, the economic value of RZV use may decline over time. This study conducted a scenario analysis to explore the possible impact of varicella vaccination on the base case estimates by adjusting HZ incidence downward for adults 19–29 years old, in proportion to the likely varicella vaccination coverage rate among this age group. The varicella vaccination scenario and the base case were both cost-saving, so the adjustment did not change our qualitative conclusions at this time. Among the many factors that can impact an individual’s HZ risk, variations in the use of prophylactic antiviral therapy among HCT recipients, variable HZ risk among patients with autoimmune and inflammatory conditions, and aging of the pediatric varicella-vaccinated cohort are particularly worthy of future research.
This study is subject to a number of limitations. Empirical information on the long-term VE of RZV (i.e., waning immunity) in the HCT population was not available. Inputs on the long-term VE were based on previous modeling efforts related to the use of RZV. An ongoing study indicates that VE remained at 84% when assessed seven years post vaccination in a general population of adults ≥50 years [36]. Estimates for the quality of life loss, given an episode of HZ and related complications, were also not available for this population. Estimates of the costs of HZ episodes, especially inpatient care, among large samples of patients were not available because HZ patients among HCT recipients is a fairly specific patient group. Our estimates attempted to capture these costs judiciously, using the average of an inclusive and restrictive approach to identifying inpatient cases. Unfortunately, a more sophisticated matching or self-controlled assessment of costs could not be conducted due to time constraints. These limitations and input uncertainty, in general, were investigated with the use of univariate and multivariate sensitivity analyses and scenario analyses. In broad terms, the findings of the sensitivity analyses were mostly consistent with the findings of the base case.
In October 2021, ACIP recommended RZV for the prevention of HZ and related complications in adults aged ≥19 years who are or will be immunodeficient or immunosuppressed because of disease or therapy [20]. While the HCT population aged 19–49 years represents a small proportion of the more broadly defined population of immunocompromised adults, the findings from the base case, sensitivity analyses, and scenario analyses of this model supported the ACIP and WG conclusion that use of RZV in immunocompromised adults is a reasonable and efficient allocation of resources. Generally favorable economic estimates support that vaccination with RZV should be incorporated into routine vaccination activities for immunocompromised adults to prevent episodes of HZ and related complications.
Supplementary Material
Highlights.
In a cost-effectiveness analysis, the use of RZV among adults aged 19–49 years who received a hematopoietic cell transplant was found to be cost-saving in the base-case scenario and in most sensitivity analyses. Scenario analyses investigating persons with other selected immunocompromising conditions resulted in a wide range of economic values.
Generally favorable economic estimates further support that vaccination of immunocompromised adults with RZV should be a clinical priority to prevent episodes of herpes zoster and related complications.
In October 2021, recombinant zoster vaccine (RZV, Shingrix) was recommended for use among immunocompromised adults aged ≥19 years.
Acknowledgement:
We greatly appreciate the helpful feedback on previous version of this manuscript provided by members of the ACIP work group on herpes zoster vaccines and from three anonymous reviewers.
Funding/support:
The authors received no financial support for this research.
Footnotes
Conflicts of interest: The authors reported no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
References
- 1.Insinga RP, et al. , The incidence of herpes zoster in a United States administrative database. Journal of General Internal Medicine, 2005. 20(8): p. 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dooling KL, et al. , Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. Morbidity and Mortality Weekly Report, 2018. 67(3): p. 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leidner AJ Overview of two economic models that assess the cost-effectiveness of herpes zoster vaccinations. in Advisory Committee on Immunization Practices, June 2017. 2017. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- 4.Prosser LA, et al. , A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Annals of Internal Medicine, 2019. 170(6): p. 380–388. [DOI] [PubMed] [Google Scholar]
- 5.Curran D, et al. , Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine, 2018. 36(33): p. 5037–5045. [DOI] [PubMed] [Google Scholar]
- 6.McKay SL, et al. , Herpes zoster risk in immunocompromised adults in the United States: A systematic review. Clinical Infectious Diseases, 2020. 71(7): p. e125–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun H, et al. , Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis & rheumatology, 2016. 68(9): p. 2328–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harpaz R and Leung JW, The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clinical Infectious Diseases, 2019. 69(2): p. 341–344. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Supplement Approval. 2021; Available from: https://www.fda.gov/media/151063/download.
