Abstract
Introduction
L-2-hydroxyglutaric aciduria (L2HGA) is a rare neurometabolic disorder marked by progressive and debilitating psychomotor deficits. Here, we report the first patient with L2HGA-related refractory dystonia that was managed with deep brain stimulation to the bilateral globus pallidus internus (GPi-DBS).
Case Presentation
We present a 17-year-old female with progressive decline in cognitive function, motor skills, and language ability which significantly impaired activities of daily living. Neurological exam revealed generalized dystonia, significant choreic movements in the upper extremities, slurred speech, bilateral dysmetria, and a wide-based gait. Brisk deep tendon reflexes, clonus, and bilateral Babinski signs were present. Urine 2-OH-glutaric acid level was significantly elevated. Brain MRI showed extensive supratentorial subcortical white matter signal abnormalities predominantly involving the U fibers and bilateral basal ganglia. Genetic testing identified a homozygous pathogenic mutation in the L-2-hydroxyglutarate dehydrogenase gene c. 164G>A (p. Gly55Asp). Following minimal response to pharmacotherapy, GPi-DBS was performed. Significant increases in mobility and decrease in dystonia were observed at 3 weeks, 6 months, and 12 months postoperatively.
Conclusion
This is the first utilization of DBS as treatment for L2HGA-related dystonia. The resulting significant improvements indicate that pallidal neuromodulation may be a viable option for pharmaco-resistant cases, and possibly in other secondary metabolic dystonias.
Keywords: Deep brain stimulation, Pediatric dystonia, L2-hydroxyglutaric aciduria
Introduction
L-2-hydroxyglutaric aciduria (L2HGA) is a rare, neurometabolic disorder that has an autosomal recessive mode of inheritance (MIM# 236792). The clinical course is slowly progressive and primarily affects the central nervous system, manifesting as ataxia, spasticity, dystonia, seizures, macrocephaly, and intellectual disability. Diagnosis is aided by biochemical analysis, neuroimaging and confirmed by identifying a homozygous variant in L2HGDH gene. Here, we reported the first patient with L2HGA-related refractory dystonia that was successfully treated with deep brain stimulation (DBS) to the bilateral globus pallidus internus (GPi).
