Abstract
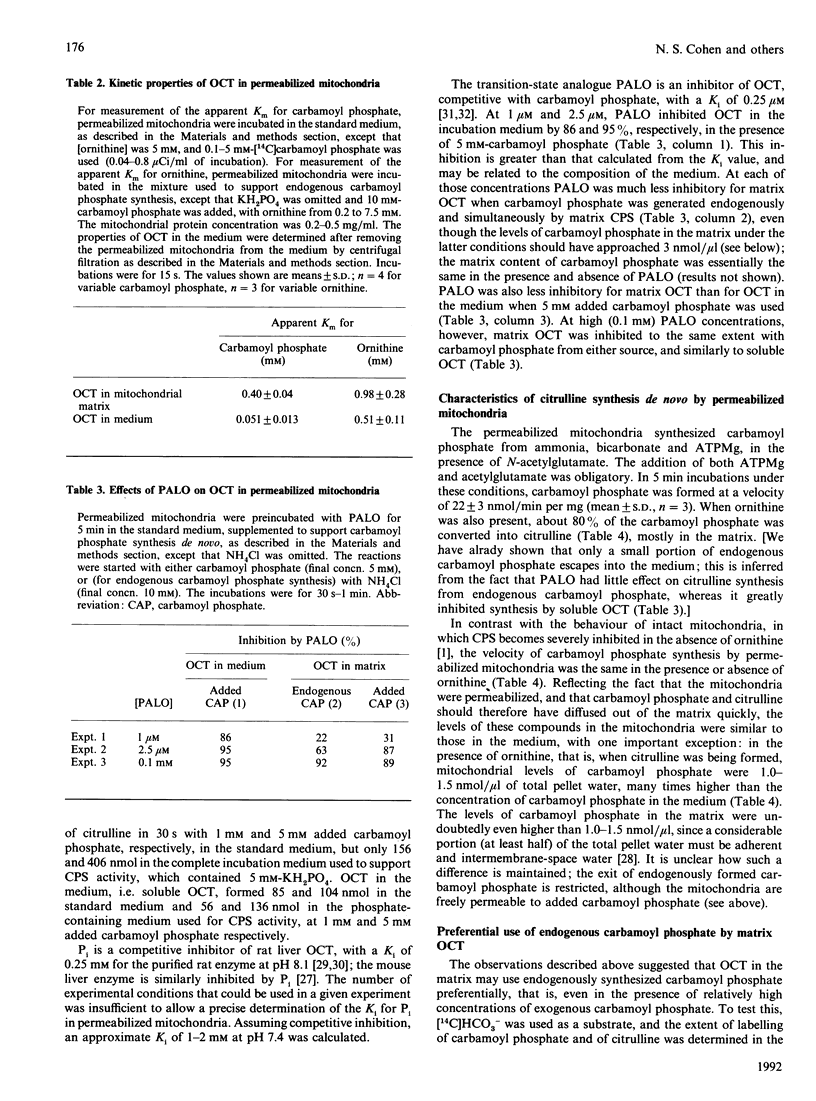
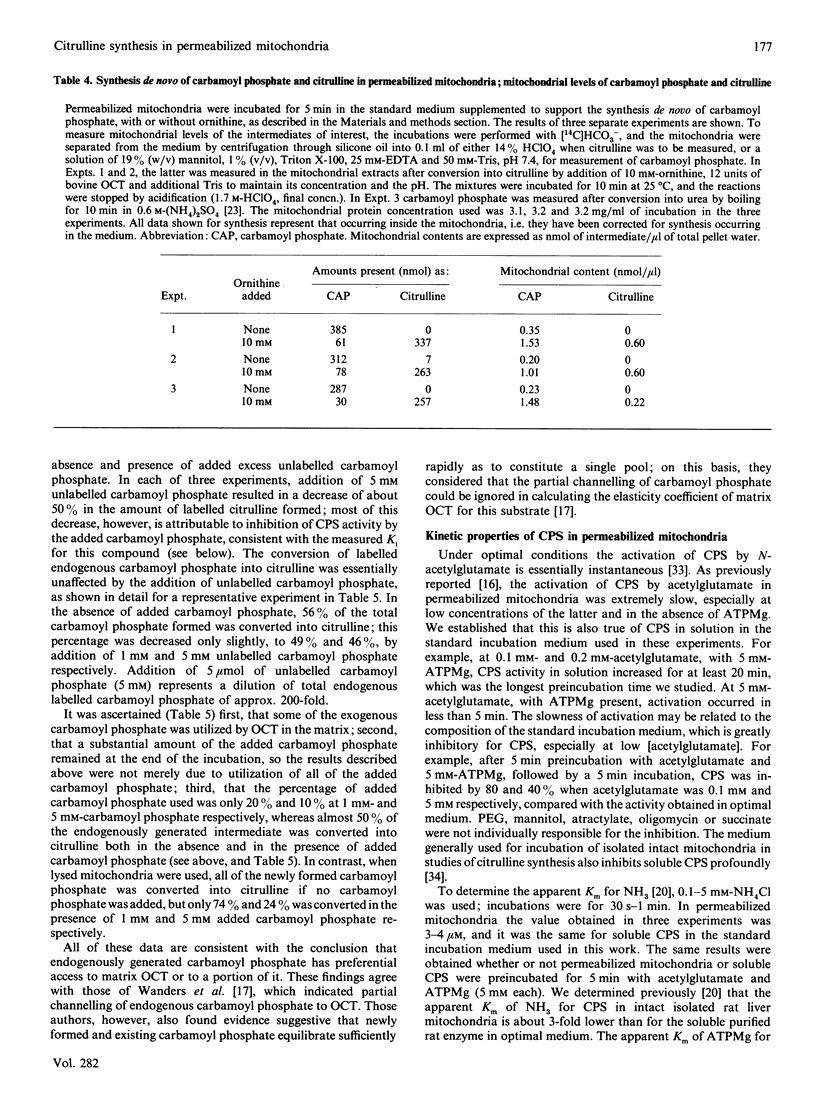
Previous studies using intact rat liver mitochondria have shown that the soluble matrix enzymes carbamoyl-phosphate synthase (ammonia) (CPS) and ornithine carbamoyltransferase (OCT) display some kinetic properties which would not be observed if they were homogeneously distributed in the matrix. In the present work we have extended these studies, using toluene-treated mitochondria which are fully permeable to substrates and inhibitors, yet retain 90% of their soluble enzymes. The results provide evidence of functional organization of CPS and OCT in situ. The major findings are as follows. (1) The apparent Km values of matrix OCT for carbamoyl phosphate and ornithine are respectively 8 and 2 times those measured for the soluble enzyme. delta-N-Phosphonacetyl-L-ornithine inhibits OCT in situ less than in solution, especially when carbamoyl phosphate is synthesized in the mitochondria rather than added to the medium. (2) During citrulline synthesis from endogenously generated carbamoyl phosphate, the concentration of the latter in permeabilized mitochondria is more than 10 times that in the medium, although the mitochondria are freely permeable to added molecules of this size. (3) Endogenously formed carbamoyl phosphate is used preferentially by OCT in situ; addition of a 200-fold excess of unlabelled carbamoyl phosphate has little effect on the conversion of labelled endogenously formed carbamoyl phosphate into citrulline by matrix OCT. (4) The synthesis de novo of carbamoyl phosphate from NH3, HCO3- and ATPMg is the same in the presence and absence of ornithine. (5) Studies with co-immobilized CPS and OCT gave results concordant with some of the above observations and with previous ones with intact mitochondria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- Cheung C. W., Cohen N. S., Raijman L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J Biol Chem. 1989 Mar 5;264(7):4038–4044. [PubMed] [Google Scholar]
- Cheung C. W., Raijman L. The regulation of carbamyl phosphate synthetase (ammonia) in rat liver mitochondria. Effects of acetylglutamate concentration and ATP translocation. J Biol Chem. 1980 Jun 10;255(11):5051–5057. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W. Differential effects of N-acetylglutamate on citrulline synthesis by coupled and uncoupled mitochondria. Arch Biochem Biophys. 1984 Oct;234(1):31–44. doi: 10.1016/0003-9861(84)90321-7. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Kyan F. S., Jones E. E., Raijman L. Mitochondrial carbamyl phosphate and citrulline synthesis at high matrix acetylglutamate. J Biol Chem. 1982 Jun 25;257(12):6898–6907. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Altered enzyme activities and citrulline synthesis in liver mitochondria from ornithine carbamoyltransferase-deficient sparse-furash mice. Biochem J. 1989 Jan 1;257(1):251–257. doi: 10.1042/bj2570251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Channeling of extramitochondrial ornithine to matrix ornithine transcarbamylase. J Biol Chem. 1987 Jan 5;262(1):203–208. