Abstract
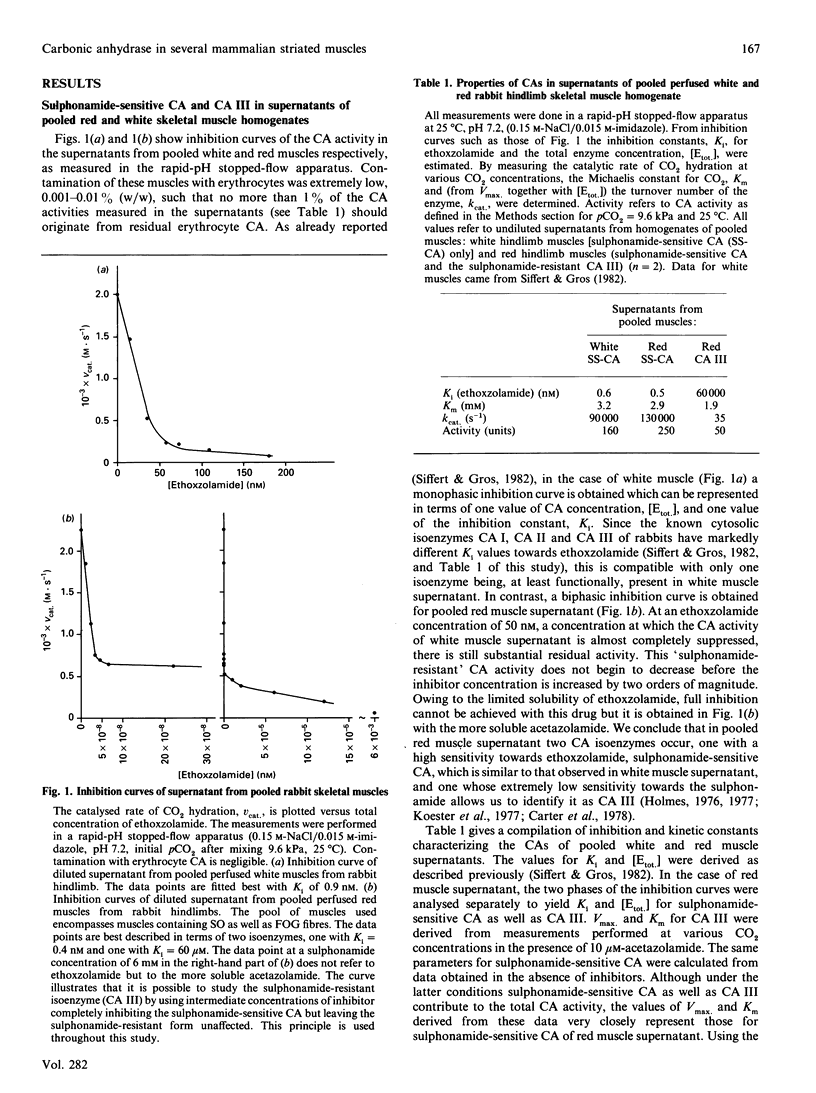
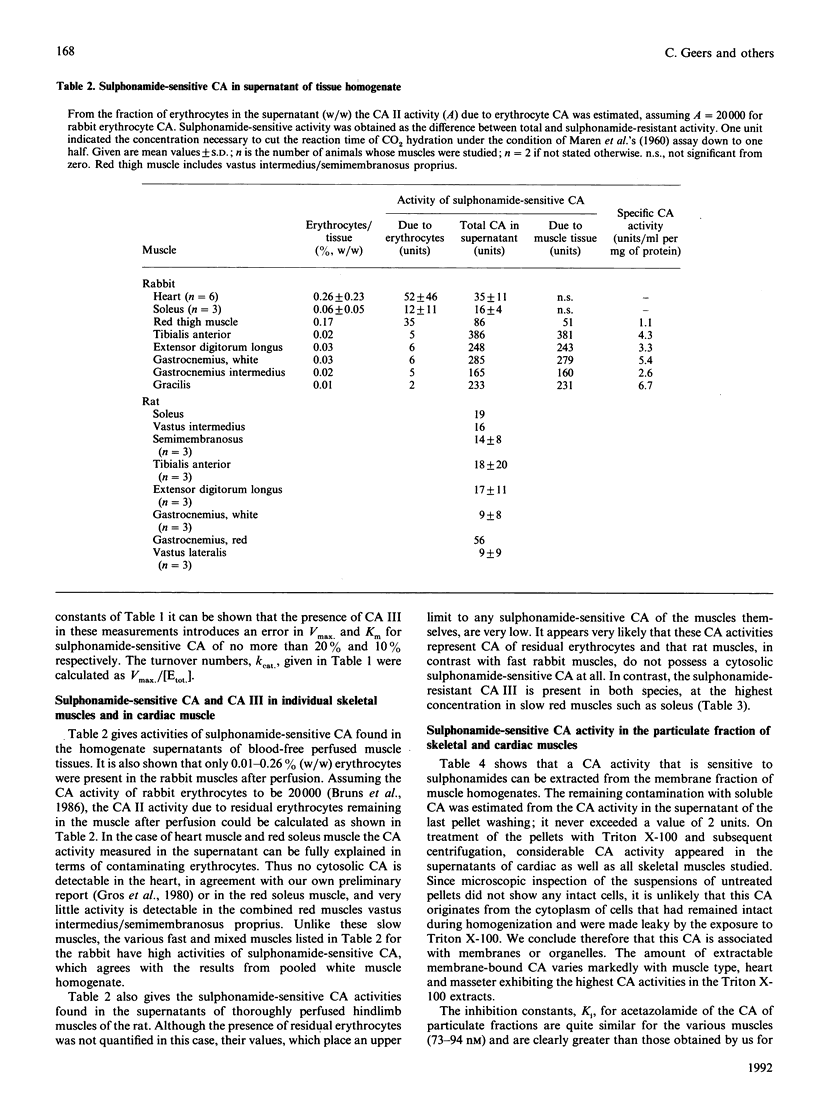
We have studied the distribution of carbonic anhydrases (CA) in several skeletal muscles of the hindlimb of rabbits and rats and in cardiac muscle of the rabbit. To remove erythrocyte CA, hindlimbs and hearts were thoroughly perfused with dextran solution, and the effectiveness of the perfusion was in most cases assessed by determining the contamination of the muscles with radioisotopes that had been used to label the erythrocytes before the perfusion was started. We observed three forms of CA: (1) cytosolic (sulphonamide-resistant) CA III; (2) a cytosolic sulphonamide-sensitive CA, probably isoenzyme II; (3) a membrane-bound form that was extracted from the particulate fraction using Triton X-100. These CA isoforms were distributed as follows. (1) CA III is located in the cytoplasm of slow, oxidative skeletal muscles and is absent from or low in fast skeletal and cardiac muscle; this holds for rabbits and rats and is identical with the pattern previously described for several other species. (2) The cytosolic sulphonamide-sensitive CA is present in fast rabbit muscles and absent from slow muscles of this species. In contrast, all skeletal muscles of the rat studied here lack, or possess only very low, activity of this isoenzyme. (3) The membrane-bound form of CA is present in all rabbit muscles studied; its activity appears somewhat higher in fast than in slow skeletal muscles. (4) Cardiac muscle constitutes an exception among all striated muscles of the rabbit as it possesses no form of cytosolic CA but a high activity of the membrane-bound form.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidani A., Crandall E. D., Forster R. E. Analysis of postcapillary pH changes in blood in vivo after gas exchange. J Appl Physiol Respir Environ Exerc Physiol. 1978 May;44(5):770–781. doi: 10.1152/jappl.1978.44.5.770. [DOI] [PubMed] [Google Scholar]
- Bruns W., Dermietzel R., Gros G. Carbonic anhydrase in the sarcoplasmic reticulum of rabbit skeletal muscle. J Physiol. 1986 Feb;371:351–364. doi: 10.1113/jphysiol.1986.sp015980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N., Jeffery S., Shiels A. Immunoassay of carbonic anhydrase III in rat tissues. FEBS Lett. 1982 Mar 22;139(2):265–266. doi: 10.1016/0014-5793(82)80866-1. [DOI] [PubMed] [Google Scholar]