Case Presentation
Our patient is a 17-year-old female born of a consanguineous union between first cousins. She is the sixth of seven siblings. Notably, two older sisters were diagnosed with L2HGA and are presently under the care of adult movement disorder specialists.
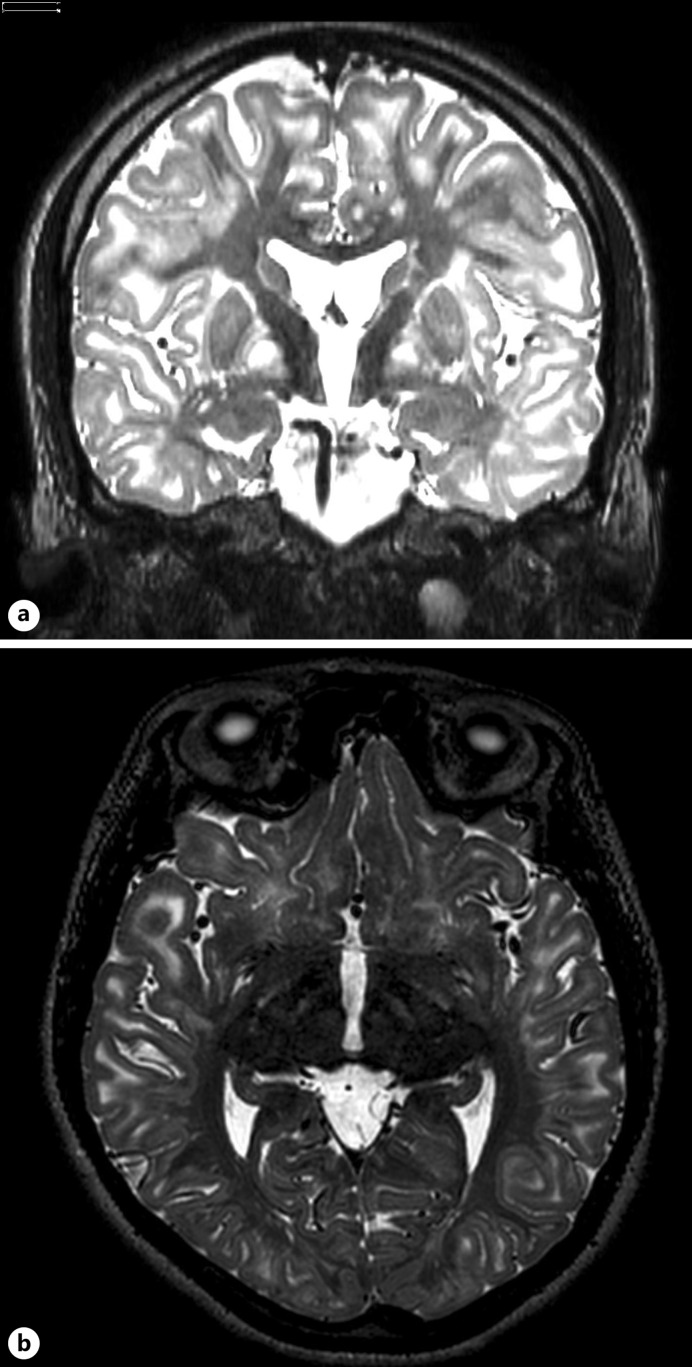
Prenatal and birth history was unremarkable. Development was at par until 4 years of age when regression in milestones were noted. There was deterioration in cognitive function, coordination, motor skills, and language ability. At 13 years of age, there was onset of abnormal body postures and gait, which significantly interfered with her activities of daily living. Brain MRI at that time showed extensive and symmetric white matter signal abnormalities, including in the deep gray nuclei, especially of the globi pallidi, as well as the red and dentate nuclei. There was note of mild cerebellar and cerebral volume loss resulting in some prominence of the ventricular system (shown in Fig. 1). Her symptoms gradually progressed with time, such that she eventually required assistance with self-care.
Fig. 1.
Preoperative brain MRI (4 years prior to surgery). T2-weighted axial sections showing extensive and symmetric white matter signal abnormalities including deep gray nuclei especially of the globi pallidi bilaterally, as well as the red and dentate nuclei.
Neurologic exam (online suppl. Video. 1, 2; for all online suppl. material, see https://doi.org/10.1159/000538418) revealed generalized dystonia most evident over the trunk as well as the upper and lower extremities. Choreic movements of the upper extremities were prominent. Brisk deep tendon reflexes, clonus, and bilateral Babinski signs were present. Her gait was wide-based and hindered by bilateral dystonic foot inversion. She was not able to perform rapid alternating movements. Dysmetria was present bilaterally. Speech was slurred. Pharmacologic treatment with levodopa, trihexyphenidyl, and tetrabenazine had limited effect on her movements and gait.
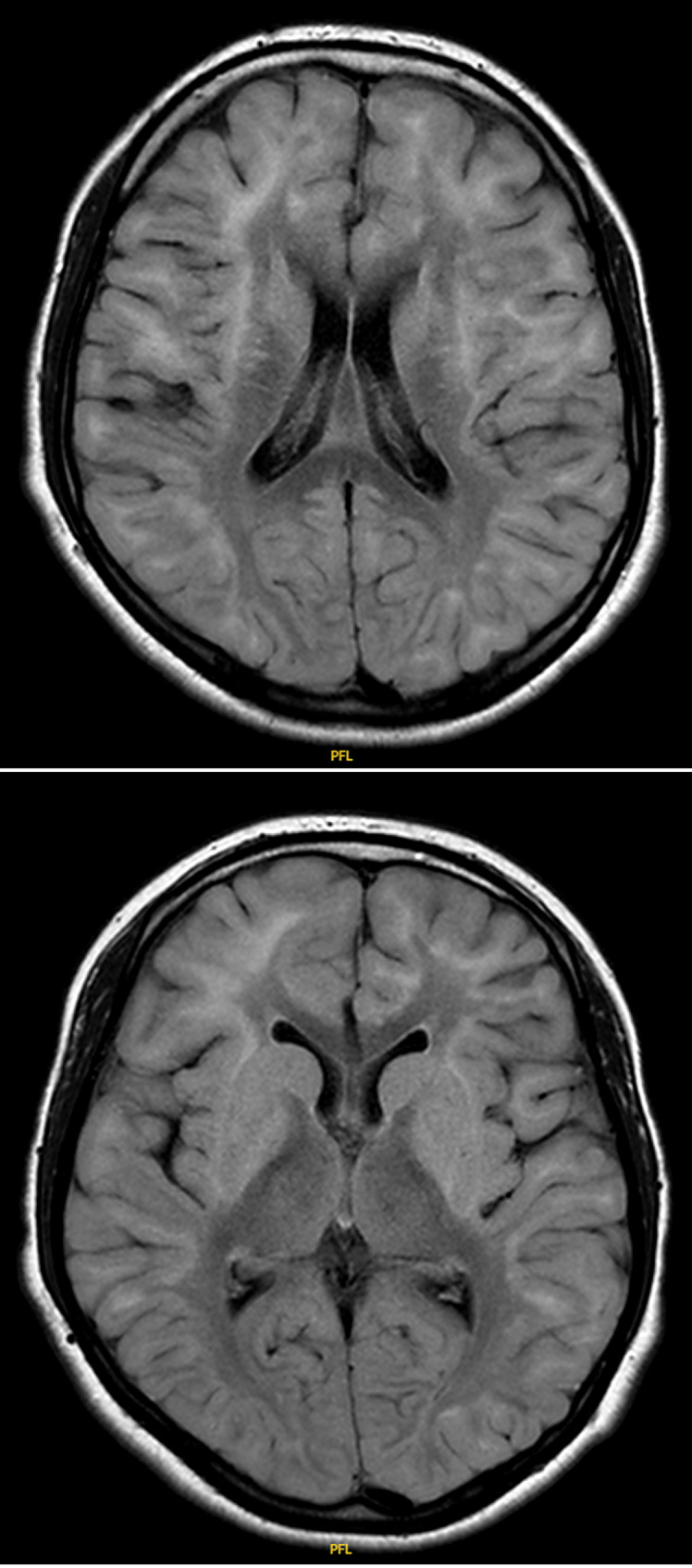