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Measurements of mitochondrial volumes are affected by the amount of mitochondria used in the determinations. Biochem J. 1987 Jul 15;245(2):375–379. doi: 10.1042/bj2450375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. The effects of ornithine on mitochondrial carbamyl phosphate synthesis. J Biol Chem. 1980 Nov 10;255(21):10248–10255. [PubMed] [Google Scholar]
- Cohen N. S., Kyan F. S., Kyan S. S., Cheung C. W., Raijman L. The apparent Km of ammonia for carbamoyl phosphate synthetase (ammonia) in situ. Biochem J. 1985 Jul 1;229(1):205–211. doi: 10.1042/bj2290205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. Kinetic studies of bovine liver carbamoyl phosphate synthetase. Biochem J. 1974 Sep;141(3):807–816. doi: 10.1042/bj1410807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthöhrlein G., Knappe J. Structure and function of carbamoylphosphate synthase. I. Transitions between two catalytically inactive forms and the active form. Eur J Biochem. 1968 Dec;7(1):119–127. doi: 10.1111/j.1432-1033.1968.tb19582.x. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci U S A. 1968 Oct;61(2):598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad N. J. Synthesis and properties of delta-N-(phosphonacetyl)-L-ornithine. A transition-state analog inhibitor of ornithine transcarbamylase. Arch Biochem Biophys. 1978 May;188(1):137–144. doi: 10.1016/0003-9861(78)90366-1. [DOI] [PubMed] [Google Scholar]
- Janski A. M., Cornell N. W. Subcellular distribution of enzymes determined by rapid digitonin fractionation of isolated hepatocytes. Biochem J. 1980 Feb 15;186(2):423–429. doi: 10.1042/bj1860423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lof C., Cohen M., Vermeulen L. P., van Roermund C. W., Wanders R. J., Meijer A. J. Properties of carbamoyl-phosphate synthetase (ammonia) in rat-liver mitochondria made permeable with toluene. Eur J Biochem. 1983 Sep 15;135(2):251–258. doi: 10.1111/j.1432-1033.1983.tb07645.x. [DOI] [PubMed] [Google Scholar]
- Lusty C. J. Catalytically active monomer and dimer forms of rat liver carbamoyl-phosphate synthetase. Biochemistry. 1981 Jun 23;20(13):3665–3674. doi: 10.1021/bi00516a001. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Matlib M. A., Boesman-Finkelstein M., Srere P. A. The kinetics of rat liver citrate synthase in situ. Arch Biochem Biophys. 1978 Dec;191(2):426–430. doi: 10.1016/0003-9861(78)90380-6. [DOI] [PubMed] [Google Scholar]
- Matlib M. A., Shannon W. A., Jr, Srere P. A. Measurement of matrix enzyme activity in isolated mitochondria made permeable with toluene. Arch Biochem Biophys. 1977 Jan 30;178(2):396–407. doi: 10.1016/0003-9861(77)90209-0. [DOI] [PubMed] [Google Scholar]