- Carter N., Shiels A., Tashian R. Carbonic anhydrase III isoenzyme from human and bovine muscle [proceedings]. Biochem Soc Trans. 1978;6(3):552–553. doi: 10.1042/bst0060552. [DOI] [PubMed] [Google Scholar]
- Crandall E. D., Bidani A., Forster R. E. Postcapillary changes in blood pH in vivo during carbonic anhydrase inhibition. J Appl Physiol Respir Environ Exerc Physiol. 1977 Oct;43(4):582–590. doi: 10.1152/jappl.1977.43.4.582. [DOI] [PubMed] [Google Scholar]
- Crandall E. D., Klocke R. A., Forster R. E. Hydroxyl ion movements across the human erythrocyte membrane. Measurement of rapid pH changes in red cell suspensions. J Gen Physiol. 1971 Jun;57(6):664–683. doi: 10.1085/jgp.57.6.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R., Leibstein A., Siffert W., Zamboglou N., Gros G. A fast screening method for histochemical localization of carbonic anhydrase. Application to kidney, skeletal muscle, and thrombocytes. J Histochem Cytochem. 1985 Feb;33(2):93–98. doi: 10.1177/33.2.3918097. [DOI] [PubMed] [Google Scholar]
- Engberg P., Millqvist E., Pohl G., Lindskog S. Purification and some properties of carbonic anhydrase from bovine skeletal muscle. Arch Biochem Biophys. 1985 Sep;241(2):628–638. doi: 10.1016/0003-9861(85)90589-2. [DOI] [PubMed] [Google Scholar]
- Forster R. E., Crandall E. D. Time course of exchanges between red cells and extracellular fluid during CO2 uptake. J Appl Physiol. 1975 Apr;38(4):710–718. doi: 10.1152/jappl.1975.38.4.710. [DOI] [PubMed] [Google Scholar]
- Geers C., Gros G., Gärtner A. Extracellular carbonic anhydrase of skeletal muscle associated with the sarcolemma. J Appl Physiol (1985) 1985 Aug;59(2):548–558. doi: 10.1152/jappl.1985.59.2.548. [DOI] [PubMed] [Google Scholar]
- Gros G., Dodgson S. J. Velocity of CO2 exchange in muscle and liver. Annu Rev Physiol. 1988;50:669–694. doi: 10.1146/annurev.ph.50.030188.003321. [DOI] [PubMed] [Google Scholar]
- Gros G., Forster R. E., Lin L. The carbamate reaction of glycylglycine, plasma, and tissue extracts evaluated by a pH stopped flow apparatus. J Biol Chem. 1976 Jul 25;251(14):4398–4407. [PubMed] [Google Scholar]