Laboratory analysis of urine organic acids showed increased 2-OH-glutaric acid. Pre-operative MRI taken 3 months prior to surgery showed similar radiologic findings. There were extensive supratentorial subcortical white matter signal abnormalities predominantly involving the subcortical U fibers and peripheral cerebral white matter. As was previously seen, there were symmetric T2 and FLAIR hyperintense signal abnormalities involving the globi pallidi, putamen, caudate, dentate, and red nuclei (shown in Fig. 2). In comparison to the first MRI taken 4 years prior, there was further increase in volume loss. Diagnosis was confirmed on genetic testing with identification of a homozygous pathogenic mutation in L2HGDH gene: c. 164G>A (p. Gly55Asp). The patient was 15 years of age at the time of genetic confirmation.
Fig. 2.
Preoperative brain MRI (3 months prior to surgery). T2-weighted coronal section (a) and T2-weighted axial section (b) at the level of the anterior and posterior commissures, showing extensive subcortical supratentorial increased white matter signal involving the subcortical U fibers and the peripheral cerebral white matter. Symmetric hyperintense signal abnormalities involving the globus pallidus (GP), putamen, and caudate nucleus bilaterally.
DBS was offered due to the medically refractory and disabling dystonia. There was an honest discussion of the risk and benefits. It was stressed that there is no cure for L2HGA, but that the DBS aims to decrease motor symptoms and that the procedure is not intended to ameliorate other co-existing conditions such as cognitive impairment. We emphasized that L2HGA is a progressive condition and the magnitude and the duration of the possible improvement after the surgery will be very hard to predict.
The patient was referred to a clinical neuropsychologist to assess appropriateness for the surgical procedure and to establish a presurgical baseline. A comprehensive assessment was completed through clinical interview and examination. Cognitive functioning across various domains such as memory, attention, and executive function was determined. Moreover, the emotional state was assessed to rule out mood disorders. Interview was also done with the mother to establish realistic expectations and a clear understanding of the risks. The neuropsychological assessment deemed the patient suitable for DBS placement.
