Abstract
Introduction
Obesity is a worldwide public health problem. Experimental animal and in vitro studies suggest that the exposure to BPA and phthalates are associated to a higher risk of obesity.
Objective
The objective of the study was to determine urinary excretion of bisphenol A and phthalates in obese and normal weight children.
Methods
A case-control study was conducted in 122 children. Sixty-six obese children, 36 girls (mean age 8.41 ± 1.27 years), and 30 boys (mean age 8.51 ± 1.33 years) and 56 normal weight children, 27 girls (mean age 7.64 ± 1.49 years), and 29 boys (mean age 7.77 ± 1.56 years) were studied. Urinary BPA and bis(2-ethylhexyl) phthalate (DEHP) metabolites (MEHP, MEHHP, and MEOHP) were measured, respectively, by gas chromatography and high-performance liquid chromatography. Individual determinants of exposure were evaluated through “ad hoc” questionnaires.
Results
BPA and DEHP metabolites were detectable in obese and normal weight children. Obese girls showed significantly higher BPA concentrations in comparison with normal weight girls (means 10.77, 95% CI = 7.02–16.53 vs. 5.50, 95% CI = 3.93–7.71 μg/g creatinine, respectively, p < 0.02). The first step of DEHP metabolic rate was significantly higher in obese girls compared with controls (p < 0.05). DEHP metabolites correlated significantly with leptin concentrations in obese girls (p < 0.03). A higher risk of obesity was found in children with BPA levels above the median values with the habit to eat food packaged (OR = 11.09, 95% CI = 1.28–95.78).
Conclusions
These findings show that a higher exposure to BPA is associated with the risk of obesity in girls. Further studies are needed to unveil the cause-effect relationship.
Keywords: Obesity, Bisphenol A, Phthalates, Children
Introduction
The prevalence of childhood obesity is increasing worldwide and it is a strong risk factor for developing comorbidities such as type 2 diabetes mellitus, gall bladder disease, sleep apnea, high blood pressure, insulin resistance, inflammation, breathlessness, metabolic syndrome, nonalcoholic fatty liver disease, and gestational diabetes mellitus in adulthood [1]. Individuals with obesity have increased risk of coronary heart disease and stroke, osteoarthritis and gout, impaired fertility, cancers, cataracts, and back problems [2]. Therefore, the prevention of obesity in childhood is crucial for reducing the risk of multiple chronic diseases in both adolescence and adulthood.
Obesity is a complex condition whose pathophysiology includes genetic, endocrine, psychological, social, and environmental factors. Endocrine disrupting chemicals (EDCs), according to the WHO definition, are “exogenous substances or mixtures that alter function(s) of the endocrine system and consequently causes adverse health effects in an intact organism or its progeny” (ref IPCS 2002). The exposure to specific EDCs has been related to the risk of obesity and these chemicals are also called “obesogens,” predisposing the individuals to obesity and its metabolic consequences. In experimental models, obesogens promote obesity by acting to increase adipocyte commitment, differentiation, and size by altering metabolic set points or altering the hormonal regulation of appetite and satiety [3, 4]. The obesogenic action of EDCs seems to be particularly effective when the exposure occurs in early life, consistently with the concept of the developmental origins of health and disease [5, 6].
Bisphenol A (BPA) is a widespread chemical that is widely used in polycarbonate plastics, can linings, and cash register receipts. The chemical structure of BPA makes it able to fit into the binding site of the estrogen receptor [7]. BPA has been widely detected in human serum, umbilical cord blood, breast milk, and urine, with human exposures occurring through oral, dermal, and inhalation routes [8]. Consistent evidence on the obesogenic effect of BPA comes from in vitro studies [9, 10]; however, a recent meta-analysis supported the association between BPA exposure and obesity in children [11].
