Abstract
Importance:
Intrapartum antibiotics greatly reduce a newborn’s risk of group B streptococcal infection (GBS) but may increase the risk of obesity during childhood due to disruption of microbiome formation.
Objective:
To examine the association between GBS intrapartum antibiotics and body mass index (BMI) trajectory during the first 5 years after birth.
Design, Setting, and Participants:
Retrospective cohort study in an integrated health care system in Southern California of 223,431 singleton-birth infants with >12 months of follow-up between 2007 and 2015.
Exposure:
We compared children exposed to GBS intrapartum antibiotic prophylaxis (IAP) defined as administration of penicillin G, ampicillin, cefazolin, clindamycin or vancomycin ≥4 hours before delivery to those unexposed or exposed to any other type or duration of antibiotics within 48 hours before delivery. Reference for vaginal delivery were unexposed children. Because virtually all children born by Cesarean delivery are exposed, other antibiotics were used as reference.
Main Outcome and Measure:
BMI measured during routine visits between 0 and 5 years of age was compared between exposure groups using non-linear multivariable models with B-spline functions (knots 0.4, 1.2, and 2.7 years of age), stratified by delivery mode (vaginal or Cesarean), and adjusted for demographic, maternal and birth-related factors, breastfeeding and childhood antibiotic exposure.
Results:
The mean follow-up time was 4.94 (SD 2.71) years (1,103,569 person-years) with 13.4 (SD 5.3) BMI measures per child; 20.0% (31,263/156,381) of vaginal and 12.5% (8,366/67,050) of Cesarean deliveries were exposed to GBS IAP. In vaginally delivered infants, GBS IAP, but not other intrapartum antibiotics, were associated with increased BMI at age 5 (0.12 kg/m2, 95% CI 0.07 to 0.16 kg/m2, P<0.001), compared to no antibiotics. In Cesarean deliveries, GBS IAP was associated with increased BMI at age 5 (0.24 kg/m2, 95% CI 0.14 to 0.34 kg/m2, P<0.001) compared to other antibiotics. Breastfeeding did not modify these associations.
Conclusions and Relevance:
With GBS IAP used in 1 in 3 U. S. births, the potential increase in BMI on a population level, although small, is relevant. While this does not support changing current practice, it is important to also pursue preventive strategies such as maternal GBS immunization.
Keywords: Perinatal group B streptococcus disease, intrapartum antibiotic prophylaxis, childhood obesity, body mass index
INTRODUCTION
Since the early 1990s, intrapartum antibiotic prophylaxis (IAP) to prevent the vertical transmission of group B Streptococcus (GBS) has resulted in an approximate 80% decline in early-onset GBS sepsis, a leading cause of neonatal sepsis in the United States.1 At the same time, the proportion of infants exposed to intrapartum antibiotics increased from 12% in the early 1990s to over 30% in 2014.2–4
Animal studies indicate that antibiotics at young age lead to long-term increases in body weight by altering the intestinal microbiota.5 GBS IAP is associated with a decreased bacterial diversity of a newborn’s microbiome.6–11 Recent meta-analyses and systematic reviews suggested a link between antibiotic use in early childhood and increased prevalence of overweight and obesity.12,13 However, the effect of intrapartum exposure to antibiotics on the risk of excess weight gain in offspring is unclear.
We examined the association between GBS IAP and body mass index (BMI) trajectory during the first 5 years of life. Additionally, we investigated whether breastfeeding modified the association.
PATIENTS AND METHODS
Study Setting and Design
Kaiser Permanente Southern California (KPSC) is a large, integrated health care delivery organization that serves approximately 4.3 million members throughout Southern California (~17% of the population) including approximately 40,000 births each year. The membership is diverse and similar in socioeconomic characteristics to the region’s census demographics.14 We conducted a retrospective cohort study among infants born to health plan members. The KPSC Institutional Review Board (IRB) approved the study and granted a waiver for informed consent. A separate IRB review of the Centers for Disease Control and Prevention (CDC) was not required because CDC staff was not engaged in human subject research.
Study population
We identified singleton infants born in KPSC hospitals between January 01, 2007 and December 31, 2015 (n=302,732). We excluded infants whose mothers did not have at least 3 months continuous health plan enrollment before delivery (n=19,443), who did not remain health plan members for 12 months after birth to allow follow-up (n=58,717) and those born before 22 weeks of gestation (n=167). The analytical cohort for this study consisted of 223,431 (74.8%) infants (eFigure 1).15 The population was a priori stratified by delivery mode (vaginal or Cesarean section).
Antibiotic Exposure
Exposure to antibacterial agents (oral, intramuscular, or intravenous) was extracted from maternal electronic medical records. We defined intrapartum as the time between maternal admission and delivery. For mothers with longer or multiple inpatient stays before delivery, we defined intrapartum as 48 hours before delivery to exclude stays for false labor or maternal observation. The intrapartum exposure to antibiotics was calculated as time from first administration to delivery and classified as: 1) no intrapartum exposure to antibiotics, 2) antibiotic prophylaxis for perinatal GBS disease (GBS IAP) defined as administration of penicillin G, ampicillin, cefazolin, clindamycin and/or vancomycin for ≥4 hours before delivery,16 or 3) any other type or duration of intrapartum antibiotic administration. Because of universal Cesarean prophylaxis (typically intravenous cefazolin administered within 60 mins before delivery), all infants delivered via Cesarean section were exposed to intrapartum antibiotics.
Follow-up for childhood body mass index
Body weight, measured by trained staff on calibrated scales at routine medical office visits during the first five years of childhood, was obtained from electronic medical records.17 Height was measured by calibrated stadiometer or horizontal length board on the same day. After data entry by staff, weight and height were visualized in growth charts with previous measurements to highlight data entry errors. In 2007 and 2008, the error rates in weight and height data of children aged <2 and 2–5 years were 0.4% and 0.7%, respectively.18 To transform recumbent length into standing height, we subtracted 0.8 cm from recumbent length in children under the age of 18 months.19 BMI was calculated as weight in kilograms divided by the square of height in meters. Children were followed for a maximum of 5 years through February 08, 2018. BMI was analyzed from birth until the first occurrence of one of the following events: reaching 5 years of age, end of KPSC health care coverage, death, or the end of follow-up.