- McConkey E. H. Molecular evolution, intracellular organization, and the quinary structure of proteins. Proc Natl Acad Sci U S A. 1982 May;79(10):3236–3240. doi: 10.1073/pnas.79.10.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley P. J., Rutter G. A., Thomas A. P., Denton R. M. Effects of Ca2+ and Mg2+ on the activity of pyruvate dehydrogenase phosphate phosphatase within toluene-permeabilized mitochondria. Biochem J. 1987 Jan 15;241(2):371–377. doi: 10.1042/bj2410371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P., Wilf J. Effect of macromolecular crowding upon the structure and function of an enzyme: glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1981 Aug 18;20(17):4821–4826. doi: 10.1021/bi00520a003. [DOI] [PubMed] [Google Scholar]
- Osawa T., Tsuji T. Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Annu Rev Biochem. 1987;56:21–42. doi: 10.1146/annurev.bi.56.070187.000321. [DOI] [PubMed] [Google Scholar]
- Powers-Lee S. G., Mastico R. A., Bendayan M. The interaction of rat liver carbamoyl phosphate synthetase and ornithine transcarbamoylase with inner mitochondrial membranes. J Biol Chem. 1987 Nov 15;262(32):15683–15688. [PubMed] [Google Scholar]
- Powers S. G. Regulation of rat liver carbamyl phosphate synthetase I. Inhibition by metal ions and activation by amino acids and other chelating agents. J Biol Chem. 1981 Nov 10;256(21):11160–11165. [PubMed] [Google Scholar]
- Raijman L., Bartulis T. Effect of ATP translocation on citrulline and oxaloacetate synthesis by isolated rat liver mitochondria. Arch Biochem Biophys. 1979 Jun;195(1):188–197. doi: 10.1016/0003-9861(79)90340-0. [DOI] [PubMed] [Google Scholar]
- Raijman L., Jones M. E. Purification, composition, and some properties of rat liver carbamyl phosphate synthetase (ammonia). Arch Biochem Biophys. 1976 Jul;175(1):270–278. doi: 10.1016/0003-9861(76)90508-7. [DOI] [PubMed] [Google Scholar]
- Rutter G. A., Denton R. M. Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem J. 1988 May 15;252(1):181–189. doi: 10.1042/bj2520181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. P., Denton R. M. Use of toluene-permeabilized mitochondria to study the regulation of adipose tissue pyruvate dehydrogenase in situ. Further evidence that insulin acts through stimulation of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1986 Aug 15;238(1):93–101. doi: 10.1042/bj2380093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. P., Diggle T. A., Denton R. M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem J. 1986 Aug 15;238(1):83–91. doi: 10.1042/bj2380083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., Van Roermund C. W., Meijer A. J. Analysis of the control of citrulline synthesis in isolated rat-liver mitochondria. Eur J Biochem. 1984 Jul 16;142(2):247–254. doi: 10.1111/j.1432-1033.1984.tb08278.x. [DOI] [PubMed] [Google Scholar]
- Yashphe J. Estimation of micro amounts of carbamyl phosphate. Anal Biochem. 1973 Mar;52(1):154–161. doi: 10.1016/0003-2697(73)90340-0. [DOI] [PubMed] [Google Scholar]
- Yokota S., Mori M. Immunoelectron microscopical localization of ornithine transcarbamylase in hepatic parenchymal cells of the rat. Histochem J. 1986 Aug;18(8):451–457. doi: 10.1007/BF01675338. [DOI] [PubMed] [Google Scholar]