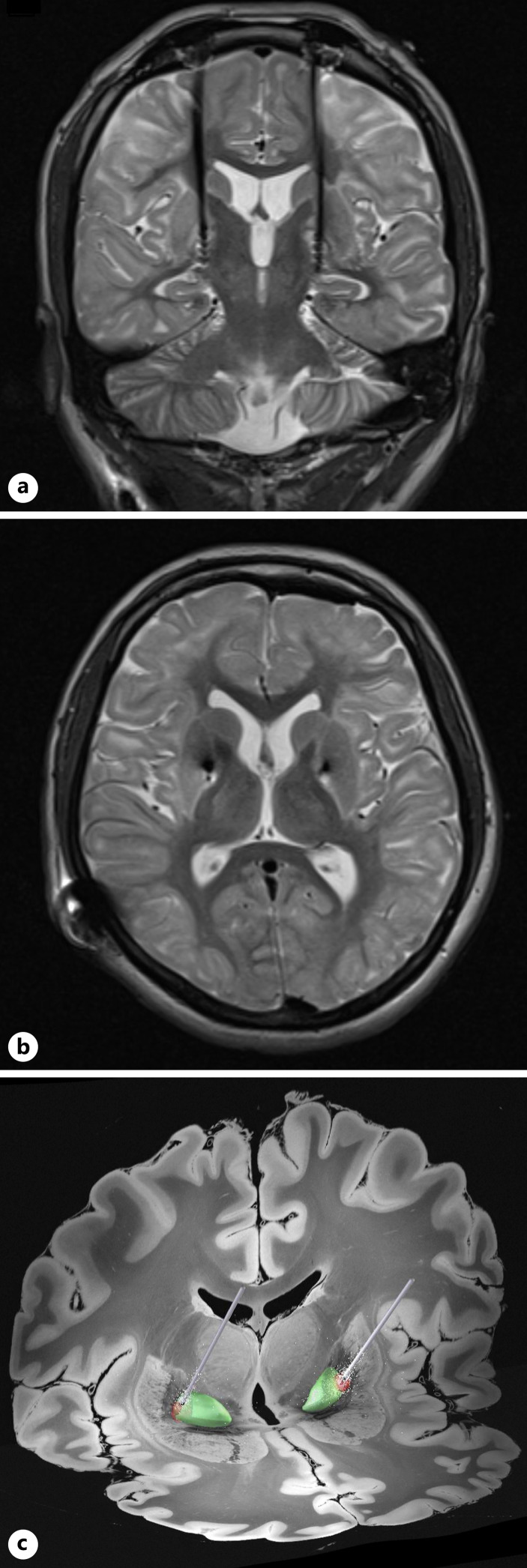
Surgical planning was performed after the neuropsychological assessment and family counseling. A stereotactic approach using a frame-based (Leksell frame) targeting technique aided by CT-MRI fusion images was utilized to target the bilateral GPi. The DBS leads were placed under direct fluoroscopic guidance. Medtronic Percept™ PC Neurostimulator was placed in the right subclavicular area. A postoperative brain MRI confirmed the position of the DBS leads in the intended target (shown in Fig. 3). The surgical procedure was uneventful. The patient was discharged on the fifth postoperative day.
Fig. 3.
Postoperative brain MRI. T2-weighted coronal section (a) and T2-weighted axial section (b) at the level of the anterior and posterior commissures, showing the tips of DBS leads implanted in the GP internus bilaterally. c Processed 3D image (Lead-DBS®) showing the DBS leads reaching the GP internus (green) with the volume of tissue activated (red) based on the last stimulation parameters.
The patient returned to the clinic after 3 weeks for initial DBS programming. The family reported approximately 50% improvement. This included less dyskinetic movements of the mouth, improved ambulation, decrease in dystonic postures, and reduced choreic movements (online Suppl. Video 3). A 19-point improvement (from 46/120 to 27/120) of the Burke Fahn Masden Dystonia Rating Scale – Motor (BFMDRS-M) (shown in Table 1) was seen prior to turning the simulation on, which likely represented the microlesional effect.
Table 1.
Masden dystonia rating scale scores
| Preoperative | Postoperative | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 weeks | 6 months | 10 months | 12 months | 13 months | ||||||||||||||||||||
| provoking factor | severity factor | weight | product | provoking factor | severity factor | weight | product | provoking factor | severity factor | weight | product | provoking factor | severity factor | weight | product | provoking factor | severity factor | weight | product | provoking factor | severity factor | weight | product | |
| Eyes | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 |
| Mouth | 1 | 2 | 0.5 | 1 | 3 | 2 | 0.5 | 3 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 |
| Speech and swallow | 2 | 1 | 1.0 | 2 | 2 | 1 | 1.0 | 2 | 1 | 1 | 1.0 | 1 | 2 | 2 | 1.0 | 4 | 1 | 3 | 1.0 | 3 | 1 | 2 | 1.0 | 2 |
| Neck | 3 | 2 | 0.5 | 3 | 2 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 |
| Arm (R) | 4 | 3 | 1.0 | 12 | 2 | 3 | 1.0 | 6 | 2 | 2 | 1.0 | 4 | 3 | 3 | 1.0 | 9 | 3 | 3 | 1.0 | 9 | 3 | 2 | 1.0 | 6 |
| Arm (L) | 4 | 3 | 1.0 | 12 | 2 | 3 | 1.0 | 6 | 2 | 3 | 1.0 | 6 | 4 | 4 | 1.0 | 16 | 4 | 3 | 1.0 | 12 | 3 | 3 | 1.0 | 9 |
| Trunk | 4 | 1 | 1.0 | 4 | 1 | 1 | 1.0 | 1 | 1 | 2 | 1.0 | 2 | 1 | 2 | 1.0 | 2 | 1 | 2 | 1.0 | 2 | 1 | 1 | 1.0 | 1 |
| Leg (R) | 3 | 2 | 1.0 | 6 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 |
| Leg (L) | 3 | 2 | 1.0 | 6 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 | 2 | 2 | 1.0 | 4 |
| Sum | 46/120 | 2/120 | 21.5/120 | 39.5/120 | 34.5/120 | 26.5/120 | ||||||||||||||||||
During subsequent follow-up visits, DBS stimulation was gradually increased using the Toronto Western Hospital Algorithm [1]. Further improvement in BFMDRS-M (21.5/120) was seen at 6 months. At 10 months, the BFMDRS-M score worsened to 39.5/120. This coincided with a skin infection over the implantable pulse generator (IPG) site. A trial of oral antibiotics was given, and the superficial infection resolved. There was no need for further intervention, surgical or otherwise. Improved scores were seen as the infection abated. At 1 year, the BFMDRS-M score was 34.5/120. At 13 months (online suppl. Video 4), the BFMDRS-M score was 26.5/120 (shown in Table 1).