Phthalates are chemicals used to impart flexibility in plastic products (plasticizers) including polyvinyl chloride to be used in floorings, car interiors, and toys [12]. These substances are also found in several household and personal care products, including food packaging and medical devices [12]. Phthalates easily leach from these products and, thus, are found in indoor air and house dust [13]. The majority of data supporting the obesogenic action of phthalates originate from animal and in vitro studies [14, 15]. There is mounting evidence suggesting a link between the exposure to phthalates and risk of obesity [16]. Phthalates exert an anti-androgenic effect through interaction with peroxisome proliferator-activated receptor alpha (PPARα) and the reduced androgen activity has been proposed to lead to obesity [17].
The aim of this case-control study, conceived in the frame of the European Commission (EC)-funded LIFE PERSUADED project [18], was to assess urinary BPA and bis(2-ethylhexyl) phthalate (DEHP) metabolite levels in a group of Italian children with obesity compared with a group of gender- and age-matched normal weight children. The impact of residential environment, lifestyle, and food habits on BPA and phthalate exposure was evaluated by ad hoc questionnaires.
Subjects and Methods
This study was conducted in the context of the EC-funded LIFE PERSUADED project whose main characteristics, aims, and methodology were previously published [18]. The project was approved by Ethics Committees of the Istituto Superiore di Sanità and “Bambino Gesù” Children’s Hospital in Rome, Italy (January 28, 2015, n. 889/2015). Written informed consent was obtained from participants (or their legal guardian in the case of minors) prior to enrollment for publication of the details of their medical case. All data generated or analyzed during this study are included in this article.
Subjects
In this case-control study, 66 obese prepubertal children (M/F, 30/36) and 56 normal weight children (M/F, 29/27) aged 5–10 years were recruited in the outpatient clinics of the “Bambino Gesù” Children’s Hospital (Rome, Italy). Children with idiopathic obesity were selected according to WHO criteria (ICP 2002). Trained pediatricians informed parents and children about the study aim and methodology with the aid of an ad hoc leaflet specifically produced for this purpose. Parents signed the informed consent form and they were asked to fill in a structured questionnaire either in paper or electronic format. For each enrolled subject, an alphanumeric code was created to guarantee anonymity. Children with obesity had a body mass index (BMI) above the 90th percentile according to the Italian reference standards [19], had no endocrine or genetic disorders, and did not take any concomitant therapy. Gender- and age-matched control children had a BMI between the 10th and 75th percentile and no endocrine disorders.
Fasting blood samples for biochemical marker analysis and urine samples, for measuring BPA and DEHP metabolites levels, were collected. Enrolled subjects provided first-morning urine samples and filled in questionnaire/food diaries to pediatrician the same day of sampling. We chose to collect the first-morning instead of 24-h urine samples, according to the procedure widely accepted in HBM study, as more suitable especially for children [20]. The urine samples were stored at −20°C until the shipping under controlled temperature to the laboratory for chemical analysis.
Anthropometry
Standing height was measured by a Harpenden stadiometer. Height, weight, BMI, and growth rate were expressed as z-scores for chronological age and sex. z-scores were calculated with the following formula: z-score = (x – average x)/SD, where x is the observed measurement, average x is the mean of this measurement at the relevant age, and SD is the standard deviation from the mean. Height, weight, and BMI z-scores were calculated using the national growth standards. The pubertal stage was assessed by trained pediatric endocrinologists according to Tanner’s criteria [21].
Metabolic Biomarkers Determination
An oral glucose tolerance test was performed with the administration of 1.75 g of glucose solution per kilogram of body weight to a maximum of 75 g. Blood samples were drawn at 0, 30, 60, 90, and 120 min for measurements of glucose. Plasma glucose was measured by enzymatic method on a Roche/Hitachi 904 analyzer (Roche Diagnostics, Mannheim, Germany; intra- and inter-assay coefficients of variation: 0.9 and 1.8%). Total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides (TGs) were measured by an enzymatic method (automated clinical chemistry analyzer Roche/Hitachi 904).
Adiponectin and leptin were determined in serum samples by commercial Quantikine ELISA (R&D Systems), having a sensitivity of 0.246 ng/mL and 7.8 pg/mL, respectively. Assays were performed according to the manufacturer’s instructions diluting serum samples 1:200 for adiponectin determination or 1:50 for leptin determination. Both kits provided a standard solution of the hormone to assay and 6 serial twofold concentrations were prepared to derive a standard curve. Each sample and standard dilution was assessed in duplicate. At the end of the test, absorbance was read at 450 nm on a Victor 3 Multilabel Reader (Perkin Elmer, MA, USA). Unknown hormone concentrations were derived from each corresponding standard curve by the GraphPad Prism 6.0 software (GraphPad Inc., La Jolla, CA, USA).