Covariates
Data regarding demographic, maternal, birth-related, and childhood factors were obtained from validated research databases derived from health plan electronic medical records, administrative records, and birth records (eTable 1).18,20–23 Covariates included antepartum and postpartum exposure to antibacterial agents.18,20–23
Statistical Analysis
We compared clinical and demographic characteristics among intrapartum antibiotic exposure groups using descriptive statistical methods including Pearson chi-square test for categorical variables and analysis of variance (ANOVA), the Kruskal-Wallis, student’s T, or the Wilcoxon rank sum tests. For summary tables, children at age 5 were categorized based on the sex-specific BMI-for-age growth charts developed by the Centers for Disease Control and Prevention (CDC) as underweight (BMI-for-age <5th percentile), normal weight (BMI-for-age ≥5th to <85th percentile), overweight (BMI-for-age ≥85th to <95th percentile), or obesity (BMI-for age ≥95th).24
Missing values in pre-pregnancy weight (n=28,420, 12.7%) and gestational weight gain (n=22,372, 10.0%) were imputed by performing a single regression imputation adjusting for race/ethnicity and parity (eTable 1).25
We examined the association between intrapartum antibiotic exposure (None, GBS IAP, Other) and longitudinal BMI using non-linear multivariable models stratified by delivery mode. B-spline functions were fitted to allow for flexible modelling of BMI trajectories over time and for varying fit by exposure group. Quadratic splines with 3 knots were selected based on initial unadjusted plots and model fit. BMI trajectories over time were similar when comparing the entire cohort, children with more than 4 BMI measurements or over at least 4 years (data not shown). For all models, we set spline knots at 0.4, 1.2, and 2.7 years of age, based on best model fit but consistent with 2000 CDC growth charts.24 We used marginal models with robust standard errors26 accounting for the repeated measures within each child to determine the mean BMI at each time point and to calculate the difference in mean population BMI between the intrapartum antibiotic exposure and the reference category. We evaluated above models against unadjusted and fully adjusted mixed effect regression models for each delivery method including a random effect to accommodate within child correlation over time with comparable results; for the latter, missing outcome data were handled through maximum likelihood estimation under the assumption of missing at random. We adjusted for demographics, maternal and birth-related covariates (eTable 1; referred to as ‘Birth Model’) and childhood covariates (referred to as ‘Full Model’). The selection of adjustment variables was informed by the development of a directed acyclic graph and the Bayesian information criterion.27 We also present comparisons of parameter estimates for antibiotic exposure antepartum (trimester 1/2, trimester 3) and postpartum (neonatal, lactation, childhood). Separate two-way interaction terms with exposure were tested for breastfeeding, maternal pre-pregnancy weight class, maternal diabetes, maternal hypertension, and chorioamnionitis. For meaningful figures of BMI trajectories, model covariates were set at the population mean for continuous and mode for categorical variables.
To translate BMI differences into weight gain differences, we transformed predicted BMIs from the fully adjusted model into weight (kg) = predicted BMI * height in m2 to determine weight in kg at age 5 years. We used the mean height at baseline and age 5 for each exposure category and delivery method from the unadjusted data. Confidence intervals (95% CI) were constructed using the standard error of the difference.
We conducted several sensitivity analyses (see eTable 2) including: 1) restricting to healthy term-born children,20,21 2) limiting the GBS IAP antibiotics to children who received no additional non-GBS agents, 3) creating antibiotic exposure groups that were homogenous with regard to GBS colonization, and 4) excluding children exposed to antibiotics during pregnancy. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R version 3.5.3.28
RESULTS
Cohort description
Of the 223,431 children included in this study, 70.0% were delivered vaginally and 30.0% were delivered via Cesarean section (Table 1). The cohort contained 1,103,569 person-years, with a mean follow-up time of 4.94 years (SD 2.71 years). A total of 122,708 (54.9%) children completed follow-up to 5 years of age (eFigure 1). Children had an average of 13.4 (SD 5.3) BMI measures during follow-up. Among vaginal deliveries, 113,693 (72.7%) were unexposed to antibiotics during the intrapartum period, 31,263 (20.0%) were exposed to GBS IAP, and 11,425 (7.3%) were exposed to other intrapartum antibiotics. Among Cesarean deliveries, 8,366 (12.5%) were exposed to GBS IAP, and 58,684 (87.5%) were exposed to antibiotics of other type or duration including antibiotic prophylaxis to prevent maternal post-Cesarean infection (eTable 3).
Table 1:
Demographic, maternal, birth-related, and childhood characteristics by delivery mode and intrapartum antibiotic exposure*
| Total Cohort | Vaginal delivery | Cesarean delivery | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Antibiotic exposure: | None | GBS | Other | P value | GBS | Other | P value | |
| N | 223431 | 113693 | 31263 | 11425 | 8366 | 58684 | ||
|
| ||||||||
| Children within delivery mode (%) | 72.7 | 20.0 | 7.3 | 12.5 | 87.5 | |||
| Total follow-up, y | <0.001 | <0.001 | ||||||
| Mean | 4.9 (2.71) | 5.1 (2.80) | 4.5 (2.43) | 4.7 (2.47) | 4.6 (2.44) | 5.0 (2.74) | ||