The PedsQL™ 4.0 [2] (Pediatric Quality of Life Inventory) score at 6 months was 62 (from a preoperative score of 74) and was 68 at 1-year post-DBS. However, the family did report significant functional improvements in her activities of daily living, especially with feeding, dressing, and self-hygiene.
The final DBS settings were as follows: right GPi case+2- 4.0 mA and left GPi case+2- 4.0 mA. A pulse width of 60 µs and a frequency of 145 Hz were used for both hemispheres.
Discussion
L2HGA is a rare cerebral organic aciduria affecting the central nervous system. Only about 150 cases have been reported to date [3]. The disease has an insidious onset [4], with a wide range in age of diagnosis (4 months–43 years) [5]. The most common presenting symptoms are seizures (26%), ataxia (26%), intellectual deficit (22%), dysarthria (11.5%), and delayed walking (11%) [5]. Movement disorders develop in half of patients. Macrocephaly, pyramidal, and extrapyramidal signs may be present [4]. Also, there is a predisposition for central nervous system neoplasms [6–9] necessitating surveillance.
Characteristic magnetic resonance imaging findings include leukodystrophic changes in subcortical cerebral white matter with T2 hyperintensities of the dentate nucleus, GPi, putamen, and caudate nucleus [10]. The diagnosis can be confirmed by elevated urinary L-2 hydroxyglutaric acid and genetic analysis of the L2HGDH gene.
Pathogenic mutations in the L2HGDH gene result in a deficiency of the mitochondrial enzyme L-2-hydroxyglutarate dehydrogenase which converts L-2-hydroxyglutarate into 2-oxoglutarate, a metabolite of the Krebs cycle. This leads to pathological accumulation of L-2-hydroxyglutarate in the nervous system which is responsible for the neurological signs and symptoms.
There are reports of clinical improvement with riboflavin and levocarnitine [5]. However, treatment is mainly supportive, with control of seizure, anti-oxidant supplementation, and physiotherapy. Most patients survive to adulthood despite a dismal outcome with a chronic progressive course of neurologic deterioration [4].
With dystonia, in general, there is wide variability in the degree of response to DBS depending on etiology. Overall, patients with secondary dystonia show about 20% improvement in BFMDRS-M scores [18]. A better point of reference may be DBS outcomes in other organic acidurias. In a recent review, 5 patients with glutaric aciduria type 1 showed a median improvement of 6.4% on BFMDRS-M [19]. Similar results were seen in another review, where 2 out of 5 patients, also with glutaric aciduria type 1, showed reduced dystonia after DBS [11]. In a case report, a 12-year-old with methylmalonic acidemia had resolution of status dystonicus after undergoing GPi-DBS [12]. Here, it is notable that follow-up at 3.5 years showed sustained motor improvement despite the disease being progressive in nature with a tendency for autopallidotomy. A similar case of methylmalonic acidemia in a 4-year-old with total liquefaction of both palladi called for an alternative DBS target. The bilateral subthalamic nucleus (STN) was targeted, resulting in significant relief of dystonia and pain [13].
Targeting the GPi for medically resistant pediatric dystonia is becoming the standard of care [14–17]. This is due to the effectiveness, low complication rates, and the ability to adjust stimulation parameters. Hale et al. [18] reviewed 19 studies involving 76 children with dystonia where the GPi was targeted in all the cases (91% were bilateral with the remaining cases being unilateral). In these cases, the BFMDRS-M scores improved by 43.8 ± 36%. The data is less robust with other targets such as STN, thalamic nuclei ventralis oralis posterior, and ventralis intermedius. Even with the presence of neurodegeneration and central nervous system pathology, DBS has still shown to have positive effects on motor scores (n = 50; 26.8% mean improvement in BFMDRS-M) [19]. Lastly, there is no literature showing that hyperintensities in the GPi are directly connected to poor outcomes after DBS.