- Hill E. P., Power G. G., Gilbert R. D. Rate of pH changes in blood plasma in vitro and in vivo. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jun;42(6):928–934. doi: 10.1152/jappl.1977.42.6.928. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Holmes L. B., Trelstad R. L. Cell polarity in precartilage mouse limb mesenchyme cells. Dev Biol. 1980 Aug;78(2):511–520. doi: 10.1016/0012-1606(80)90350-4. [DOI] [PubMed] [Google Scholar]
- Holmes R. S. Mammalian carbonic anhydrase isozymes: evidence for a third locus. J Exp Zool. 1976 Aug;197(2):289–295. doi: 10.1002/jez.1401970210. [DOI] [PubMed] [Google Scholar]
- Jeffery S., Carter N. D., Smith A. Immunocytochemical localization of carbonic anhydrase isozymes I, II, and III in rat skeletal muscle. J Histochem Cytochem. 1986 Apr;34(4):513–516. doi: 10.1177/34.4.2936798. [DOI] [PubMed] [Google Scholar]
- Koester M. K., Register A. M., Noltmann E. A. Basic muscle protein, a third genetic locus isoenzyme of carbonic anhydrase? Biochem Biophys Res Commun. 1977 May 9;76(1):196–204. doi: 10.1016/0006-291x(77)91686-2. [DOI] [PubMed] [Google Scholar]
- Lobley G. E., Wilson A. B., Bruce A. S. An estimation of the fibre type compostion of eleven skeletal muscles from New Zealand White rabbits between weaning and early maturity. J Anat. 1977 Apr;123(Pt 2):501–513. [PMC free article] [PubMed] [Google Scholar]
- MAREN T. H., PARCELL A. L., MALIK M. N. A kinetic analysis of carbonic anhydrase inhibition. J Pharmacol Exp Ther. 1960 Dec;130:389–400. [PubMed] [Google Scholar]
- Maren T. H., Rayburn C. S., Liddell N. E. Inhibition by anions of human red cell carbonic anhydrase B: physiological and biochemical implications. Science. 1976 Feb 6;191(4226):469–472. doi: 10.1126/science.813299. [DOI] [PubMed] [Google Scholar]
- Metz A., Reinert K. Uber den Einfluss von Fett auf die Proteinbestimmung mit Biuret- und Folin-Ciocalteus Phenol-Reagenz in Leberhomogenaten. Z Klin Chem Klin Biochem. 1974 Aug;12(8):361–366. [PubMed] [Google Scholar]
- Moynihan J. B. Carbonic anhydrase activity in mammalian skeletal and cardiac muscle. Biochem J. 1977 Dec 15;168(3):567–569. doi: 10.1042/bj1680567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan L. M., Noltmann E. A. Simultaneous and independent versus antagonistic inhibition of muscle carbonic anhydrase (CA III) by acetazolamide and cyanate. Biochem Pharmacol. 1984 Aug 15;33(16):2641–2645. doi: 10.1016/0006-2952(84)90638-5. [DOI] [PubMed] [Google Scholar]