- 10.Chen S-Y, et al. , Incidence of herpes zoster in patients with altered immune function. Infection, 2014. 42(2): p. 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, et al. , Duration of antiviral prophylaxis and risk of herpes zoster among patients receiving autologous hematopoietic stem cell transplants: a retrospective, observational study. Advances in therapy, 2017. 34(7): p. 1610–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo H-M, et al. , Antiviral prophylaxis for preventing herpes zoster in hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Antiviral research, 2017. 140: p. 106–115. [DOI] [PubMed] [Google Scholar]
- 13.Weinmann S, et al. , Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. The Journal of infectious diseases, 2013. 208(11): p. 1859–1868. [DOI] [PubMed] [Google Scholar]
- 14.Harpaz R and Leung JW, The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among children. Clinical Infectious Diseases, 2019. 69(2): p. 345–347. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. ACIP Evidence to Recommendations Framework for Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years. 2022. [cited 2022 January 28]; Available from: https://www.cdc.gov/vaccines/acip/recs/grade/recombinant-zoster-immunocompromised-etr.html.
- 16.Bastidas A, et al. , Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. Jama, 2019. 322(2): p. 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega-Sanchez I, Economics of vaccinating immunocompromised 19–49-years old adults against herpes zoster in the US, in Meeting of the Advisory Committee on Immunization Practices, September 29, 2021. 2021, CDC: Atlanta. [Google Scholar]
- 18.Centers for Disease Control & Prevention. Evidence to Recommendations Frameworks. 2021. [cited 2021 November 30]; Available from: https://www.cdc.gov/vaccines/acip/recs/grade/etr.html.
- 19.Anderson TC WG interpretation of the EtR regarding use of RZV in immunocompromised adults, considerations for use and proposed policy changes. in Meeting of the Advisory Committee on Immunization Practices, October 20, 2021. 2021. Atlanta: CDC. [Google Scholar]
- 20.Anderson TC, et al. , Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep, 2022. 71(3): p. 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Center for International Blood and Marrow Transplant Research, Transplant Activity Report Covering 2013–2017. 2018. [Google Scholar]
- 22.Izurieta HS, et al. , Recombinant Zoster Vaccine (Shingrix) real-world effectiveness in the first two years post-licensure. Clinical Infectious Diseases, 2021. [DOI] [PubMed] [Google Scholar]
- 23.Dagnew AF, et al. , Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. The Lancet Infectious diseases, 2019. 19(9): p. 988–1000. [DOI] [PubMed] [Google Scholar]
- 24.Curran D, et al. , Recombinant Zoster Vaccine Significantly Reduces the Impact on Quality of Life Caused by Herpes Zoster in Adult Autologous Hematopoietic Stem Cell Transplant Recipients: A Randomized Placebo-Controlled Trial (ZOE-HSCT). Biology of Blood and Marrow Transplantation, 2019. 25(12): p. 2474–2481. [DOI] [PubMed] [Google Scholar]
- 25.Leidner AJ, et al. , Insurance Reimbursements for Recombinant Zoster Vaccine in the Private Sector. Vaccine, 2022. 39(36): p. 5091–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IBM Watson Health, IBM(R) MarketScan(R) Research Databases for Health Services Researchers. 2019, IBM Corporation: Somers, NY. [Google Scholar]
- 27.Harvey M, et al. , Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain, 2020. 161(2): p. 361–368. [DOI] [PubMed] [Google Scholar]
- 28.Lieu TA, et al. , Community and patient values for preventing herpes zoster. Pharmacoeconomics, 2008. 26(3): p. 235–249. [DOI] [PubMed] [Google Scholar]
- 29.Hanmer J, et al. , Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Medical Decision Making, 2006. 26(4): p. 391–400. [DOI] [PubMed] [Google Scholar]
- 30.Sisk JE, et al. , Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Annals of internal medicine, 2003. 138(12): p. 960–968. [DOI] [PubMed] [Google Scholar]
- 31.Neumann PJ, et al. , Cost-effectiveness in health and medicine. 2016: Oxford University Press. [Google Scholar]
- 32.Brisson M, et al. , Estimating the number needed to vaccinate to prevent diseases and death related to human papillomavirus infection. Cmaj, 2007. 177(5): p. 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leidner AJ, et al. , Guidance for health economics studies presented to the Advisory Committee on Immunization Practices (ACIP), 2019 update. 2019, National Center for Immunization and Respiratory Diseases. [Google Scholar]
- 34.National Center for Immunization and Respiratory Diseases. 1996 through 2017 Childhood Varicella Vaccination Coverage Trend Report. ChildVaxView 2018. [cited 2021 September 1]; Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/data-reports/varicella/trend/index.html.