During the programming process, we appreciated better clinical outcomes with higher voltage/current settings. A recent review of DBS in pediatric dystonia [16] indicates that amplitude is commonly increased as tolerated until reaching 4–5 mA. Our patient is at the high end of that at 4.0. The reason for the higher settings in not fully understood. An important factor may be the significant leukodystrophy. As for the other parameters, the review notes that pulse width characteristically ranges from 60 to 200 μs (our patient is at 60 μs), and frequency can be as high as 190 Hz (our patient is at 145 Hz).
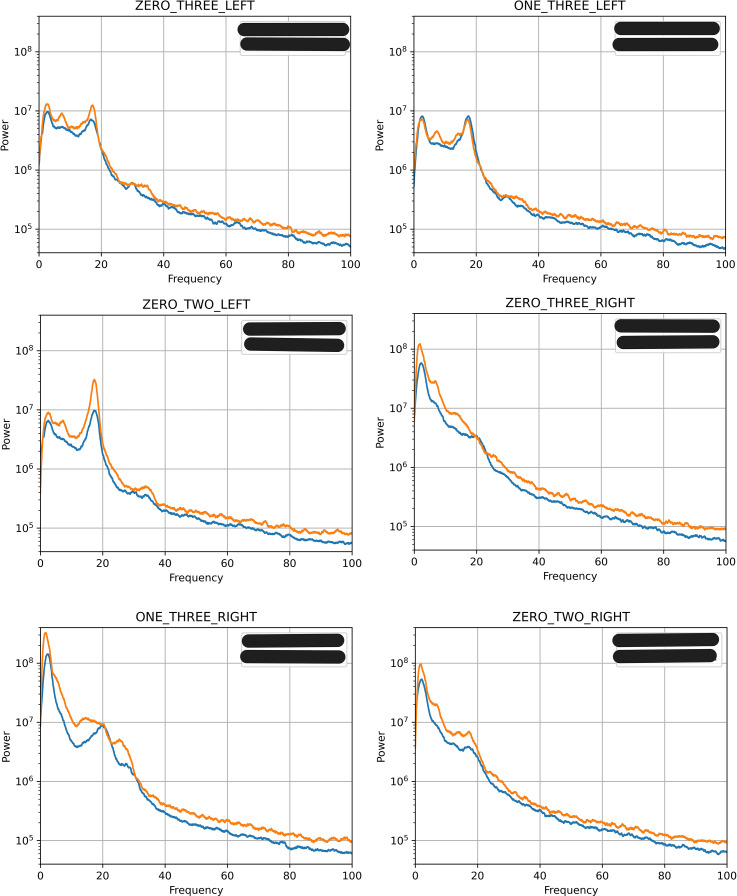
The Medtronic system allowed us to retrieve the local field potentials (LFPs) from the 4-month and 13-month postoperative periods (shown in Fig. 4). During both sessions, the right hemisphere showed accentuated spectral peaks in the delta (channel 0–3) and beta (channels 1–3 and 0–2) bands. The left hemisphere showed accentuated spectral peaks in the delta, theta, and beta bands across all channels. At both postoperative sessions, the right hemisphere exhibited more power than the left. The increased power over low frequencies is consistent with observations seen in other patients with dystonia [20, 21].
Fig. 4.
Local field potentials (LFPs). Graphical representation of LFP recordings from the left and right GPi at channels zero-three, one-three, and zero-two, with respect to the power and frequency (Hz). The spectra at the 4-month postoperative period are represented by blue lines. The spectra at the 13-month postoperative period are represented by orange lines.
The patient at the 4-month session had a BFMDRS-M score of 21.5/120, whereas at 13 months, the BFMDRS-M score was 26.5/120. The slight worsening of the dystonia scores corresponds to the increase in spectral power across all six channels during the 13-month postoperative session. Although there has been no correlation found between BFMDRS-M scores and spectral power [22], it appears that there is an association in this case.
Due to the relatively short follow-up time that we present here, the long-term outcomes are still to be determined. Longitudinal DBS data for pediatric dystonia have shown sustained improvement more than 5 years post-surgery [23]. However, less is known for dystonia caused by progressive degenerative disorders. Sustained motor gains have been shown in methylmalonic acidemia [12], but there are no data with L2HGA. Thus, we continue to monitor this patient regularly at the clinic. Nonetheless, the short- and medium-term results are promising.