| Median (IQR) | 4.4 (2.7, 7.0) | 4.6 (2.7, 7.2) | 4.0 (2.6, 6.4) | 4.2 (2.7, 6.5) | 4.1 (2.6, 6.4) | 4.5 (2.7, 7.1) | ||
| Demographics | ||||||||
| Sex, | <0.001 | <0.001 | ||||||
| Male | 108967 (48.8) | 56801 (50.0) | 15198 (48.6) | 5551 (48.6) | 3682 (44.0) | 27735 (47.3) | ||
| Female | 114464 (51.2) | 56892 (50.0) | 16065 (51.4) | 5874 (51.4) | 4684 (56.0) | 30949 (52.7) | ||
| Race/Ethnicity | <0.001 | <0.001 | ||||||
| White | 63085 (28.2) | 32554 (28.6) | 8494 (27.2) | 2946 (25.8) | 2112 (25.2) | 16979 (28.9) | ||
| Hispanic | 105699 (47.3) | 55662 (49.0) | 14200 (45.4) | 5475 (47.9) | 3701 (44.2) | 26661 (45.4) | ||
| Black | 18934 (8.5) | 8157 (7.2) | 3277 (10.5) | 989 (8.7) | 1080 (12.9) | 5431 (9.3) | ||
| Asian/Pacific Islander | 27937 (12.5) | 13394 (11.8) | 4123 (13.2) | 1632 (14.3) | 1187 (14.2) | 7601 (13) | ||
| Other, unknown | 7776 (3.5) | 3926 (3.5) | 1169 (3.7) | 383 (3.4) | 286 (3.4) | 2012 (3.4) | ||
| State-subsidized health plan | 20071 (9.0) | 10273 (9.0) | 3275 (10.5) | 1082 (9.5) | <0.001 | 688 (8.2) | 4753 (8.1) | 0.697 |
| Maternal and pregnancy-related factors | ||||||||
| Maternal education | <0.001 | 0.002 | ||||||
| Less than HS | 10969 (4.9) | 6275 (5.5) | 1263 (4.0) | 547 (4.8) | 295 (3.5) | 2589 (4.4) | ||
| High School | 55668 (25.0) | 29347 (25.9) | 7857 (25.2) | 2874 (25.2) | 1921 (23.0) | 13669 (23.3) | ||
| Some college | 67772 (30.4) | 33994 (30.0) | 9783 (31.3) | 3426 (30.0) | 2608 (31.2) | 17961 (30.6) | ||
| Bachelor degree or higher | 86037 (38.5) | 42565 (37.5) | 11950 (38.2) | 4418 (38.7) | 3423 (41.0) | 23681 (40.4) | ||
| Unknown | 2985 (1.2) | 1512 (1.1) | 410 (1.3) | 160 (1.3) | 119 (1.3) | 784 (1.3) | ||
| Parity | <0.001 | <0.001 | ||||||
| 1 | 168219 (75.3) | 83883 (73.8) | 24256 (77.6) | 8440 (73.9) | 7468 (89.3) | 44172 (75.3) | ||
| 2 | 36208 (16.2) | 19336 (17.0) | 4353 (13.9) | 1938 (17.0) | 578 (6.9) | 10003 (17.0) | ||
| 3+ | 19004 (8.5) | 10474 (9.2) | 2654 (8.5) | 1047 (9.2) | 320 (3.8) | 4509 (7.7) | ||
| Diabetes | 13542 (6.1) | 4962 (4.4) | 2104 (6.7) | 671 (5.9) | <0.001 | 791 (9.5) | 5014 (8.5) | 0.010 |
| Pre-pregnancy BMI (kg/m2) | ||||||||
| Mean (SD) | 26.9 (6.15) | 26.2 (5.64) | 26.9 (6.23) | 26.3 (5.79) | <0.001 | 28.6 (7.12) | 28.2 (6.70) | <0.001 |
| Pregnancy weight gain (lbs) | ||||||||
| Mean (SD) | 27.5 (13.53) | 27.6 (12.86) | 26.8 (14.11) | 27.2 (13.25) | <0.001 | 28.0 (15.41) | 27.6 (14.22) | <0.001 |
| Chorioamnionitis | 10869 (4.9) | 1203 (1.1) | 2213 (7.1) | 2604 (22.8) | <0.001 | 1806 (21.6) | 3043 (5.2) | <0.001 |
| Smoking during pregnancy | 24231 (10.8) | 11610 (10.2) | 3644 (11.7) | 1177 (10.3) | <0.001 | 1075 (12.8) | 6725 (11.5) | <0.001 |
| GBS culture status | <0.001 | <0.001 | ||||||
| Negative | 163026 (73.0) | 106423 (93.6) | 4662 (14.9) | 5384 (47.1) | 2173 (26) | 44384 (75.6) | ||
| Positive | 38258 (17.1) | 3204 (2.8) | 19535 (62.5) | 4305 (37.7) | 4469 (53.4) | 6745 (11.5) | ||
| Unknown | 22147 (9.9) | 4066 (3.6) | 7066 (22.6) | 1736 (15.2) | 1724 (20.6) | 7555 (12.9) | ||
| Antibiotic exposure | ||||||||
| Antepartum, trimester 1/2 | 65276 (29.2) | 30040 (26.4) | 11055 (35.4) | 3567 (31.2) | <0.001 | 2976 (35.6) | 17638 (30.1) | <0.001 |
| Antepartum, trimester 3 | 26710 (11.9) | 12053 (10.6) | 4589 (14.7) | 1502 (13.1) | <0.001 | 1319 (15.8) | 7247 (12.3) | <0.001 |
| Birth | ||||||||
| Birth weight (g) | ||||||||
| Mean (SD) | 3346 (546) | 3374 (458) | 3252 (577) | 3281 (543) | <0.001 | 3261 (760) | 3364 (634) | <0.001 |
| Gestational age (weeks) | ||||||||
| Mean (SD) | 38.7 (1.81) | 39.0 (1.33) | 38.4 (2.21) | 38.6 (2.02) | <0.001 | 38.2 (2.95) | 38.5 (2.04) | <0.001 |
| Preterm birth (<37th week) | 17191 (7.7) | 3638 (3.2) | 4880 (15.6) | 1173 (10.3) | <0.001 | 1546 (18.5) | 5954 (10.1) | <0.001 |
| Childhood | ||||||||
| Any Breastfeeding | ||||||||
| at 3 months of age | 147716 (66.1) | 75710 (70.3) | 21236 (70.1) | 7817 (70.6) | 0.717 | 5481 (68.0) | 37472 (67.8) | 0.634 |
| at 6 months of age | 125523 (56.2) | 64469 (59.8) | 18182 (60.1) | 6720 (60.6) | 0.232 | 4644 (57.6) | 31508 (57) | 0.262 |
| Antibiotic exposure | ||||||||
| Neonatal | ||||||||
| first 4 days (72 h) | 12953 (5.8) | 2528 (2.2) | 2851 (9.1) | 1360 (11.9) | <0.001 | 1534 (18.3) | 4680 (8.0) | <0.001 |
| 4–28 days | 7438 (3.3) | 2415 (2.1) | 1358 (4.3) | 548 (4.8) | <0.001 | 691 (8.3) | 2426 (4.1) | <0.001 |
| Indirect during lactation | ||||||||
| 0–3 mo | 30232 (13.5) | 13392 (11.8) | 4389 (14.0) | 1716 (15) | <0.001 | 1742 (20.8) | 8993 (15.3) | <0.001 |
| 3–6 mo | 13473 (6.0) | 6417 (5.6) | 2072 (6.6) | 706 (6.2) | 0.736 | 528 (6.3) | 3750 (6.4) | 0.782 |
| Cumulative childhood episodes | ||||||||
| Mean (SD) | 2.7 (3.11) | 2.7 (3.04) | 2.6 (2.99) | 2.6 (2.95) | 0.153 | 2.9 (3.26) | 2.9 (3.32) | 0.159 |
| BMI class at age 5# | <0.001 | 0.014 | ||||||
| N | 104394 | 55005 | 12714 | 4945 | 3556 | 28174 | ||
| Underweight | 4011 (3.8) | 2171 (3.9) | 509 (4.0) | 215 (4.3) | 147 (4.1) | 969 (3.4) | ||
| Normal weight | 73214 (70.1) | 39439 (71.7) | 8863 (69.7) | 3477 (70.3) | 2326 (65.4) | 19109 (67.8) | ||
| Overweight | 14164 (13.6) | 7185 (13.1) | 1781 (14.0) | 678 (13.7) | 524 (14.7) | 3996 (14.2) | ||
| Obesity | 13005 (12.5) | 6210 (11.3) | 1561 (12.3) | 675 (11.6) | 559 (15.7) | 4100 (14.6) | ||
Antibiotic exposure: none, no intrapartum exposure to antibiotics; GBS, GBS intrapartum antibiotic prophylaxis as recommended for the prevention of perinatal group B streptococcal disease; other, any other type or duration of intrapartum antibiotic administration
Extrapolated based on the nearest 2 weight measures within 6 months of a child’s 5th birthday (N=104394).