- 35.Chiyaka ET, et al. , Cost-effectiveness of herpes zoster vaccination: a systematic review. Pharmacoeconomics, 2019. 37(2): p. 169–200. [DOI] [PubMed] [Google Scholar]
- 36.Boutry C, et al. 8. The Adjuvanted Recombinant Zoster Vaccine (RZV) Confers Long-term Protection Against Herpes Zoster: Interim Results of an Extension Study (ZOSTER-049) of Two Clinical Trials (ZOE-50 and ZOE-70). in Open Forum Infectious Diseases. 2020. Oxford University Press US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanni EA, et al. , Burden of herpes zoster in 16 selected immunocompromised populations in England: a cohort study in the Clinical Practice Research Datalink 2000–2012. BMJ open, 2018. 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arias E and Xu J, United States life tables, 2007. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 2017. 68(7): p. 1–65. [PubMed] [Google Scholar]
- 39.Ashton LJ, et al. , A population-based cohort study of late mortality in adult autologous hematopoietic stem cell transplant recipients in Australia. Biology of Blood and Marrow Transplantation, 2014. 20(7): p. 937–945. [DOI] [PubMed] [Google Scholar]
- 40.D’Souza A, et al. , Current use and trends in hematopoietic cell transplantation in the united states. Biology of Blood and Marrow Transplantation, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee GM, et al. , Health-state valuations for pertussis: methods for valuing short-term health states. Health and Quality of Life Outcomes, 2005. 3(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le P and Rothberg MB, Cost-effectiveness of the adjuvanted herpes zoster subunit vaccine in older adults. JAMA internal medicine, 2018. 178(2): p. 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prosser LA, et al. , Valuing health across the lifespan: health state preferences for seasonal influenza illnesses in patients of different ages. Value in Health, 2011. 14(1): p. 135–143. [DOI] [PubMed] [Google Scholar]
- 44.Johnson BH, et al. , Healthcare resource utilization and costs associated with herpes zoster in the US. Journal of medical economics, 2016. 19(10): p. 928–935. [DOI] [PubMed] [Google Scholar]
- 45.Yawn BP, et al. , Health care utilization and cost burden of herpes zoster in a community population. Mayo Clinic Proceedings, 2009. 84(9): p. 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai Y, Zhou F, and Lindley MC, Insurance Reimbursements for Routinely Recommended Adult Vaccines in the Private Sector. American Journal of Preventive Medicine, 2019. 57(2): p. 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poudel DR, et al. , Burden of hospitalizations related to adverse drug events in the USA: a retrospective analysis from large inpatient database. Pharmacoepidemiology and drug safety, 2017. 26(6): p. 635–641. [DOI] [PubMed] [Google Scholar]
- 48.Linley L, et al. , Estimated HIV incidence and prevalence in the United States, 2014–2018. 2020. [Google Scholar]
- 49.Lesko CR, et al. , Ten-year survival by race/ethnicity and sex among treated, HIV-infected adults in the United States. Clinical Infectious Diseases, 2015. 60(11): p. 1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habel LA, et al. , The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiology and Prevention Biomarkers, 2013. 22(1): p. 82–90. [DOI] [PubMed] [Google Scholar]
- 51.Hill HA, et al. , Vaccination coverage among children aged 19–35 months—United States, 2017. Morbidity and Mortality Weekly Report, 2018. 67(40): p. 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchan SA, et al. , Incidence of hospitalizations and emergency department visits for herpes zoster in immunocompromised and immunocompetent adults in Ontario, Canada, 2002–2016. Clinical Infectious Diseases, 2020. 71(1): p. 22–29. [DOI] [PubMed] [Google Scholar]
- 53.Li Q, et al. Cost of herpes zoster in patients with selected immune-compromised conditions in the United States. in Open forum infectious diseases. 2016. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lal H, et al. , Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. New England Journal of Medicine, 2015. 372(22): p. 2087–2096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