To the best of our knowledge, this is the first report of DBS in a patient with L2HGA-related dystonia. Our patient experienced a significant decrease in dystonia as evidenced by the improvement in the BFMDRS-M scores. It is interesting to note that the PedsQL™ 4.0 scores showed slight worsening despite the motor and functional improvements. Previous studies found no correlation between BFMDRS improvement and QoL suggesting that these measures assess different aspects of the patient’s life and may not provide a comprehensive picture of treatment response [24]. Nonetheless, as further evidence of improvement, the patient was able to stop all oral anti-dystonia medications during the first year on DBS.
Over the last decade, DBS has gained increased popularity in rare pediatric conditions. There are reports of other rare genetic and metabolic conditions that variably responded to DBS for dystonia. For example, homocystinuria [25], neuronal ceroid lipofuscinosis, X-linked adrenoleukodystrophy, Wilson’s disease, and others [26]. Furthermore, Beneto et al. [12] reported a patient with methylmalonic acidemia who had a good response to stimulation of the STN that lasted more than 3 years. Although our observation only applies to a single case, it is important to report DBS outcome – positive or negative – as this can further assist physicians, patients, and families worldwide [26].
Finally, although the long-term outcome of our patient remains unknown, and the integrity of deep gray nuclei and neural networks are important for chronic DBS to show a good effect, we suggest that pallidal neuromodulation is a viable option for properly selected patients, even with secondary metabolic dystonias.
Acknowledgments
The authors thank the patient and her parents for their agreement to the study and for consenting to the publication of videos.
Statement of Ethics
The Research Ethics Boards at the Hospital for Sick Children has reviewed and approved this study (DBS Registry REB #1000068019). Written informed consent was obtained from the parent of the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
A.A.: conception, organization, execution, and writing of the first draft; S.B.: organization and review and critique; N.W.: execution and review and critique; E.R.: review and critique, writing – review and editing, and writing – abstract; G.I.: conception and review and critique; and A.F. and C.G.: conception, organization, and review and critique.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The data generated during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Picillo M, Lozano AM, Kou N, Munhoz RP, Fasano A. Programming deep brain stimulation for tremor and dystonia: the Toronto western hospital algorithms. Brain Stimul. 2016;9(3):438–52. [DOI] [PubMed] [Google Scholar]
- 2. Varni JW, Seid M, Kurtin PS. PedsQLTM 4.0: reliability and validity of the pediatric quality of life InventoryTM version 4.0 generic core scales in healthy and patient populations. [Online]. Available from: https://www.pedsql.org. [DOI] [PubMed]
- 3. Shah H, Chandarana M, Sheth J, Shah S. A case report of chronic progressive pancerebellar syndrome with leukoencephalopathy:L-2 hydroxyglutaric aciduria. Mov Disord Clin Pract. 2020;7(5):560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kranendijk M, Struys EA, Salomons GS, Van Der Knaap MS, Jakobs C. Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis. 2012;35(4):571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed S, Siddiqui A, DeBerardinis RJ, Ni M, Gu Lai W, Cai F, et al. L-2-hydroxyglutaric aciduria: review of literature and case series. Ann Med Surg. 2023;85(4):712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patay Z, Mills JC, Lö U, Lambert A, Sablauer A, Ellison DW. Cerebral neoplasms in L-2 hydroxyglutaric aciduria: 3 new cases and meta-analysis of literature data. Am J Neuroradiol. 2012;33(5):940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moroni I, Bugiani M, D’Incerti L, Maccagnano C, Rimoldi M, Bissola L, et al. L-2-hydroxyglutaric aciduria and brain malignant tumors: a predisposing condition? Neurology. 2004;62(10):1882–4. [DOI] [PubMed] [Google Scholar]