Infants born to mothers exposed to GBS IAP were more likely to be born preterm (P <0.001 for vaginal and Cesarean section deliveries), born to a mother with diabetes (P < 0.001 for vaginal, P =0.010 for CS), or of Black race (P <0.001 for both delivery methods) than were infants exposed to no or other intrapartum antibiotics. They were also more likely to be exposed to antibiotics antepartum for both delivery methods (P <0.001 for both), during the neonatal period for Cesarean section deliveries (P < 0.001), and more likely to be on a state subsidized health plan for vaginal deliveries (P< 0.001).
Vaginal delivery
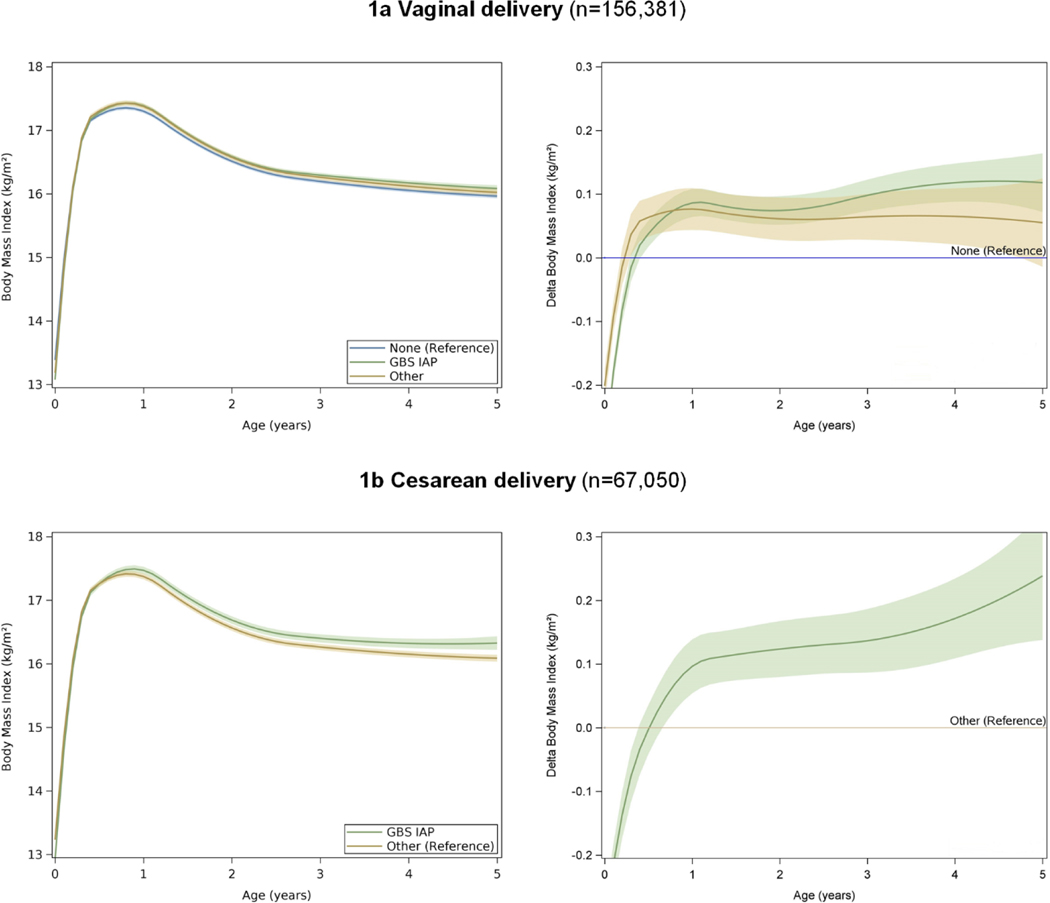
We observed significant differences in BMI between the GBS IAP and unexposed groups (unadjusted Δ BMI at age 5 = 0.09 kg/m2, 95% CI 0.04 kg/m2 to 0.13 kg/m2, P<0.001, Table 2, crude models, eFigure 2). BMI did not differ between the other intrapartum antibiotic and unexposed groups. Adjustment for sociodemographic, maternal and delivery-related factors (Birth Model – covariates up to delivery), breastfeeding, and exposure to antibiotics during childhood (Full Model – covariates through childhood) slightly strengthened the association between GBS IAP and BMI starting after the age of 0.5 years (Full Model, adjusted Δ BMI at age 5 = 0.12 kg/m2, 95% CI 0.07 kg/m2 to 0.16 kg/m2, P<0.001, Figure 1a). No significant interactions between breastfeeding and other covariates with antibiotic exposure were observed, indicating that breastfeeding and other covariates did not modify the association of antibiotic exposure and BMI.