- Riley D. A., Ellis S., Bain J. Carbonic anhydrase activity in skeletal muscle fiber types, axons, spindles, and capillaries of rat soleus and extensor digitorum longus muscles. J Histochem Cytochem. 1982 Dec;30(12):1275–1288. doi: 10.1177/30.12.6218195. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Pessah N. I., Maren T. H. Kinetics and inhibition of membrane-bound carbonic anhydrase from canine renal cortex. Biochim Biophys Acta. 1981 Jan 15;657(1):128–137. doi: 10.1016/0005-2744(81)90136-4. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Swenson E. R., Pessah N. I., Maren T. H. The carbon dioxide hydration activity of skeletal muscle carbonic anhydrase. Inhibition by sulfonamides and anions. Mol Pharmacol. 1982 Jul;22(1):211–220. [PubMed] [Google Scholar]
- Sanyal G. The carbon dioxide hydration activity of the sulfonamide-resistant carbonic anhydrase from the liver of male rat: pH independence of the steady-state kinetics. Arch Biochem Biophys. 1984 Nov 1;234(2):576–579. doi: 10.1016/0003-9861(84)90306-0. [DOI] [PubMed] [Google Scholar]
- Shima K., Tashiro K., Hibi N., Tsukada Y., Hirai H. Carbonic anhydrase-III immunohistochemical localization in human skeletal muscle. Acta Neuropathol. 1983;59(3):237–239. doi: 10.1007/BF00703210. [DOI] [PubMed] [Google Scholar]
- Siffert W., Gros G. Carbonic anhydrase C in white-skeletal-muscle tissue. Biochem J. 1982 Sep 1;205(3):559–566. doi: 10.1042/bj2050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vänänen H. K., Takala T., Morris D. C. Immunoelectron microscopic localization of carbonic anhydrase III in rat skeletal muscle. Histochemistry. 1986;86(2):175–179. doi: 10.1007/BF00493384. [DOI] [PubMed] [Google Scholar]
- Wetzel P., Gros G. Sarcolemmal carbonic anhydrase in red and white rabbit skeletal muscle. Arch Biochem Biophys. 1990 Jun;279(2):345–354. doi: 10.1016/0003-9861(90)90501-o. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]
- Zborowska-Sluis D. T., L'Abbate A., Mildenberger R. R., Klassen G. A. The effect of acetazolamide on myocardial carbon dioxide space. Respir Physiol. 1975 Apr;23(3):311–316. doi: 10.1016/0034-5687(75)90081-x. [DOI] [PubMed] [Google Scholar]
- de Hemptinne A., Marrannes R., Vanheel B. Surface pH and the control of intracellular pH in cardiac and skeletal muscle. Can J Physiol Pharmacol. 1987 May;65(5):970–977. doi: 10.1139/y87-154. [DOI] [PubMed] [Google Scholar]