- 8. Faiyaz-Ul-Haque M, Al-Sayed MD, Faqeih E, Jamil M, Saeed A, Amoudi MS, et al. Clinical, neuroimaging, and genetic features of L-2-hydroxyglutaric aciduria in Arab kindreds. Ann Saudi Med. 2014;34(2):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haliloglu G, Jobard F, Oguz K, Anlar B, Akalan N, Coskun T, et al. L-2-hydroxyglutaric aciduria and brain tumors in children with mutations in the L2HGDH gene: neuroimaging findings. Neuropediatrics. 2008;39(02):119–22. [DOI] [PubMed] [Google Scholar]
- 10. Steenweg ME, Salomons GS, Yapici Z, Uziel G, Scalais E, Zafeiriou DI, et al. l-2-Hydroxyglutaric aciduria: pattern of MR imaging abnormalities in 56 Patients. Radiology. 2009;251(3):856–65. [DOI] [PubMed] [Google Scholar]
- 11. Shlobin NA, Hofmann K, Keating RF, Oluigbo CO. Deep brain stimulation and intrathecal/intraventricular baclofen for glutaric aciduria type 1: a scoping review, individual patient data analysis, and clinical trials review. J Inherit Metab Dis. 2023;46(4):543–53. [DOI] [PubMed] [Google Scholar]
- 12. Benato A, Carecchio M, Burlina A, Paoloni F, Sartori S, Nosadini M, et al. Long-term effect of subthalamic and pallidal deep brain stimulation for status dystonicus in children with methylmalonic acidemia and GNAO1 mutation. J Neural Transm. 2019;126(6):739–57. [DOI] [PubMed] [Google Scholar]
- 13. Chakraborti S, Hasegawa H, Lumsden DE, Ali W, Kaminska M, Lin JP, et al. Bilateral subthalamic nucleus deep brain stimulation for refractory total body dystonia secondary to metabolic autopallidotomy in a 4-year-old boy with infantile methylmalonic acidemia: case report. J Neurosurg Pediatr. 2013;12(4):374–9. [DOI] [PubMed] [Google Scholar]
- 14. Gorodetsky C, Fasano A. Approach to the treatment of pediatric dystonia. Dystonia. 2022;1(Aug). [Google Scholar]
- 15. Tisch S, Kumar KR. Pallidal deep brain stimulation for monogenic dystonia: the effect of gene on outcome. Front Neurol. 2020;11:630391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gelineau-Morel R, Kruer MC, Garris JF, Abu Libdeh A, Barbosa DAN, Coffman KA, et al. Deep brain stimulation for pediatric dystonia: a review of the literature and suggested programming Algorithm. J Child Neurol. 2022;37(10–11):813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandarano R, Danieli A, Petacchi E, Di Pede C, Mondani M, Armellin MT, et al. Deep Brain Stimulation in childhood-onset dystonia due to brain pathology. A long-term study. Eur J Paediatr Neurol. 2022;37:62–7. [DOI] [PubMed] [Google Scholar]
- 18. Hale AT, Monsour MA, Rolston JD, Naftel RP, Englot DJ. Deep brain stimulation in pediatric dystonia: a systematic review. Neurosurg Rev. 2020;43(3):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elkaim LM, Alotaibi NM, Sigal A, Alotaibi HM, Lipsman N, Kalia SK, et al. Deep brain stimulation for pediatric dystonia: a meta-analysis with individual participant data. Dev Med Child Neurol. 2019. 61(1):49–56. [DOI] [PubMed] [Google Scholar]
- 20. Chen CC, Kühn AA, Hoffmann KT, Kupsch A, Schneider GH, Trottenberg T, et al. Oscillatory pallidal local field potential activity correlates with involuntary EMG in dystonia. Neurology. 2006;66(3):418–20. [DOI] [PubMed] [Google Scholar]
- 21. Silberstein P, Kühn AA, Kupsch A, Trottenberg T, Krauss JK, Wöhrle JC, et al. Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain. 2003;126(Pt 12):2597–608. [DOI] [PubMed] [Google Scholar]
- 22. Ebden M, Elkaim LM, Breitbart S, Yan H, Warsi N, Huynh M, et al. Chronic pallidal local field potentials are associated with dystonic symptoms in children. Neuromodulation. 2024;27(3):551–6. [DOI] [PubMed] [Google Scholar]
- 23. Malatt C, Tagliati M. Long-term outcomes of deep brain stimulation for pediatric dystonia. Pediatr Neurosurg. 2022;57(4):225–37. [DOI] [PubMed] [Google Scholar]
- 24. Koy A, Kühn AA, Huebl J, Schneider GH, van Riesen AK, Eckenweiler M, et al. Quality of life after deep brain stimulation of pediatric patients with dyskinetic cerebral palsy: a prospective, single-arm, multicenter study with a subsequent randomized double-blind crossover (STIM-CP). Mov Disord. 2022;37(4):799–811. [DOI] [PubMed] [Google Scholar]
- 25. Aydin S, Abuzayed B, Varlibas F, Apaydin H, Mengi M, Kucukyuruk B, et al. Treatment of homocystinuria-related dystonia with deep brain stimulation: a case report. Stereotact Funct Neurosurg. 2011;89(4):210–3. [DOI] [PubMed] [Google Scholar]
- 26. Beaulieu-Boire I, Aquino CC, Fasano A, Poon YY, Fallis M, Lang AE, et al. Deep brain stimulation in rare inherited dystonias. Brain Stimul. 2016;9(6):905–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.