Table 2:
Body mass index difference between children unexposed to intrapartum antibiotics (None), children exposed to intrapartum antibiotic prophylaxis as recommended for the prevention of perinatal group B streptococcal disease (GBS) and any other type or duration of intrapartum antibiotic administration (Other) stratified by delivery mode.
| Vaginal delivery | Cesarean delivery | ||||
|---|---|---|---|---|---|
|
|
|||||
| Child’s age (years) | None (BMI, kg/m2) | GBS (Δ BMI, kg/m2) | Other (Δ BMI, kg/m2) | Other (BMI, kg/m2) | GBS (Δ BMI, kg/m2) |
|
| |||||
| Total cohort, N | 113693 | 31263 | 11425 | 58684 | 8366 |
| Crude model | Reference | Reference | |||
| 0.0 | 13.35 (0.004) | −0.36 (−0.38, −0.34) | −0.26 (−0.29, −0.23) | 13.29 (0.007) | −0.37 (−0.41, −0.32) |
| 1.0 | 17.29 (0.005) | 0.05 (0.03, 0.07) | 0.02 (−0.01, 0.05) | 17.46 (0.007) | 0.04 (0.00, 0.08) |
| 2.0 | 16.53 (0.005) | 0.03 (0.01, 0.06) | 0.01 (−0.03, 0.04) | 16.69 (0.007) | 0.07 (0.03, 0.11) |
| 3.0 | 16.23 (0.006) | 0.06 (0.04, 0.08) | 0.01 (−0.03, 0.04) | 16.43 (0.008) | 0.08 (0.04, 0.13) |
| 4.0 | 16.11 (0.007) | 0.08 (0.05, 0.11) | 0.01 (−0.03, 0.05) | 16.35 (0.010) | 0.12 (0.06, 0.18) |
| 5.0 | 16.03 (0.010) | 0.09 (0.04, 0.13) | 0.00 (−0.07, 0.07) | 16.31 (0.015) | 0.18 (0.09, 0.28) |
| Birth Model (adjusting for covariates through delivery) # | |||||
| 0.0 | 13.25 (0.014) | −0.31 (−0.33, −0.29) | −0.20 (−0.23, −0.18) | 13.11 (0.021) | −0.31 (−0.36, −0.27) |
| 1.0 | 17.17 (0.014) | 0.08 (0.06, 0.10) | 0.07 (0.04, 0.10) | 17.25 (0.021) | 0.09 (0.05, 0.13) |
| 2.0 | 16.40 (0.014) | 0.07 (0.05, 0.09) | 0.06 (0.02, 0.09) | 16.49 (0.021) | 0.12 (0.07, 0.16) |
| 3.0 | 16.11 (0.014) | 0.09 (0.07, 0.12) | 0.06 (0.02, 0.09) | 16.23 (0.021) | 0.13 (0.08, 0.18) |
| 4.0 | 15.98 (0.014) | 0.12 (0.09, 0.15) | 0.06 (0.01, 0.10) | 16.15 (0.022) | 0.16 (0.10, 0.23) |
| 5.0 | 15.91 (0.016) | 0.12 (0.07, 0.16) | 0.05 (−0.02, 0.12) | 16.12 (0.025) | 0.23 (0.13, 0.33) |
| Full Model (adjusting for covariates through childhood) $ | |||||
| 0.0 | 13.38 (0.015) | −0.31 (−0.33, −0.29) | −0.20 (−0.23, −0.17) | 13.23 (0.023) | −0.31 (−0.35, −0.27) |
| 1.0 | 17.30 (0.015) | 0.09 (0.06, 0.10) | 0.08 (0.04, 0.11) | 17.37 (0.023) | 0.10 (0.05, 0.14) |
| 2.0 | 16.51 (0.015) | 0.07 (0.05, 0.10) | 0.06 (0.03, 0.09) | 16.56 (0.023) | 0.12 (0.08, 0.17) |
| 3.0 | 16.20 (0.016) | 0.10 (0.07, 0.12) | 0.06 (0.03, 0.10) | 16.26 (0.024) | 0.14 (0.09, 0.19) |
| 4.0 | 16.05 (0.016) | 0.12 (0.09, 0.15) | 0.07 (0.02, 0.11) | 16.15 (0.025) | 0.17 (0.11, 0.23) |
| 5.0 | 15.97 (0.018) | 0.12 (0.07, 0.16) | 0.06 (−0.01, 0.12) | 16.09 (0.028) | 0.24 (0.14, 0.34) |
Birth Model (covariates through delivery) adjustment for demographics, maternal and birth-related factors included infant sex, gestational age at birth, birth weight, infant’s race/ethnicity (White, Black, Hispanic, Asian or Pacific Islander, other or unknown), year of birth, medical center of birth, maternal education, parity, maternal diabetes, maternal pre-pregnancy BMI, maternal gestational weight gain, maternal smoking during pregnancy, antepartum antibiotic exposure.
Full Model (covariates through childhood) adjustment included previously listed Birth Model variables plus neonatal antibiotic exposure, breastfeeding, indirect antibiotic exposure during breastfeeding, and childhood antibiotic exposure.
Sensitivity analysis cohort excludechildren with a birth weight above and below the 5th percentile, born before 37th week of gestation, infants with a positive blood or cerebrospinal fluid culture in first 72 hours with > 5 days of antibiotic therapy, prolonged birth admission (ICU admission > 5 days or total stay > 7 days, infants with any pediatric complex care conditions during follow-up.
Figure 1:
Adjusted body mass index (BMI), delta BMI, and their 95% CIs for children unexposed to intrapartum antibiotics (None), children exposed to intrapartum antibiotic prophylaxis as recommended for the prevention of perinatal group B streptococcal disease (GBS) and any other type or duration of intrapartum antibiotic administration (Other) stratified by delivery mode
Covariate adjustments included demographics, maternal and birth-related factors included infant sex, gestational age at birth, birth weight, infant’s race/ethnicity (White, Black, Hispanic, Asian or Pacific Islander, other or unknown), year of birth, medical center of birth, maternal education, parity, maternal diabetes, maternal pre-pregnancy BMI, maternal gestational weight gain, maternal smoking during pregnancy, antepartum antibiotic exposure, neonatal antibiotic exposure, any breastfeeding, indirect antibiotic exposure during breastfeeding, and childhood antibiotic exposure.
Our results translated into a difference in attributable weight gain from birth to 5 years of age of 0.32 kg (95% CI 0.31 kg to 0.33 kg, P <0.001) for GBS IAP and 0.16 kg (95% CI 0.15 kg to 0.18 kg, P <0.001) for other intrapartum antibiotics compared to no antibiotics (Table 3).
Table 3:
Attributable body weight difference from birth to 5 years of age in children unexposed to intrapartum antibiotics (None), children exposed to intrapartum antibiotic prophylaxis as recommended for the prevention of perinatal group B streptococcal disease (GBS) and any other type or duration of intrapartum antibiotic administration (Other) stratified by delivery mode.
| Vaginal delivery | Cesarean delivery | ||||
|---|---|---|---|---|---|
|
|
|||||
| None (weight gain, kg) | GBS (Δ weight gain, kg) | Other (Δ weight gain, kg) | Other (weight gain, kg) | GBS (Δ weight gain, kg) | |
|
| |||||
| Total cohort, N | 113693 | 31263 | 11425 | 58684 | 8366 |
| Reference | Reference | ||||
| Crude model | 16.08 | 0.30 (0.29, 0.30) | 0.12 (0.10, 0.13) | 16.47 | 0.38 (0.36, 0.39) |
| Full Model | 16.00 | 0.32 (0.31, 0.33) | 0.16 (0.15, 0.18) | 16.21 | 0.43 (0.41, 0.45) |
Using the Full Model (Covariates through childhood) adjustment for demographics, maternal and birth-related factors included infant sex, gestational age at birth, birth weight, infant’s race/ethnicity (White, Black, Hispanic, Asian or Pacific Islander, other or unknown), year of birth, medical center of birth, maternal education, parity, maternal diabetes, maternal pre-pregnancy BMI, maternal gestational weight gain, maternal smoking during pregnancy, antepartum antibiotic exposure, neonatal antibiotic exposure, breastfeeding, indirect antibiotic exposure during breastfeeding, and childhood antibiotic exposure.
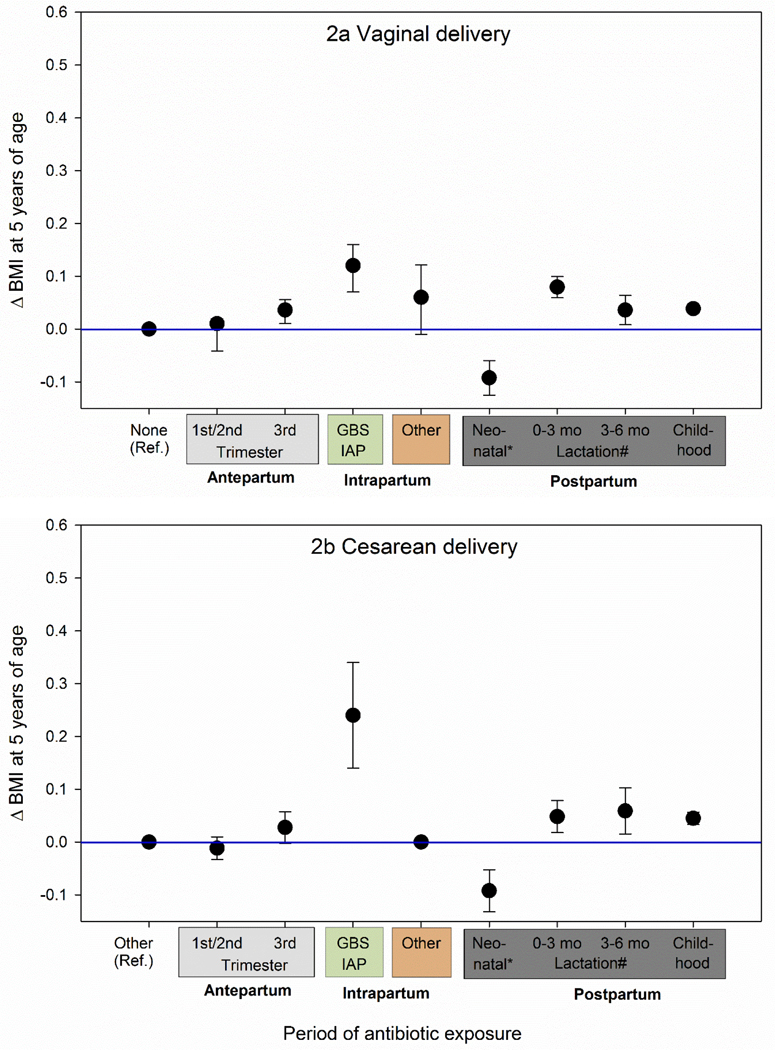
In contrast, antepartum exposure to antibiotics was associated with an adjusted BMI of 0.01 kg/m2 (95% CI 0.00 kg/m2 to 0.02 kg/m2, P =0.178) for the first or second trimester of pregnancy and 0.04 kg/m2 (95% CI 0.02 kg/m2 to 0.06 kg/m2, P <0.001) for the third trimester of pregnancy (Figure 2a). The indirect antibiotic exposure during lactation with an adjusted Δ BMI of 0.08 kg/m2 (95% CI 0.06 kg/m2 to 0.10 kg/m2, P <0.001) during the first 3 months of life and of 0.04 kg/m2 (95% CI 0.01 kg/m2 to 0.06 kg/m2, P <0.012) during the first 3 to 6 months of life. Each 14-day antibiotic treatment episode during follow-up was associated with an adjusted Δ BMI of 0.04 kg/m2 (95% CI 0.03 kg/m2 to 0.05 kg/m2, P <0.001).
Figure 2:
Comparison of adjusted excess BMI at 5 years of age associated with exposure to antibiotics during antepartum, intrapartum and postpartum periods
*Δ BMI attributable to neonatal antibiotics is no longer significant when excluding children with low or high birth weight and/or complex care conditions (Vaginal delivery: −0.05 kg/m2, 95% CI −0.11 kg/m2 to 0.01 kg/m2, P =0.090; Cesarean delivery: 0.02 kg/m2, 95% CI −0.04 kg/m2 to 0.09 kg/m2, P =0.499; see eFigure 3).
#Indirect antibiotic exposure through breastfeeding by a mother using antibiotics during the first 3 months after birth. The estimate for childhood exposure is per 14-day episode of antibiotic use.
Covariates adjusted for included demographics, maternal and birth-related factors included infant sex, gestational age at birth, birth weight, infant’s race/ethnicity (White, Black, Hispanic, Asian or Pacific Islander, other or unknown), year of birth, medical center of birth, maternal education, parity, maternal diabetes, maternal pre-pregnancy BMI, maternal gestational weight gain, maternal smoking during pregnancy, antepartum antibiotic exposure, neonatal antibiotic exposure, any breastfeeding, indirect antibiotic exposure during breastfeeding, and childhood antibiotic exposure.
The reference point reflects a population mean BMI at age 5 of 15.97 (SD 0.018) kg/m2 for vaginally delivered children unexposed to intrapartum antibiotics (None) and 16.09 (SD 0.028) kg/m2 for children delivered by Cesarean section and exposed to other intrapartum antibiotics (Other).
Cesarean delivery
Children delivered via Cesarean section had a significantly higher BMI if exposed to GBS IAP compared to children exposed to other intrapartum antibiotics (unadjusted Δ BMI at age 5 = 0.18 kg/m2, 95% CI 0.09 kg/m2 to 0.28 kg/m2, P<0.001, Table 2, crude analysis). Further adjustment for covariates slightly strengthened the association between GBS IAP and childhood BMI after the age of 0.7 years (Full Model adjusted Δ BMI at age 5 = 0.24 kg/m2, 95% CI 0.14 kg/m2 to 0.34 kg/m2, P <0.001, Figure 1b). No significant interactions between breastfeeding and other covariates with antibiotic exposure were observed (P >0.150).
This translated into a difference in attributable weight gain from birth to 5 years of age of 0.43 kg (95% CI 0.41 kg to 0.45 kg, P <0.001) for GBS IAP compared to other intrapartum antibiotics (Table 3).
The association between GBS IAP and childhood BMI was stronger than the association observed with exposure to antibiotics at other time points during pregnancy and childhood (Figure 2b).
Sensitivity analyses
Restricting the cohort to healthy, term children slightly attenuated the association between GBS IAP and childhood BMI for vaginally delivered children (sensitivity analyses 1, full model adjusted Δ BMI at age 5 = 0.16 kg/m2, 95% CI 0.15 kg/m2 to 0.17 kg/m2, P<0.001, eTable 4, 5 and eFigures 2 and 3) and children delivered by Cesarean section (full model adjusted Δ BMI at age 5 = 0.23 kg/m2, 95% CI 0.21 kg/m2 to 0.26 kg/m2, P <0.001). In contrast to the main cohort, the association between exposure to antibiotics during the neonatal period and childhood BMI was not significant. Sensitivity analysis limiting the GBS IAP antibiotics to children who received no additional non-GBS agents, creating homogenous exposure groups with regard to GBS colonization, or excluding infants who were exposed to antibiotics antepartum did not alter the results (eTable 4).
DISCUSSION
This is currently the largest study investigating the impact of intrapartum antibiotic exposure as recommended for perinatal GBS disease prevention on childhood BMI. Exposure to GBS IAP was associated with a higher BMI starting at 5 to 7 months of age, depending on the delivery mode and translated at 5 years of age into an excess weight gain of 0.12 to 0.24 kg/m2. Our study was conducted in parallel with a retrospective cohort study in Philadelphia (N =13,804) using the same exposure definition and a similar analysis approach.29 In that study, GBS IAP was associated with an adjusted attributable difference in BMI gain from birth to age 5 years of 0.24 kg (95% CI 0.04 kg to 0.44 kg) for vaginal deliveries and 0.60 kg (95% CI 0.32 kg to 0.88 kg) for Cesarean deliveries.29
Small changes in BMI at the individual level can have significant effects at the population level.30,31 Early childhood BMI trajectories are crucial factors in the development of adult obesity from early youth. Several studies suggest that the majority of excess body weight in children who develop obesity is often gained before age 5 or 6 years.32,33 A recent modeling study also suggested that the chance a child with obesity at age 2 years resolves their obesity decreases with every year that they have obesity,34 underscoring the importance of preventing early excess weight gain.
Associations between antibiotic exposure in early childhood and childhood obesity have received recent attention.12,13 Our findings suggest the association between GBS IAP and childhood BMI was stronger than for other (primarily shorter) intrapartum exposures as well as antepartum and postpartum antibiotic exposures. This may be consistent with hypotheses that disruptions at the time of initial microbiome formation may have lasting effects.35–40
It is currently believed that an infant’s microbiome is seeded by selected maternal bacteria.41 GBS colonization of the mother as well as GBS IAP are associated with a shift in microbiome composition42 and a decreased bacterial diversity in a newborn’s microbiome.6–11,43 Rodent studies indicate that antibiotics at young age lead to long-term increases in body weight by altering the intestinal microbiota in a way that may cross generations.5,44 These downstream health consequences may be intensified when combined with other factors associated with microbiome disruption such as Cesarean delivery9,11,45–48 and formula feeding.9,11 While results from our study showed stronger associations between GBS IAP and childhood BMI in children born via Cesarean delivery, breastfeeding did not alter the association. Our sensitivity analyses suggested that GBS colonization alone was not an important factor in the association between antibiotic exposure and childhood BMI. Prospective studies are necessary to examine if the early life disruption in an infant’s microbiome caused by GBS IAP may lead to strong or sustained shifts in microbiome composition or microbiome diversity and to understand how these may lead to an increase in BMI.35–38
Strengths of the study include medical record data from a large, community-based, average risk population with a high screening rate for GBS colonization.2 The study population is generally reflective of Southern California and includes a high proportion of children born to low income families.14 The cohort study design reduced the chance of possible bias inherent in case-control and hospital-based studies. An extensive number of potential covariates was available including demographics, maternal health, birth-related factors, and childhood information with a high proportion of children who remained in the study for over 4 years. Exposure to antibiotics antepartum, intrapartum, and postpartum was captured comprehensively via electronic medical records, enabling adjustment for the contributions of antibiotic exposure during pregnancy and childhood. Antibiotic exposure groups were compared by delivery mode with unexposed infants from the same background population. The study benefitted from the high frequency of BMI measures throughout childhood, a high quality of weight and height measured due to rigorous training.17 The large sample size enabled evaluation of the BMI trajectories with higher precision than previous studies and allowed us to estimate continuous differences in BMI rather than risk of obesity as binary outcome.
Limitations include the possibility of residual confounding inherent to the observational design, including the possibility of differential distribution of unmeasured or incompletely measured confounders. Assessment of the duration of intrapartum antibiotic exposure from electronic medical records may have resulted in reduced capture of women who received GBS IAP due to underestimation of the antibiotic exposure duration.
Conclusions
Exposure of infants to GBS IAP compared to no intrapartum antibiotics was associated with a small but statistically significant and sustained increase in BMI starting at a very early age. On an individual level, the benefit from GBS intrapartum antibiotic prophylaxis to decrease the risk of neonatal sepsis may outweigh the potential risk of weight gain later. On a population level, the potential increase in body weight may be more relevant as GBS IAP is used in 1 in 3 U. S. births. While the potential effects do not support changing current practice, it is important to also pursue preventive strategies such as maternal GBS immunization.
Supplementary Material
Key Points.
Question
Do children exposed to intrapartum antibiotics for the prevention of group B streptococcal (GBS) infection have a higher body-mass index (BMI) than unexposed children?
Findings
In this population-based study of 223,431 singleton-births, children exposed to GBS intrapartum antibiotic prophylaxis had a higher BMI at 5 years of age compared to unexposed children, after controlling for key covariates including maternal BMI and breastfeeding. The increase was more pronounced in children delivered via Cesarean section (0.24 kg/m2) than in those delivered vaginally (0.12 kg/m2).
Meaning
GBS intrapartum antibiotic prophylaxis was associated with a small but statistically significant and sustained increase in BMI starting at a very early age.
Acknowledgments
This study was supported by a contract from the Centers of Disease Control and Prevention (HCVLD-2017-16907) and by Kaiser Permanente Community Benefit Funds.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or Kaiser Permanente Community Benefit Funds.
Abbreviations:
- BMI
body mass index
- GBS
Group B Streptococcus
- KPSC
Kaiser Permanente Southern California
REFERENCES
- 1.Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31 Suppl 4:D20–26. [DOI] [PubMed] [Google Scholar]
- 2.Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;360(25):2626–2636. [DOI] [PubMed] [Google Scholar]
- 3.Schrag SJ, Farley MM, Petit S, et al. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatr. 2016;138(6). [DOI] [PubMed] [Google Scholar]
- 4.Stokholm J, Schjorring S, Pedersen L, et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLoS One. 2013;8(12):e82932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aloisio I, Mazzola G, Corvaglia LT, et al. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol. 2014;98(13):6051–6060. [DOI] [PubMed] [Google Scholar]
- 7.Jaureguy F, Carton M, Panel P, Foucaud P, Butel MJ, Doucet-Populaire F. Effects of intrapartum penicillin prophylaxis on intestinal bacterial colonization in infants. J Clin Microbiol. 2004;42(11):5184–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keski-Nisula L, Kyynarainen HR, Karkkainen U, Karhukorpi J, Heinonen S, Pekkanen J. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013;102(5):480–485. [DOI] [PubMed] [Google Scholar]
- 9.Fallani M, Young D, Scott J, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51(1):77–84. [DOI] [PubMed] [Google Scholar]
- 10.Corvaglia L, Tonti G, Martini S, et al. Influence of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on Gut Microbiota in the First Month of Life. J Pediatr Gastroenterol Nutr. 2016;62(2):304–308. [DOI] [PubMed] [Google Scholar]
- 11.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partap U, Allcock SH, Parker E, Gurdasani D, Young EH, Sandhu MS. Association between early life antibiotic use and childhood overweight and obesity: a narrative review. Glob Health Epidemiol Genom. 2018;3:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller SA, Wu RKS, Oremus M. The association between antibiotic use in infancy and childhood overweight or obesity: a systematic review and meta-analysis. Obes Rev. 2018;19(11):1463–1475. [DOI] [PubMed] [Google Scholar]
- 14.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koebnick C, Tartof SY, Sidell MA, et al. The Fetal Antibiotic EXposure (FAX) Study: Research on the Effect of in-utero antibiotic exposure on childhood outcomes. JMIR Res Protoc. 2018, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 485: Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2011;117(4):1019–1027. [DOI] [PubMed] [Google Scholar]
- 17.Koebnick C, Mohan YD, Li X, Young DR. Secular Trends of Overweight and Obesity in Young Southern Californians 2008–2013. J Pediatr. 2015;167(6):1264–1271 e1262. [DOI] [PubMed] [Google Scholar]
- 18.Smith N, Coleman KJ, Lawrence JM, et al. Body weight and height data in electronic medical records of children. Int J Pediatr Obes. 2010;5(3):237–242. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Technical Report Series 894: Obesity: Preventing and managing the global epidemic. World Health Organization, Geneva. 2000; ISBN 92–4-120894–5 [PubMed] [Google Scholar]
- 20.Savitz DA, Terry JW Jr., Dole N, Thorp JM Jr., Siega-Riz AM, Herring AH Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187(6):1660–1666. [DOI] [PubMed] [Google Scholar]
- 21.Getahun D, Demissie K, Marcella SW, Rhoads GG. The impact of changes in preterm birth among twins on stillbirth and infant mortality in the United States. J Perinatol. 2014;34(11):823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer MS, Ananth CV, Platt RW, Joseph KS. US Black vs White disparities in foetal growth: physiological or pathological? Int J Epidemiol. 2006;35(5):1187–1195. [DOI] [PubMed] [Google Scholar]
- 23.Koebnick C, Smith N, Huang K, Martinez MP, Clancy HA, Kushi LH. The prevalence of obesity and obesity-related health conditions in a large, multiethnic cohort of young adults in California. Ann Epidemiol. 2012;22(9):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 25.Zhang Z. Missing data imputation: focusing on single imputation. Ann Transl Med. 2016;4(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiol. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. In. Vienna, Austria: R Foundation for Statistical Computing; 2018:URL https://www.R-project.org/. [Google Scholar]
- 29.Mukhopadhyay S, Bryan M, Dhudasia MD, et al. GBS Intrapartum Antibiotic Prophylaxis and Early Childhood Weight Gain. 2019, submitted. [Google Scholar]
- 30.Ludwig DS. Childhood obesity--the shape of things to come. NEnglJMed. 2007;357(23):2325–2327. [DOI] [PubMed] [Google Scholar]
- 31.Fagot-Campagna A. Emergence of type 2 diabetes mellitus in children: epidemiological evidence. J Pediatr Endocrinol Metab. 2000;13 Suppl 6:1395–1402. [DOI] [PubMed] [Google Scholar]
- 32.Buscot MJ, Thomson RJ, Juonala M, et al. BMI Trajectories Associated With Resolution of Elevated Youth BMI and Incident Adult Obesity. Pediatrics. 2018;141(1). [DOI] [PubMed] [Google Scholar]
- 33.Taveras EM, Rifas-Shiman SL, Sherry B, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–998. [DOI] [PubMed] [Google Scholar]
- 34.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N Engl J Med. 2017;377(22):2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stokholm J, Schjorring S, Eskildsen CE, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect. 2014;20(7):629–635. [DOI] [PubMed] [Google Scholar]
- 36.Kuperman AA, Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med. 2016;14(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11(3):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaser MJ. The Past and Future Biology of the Human Microbiome in an Age of Extinctions. Cell. 2018;172(6):1173–1177. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun. 2017;8(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korpela K, Costea P, Coelho LP, et al. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 2018;28(4):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassidy-Bushrow AE, Sitarik A, Levin AM, et al. Maternal group B Streptococcus and the infant gut microbiota. J Dev Orig Health Dis. 2016;7(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roesch LF, Silveira RC, Corso AL, et al. Diversity and composition of vaginal microbiota of pregnant women at risk for transmitting Group B Streptococcus treated with intrapartum penicillin. PLoS One. 2017;12(2):e0169916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol. 2018;3(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53(9):1388–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. [DOI] [PubMed] [Google Scholar]
- 47.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.