Phthalates and BPA Level Determination
The analytical methods for measuring BPA and the DEHP metabolites mono-(2-ethylhexyl) phthalate (MEHP), 2-ethyl-5-hydroxy-hexylphthalate (MEHHP), 2-ethyl-5-oxo-hexylphthalate (MEOHP) were previously described [22–24]. Briefly, upon arrival to the CNR laboratory, the urine samples were checked for integrity, aliquoted in polypropylene tubes (BPA- and phthalate-free materials) for creatinine, BPA, and DEHP metabolites determination, and stored at −20°C before being analyzed. Additional aliquots were stored in a biobank (https://www.ifc.cnr.it/index.php/it/biologia-preclinica/biobanca accessed on October 20, 2021). DEHP metabolite analyses were carried on using UHPLC 1290 infinity coupled with quadrupole time of flight (QTOF) 6540 (Agilent, Santa Clara, CA, USA) and BPA analysis by gas chromatography/mass spectrometry (GC 7890A coupled with MS 5975 Agilent, Santa Clara, CA, USA) as previously described in details [23].
Quantification of concentration of DEHP metabolites and BPA were determined using labelled internal standards and the analytical method was validated in a proficiency test organized by the HBM4EU Project within the quality assurance and quality control program, with good results [23, 25]. BPA and DEHP metabolite concentrations were normalized to urinary creatinine concentrations measured by Jaffe’s method (Beckman Coulter AU400, Brea, CA, USA) according to the manufacturer’s procedure. Creatinine levels were in the range 0.3–3 g/L in all urine samples included in the study. Limit of detection and quantification determination and values, background levels measured in water and urine, reagent blank, spike recovery, calibration curves, and quality control procedures have been previously reported [22–24].
Calculation of phthalate relative metabolic rates and percentages have been previously described [22]. Briefly, RMR1 = ([MEHHP] + [MEOHP])/[MEHP] represents the rate of hydroxylation from MEHP to MEHHP; RMR2 = ([MEOHP]/[MEHHP]) ×10 represents the rate of oxidation of MEHHP to MEOHP. The ratio of the molar concentration of each metabolite to the sum of all the metabolites is the percentage fraction of each metabolite.
Questionnaire and Food Diary
Parents of each enrolled children filled in a structured questionnaire and a food diary of the 2 days before the urine sampling [18]. Questionnaires provided information on residential environment, lifestyle, and food habits including specific questions concerning the handling, cooking, and storage of the food items consumed, in order to consider any potential source of BPA and phthalate exposure. Both paper and electronic versions of the questionnaires were made available to pediatricians and parents. Questionnaire data filled in paper format were entered by professionals in the electronic form to store the overall data and transfer them to a centralized database. Quality control of the data entry on both questionnaires and diaries was performed to ensure the completeness and the quality of data.
Statistical Analysis
Data analysis was performed using Stata ver14.2 (StataCorp, Lakeway Drive College Station, TX, USA) setting significance at p < 0.05 for all the statistical tests performed. Due to non-normality of the distributions, non-parametric Mann-Whitney and Kruskal-Wallis tests (with Dunn’s post hoc evaluation where applicable) were used to assess statistical differences between cases and controls. Differences between means in the different groups were assessed using general multivariate regression model MANOVA and Bonferroni’s post hoc test. Multivariable logistic analysis was performed to assess the relationships between metabolic and auxological (height, weight, and BMI) parameters, lifestyle or food habits, and urinary excretion of BPA and DEHP metabolites (expressed as creatinine-adjusted value or molar percentages). The study design matched by age and sex of cases and controls, but due to the statistically significant correlation between age and BMI, all the analyses included age as a covariate. The variable age was categorized in “0” for children 5–7 years old and “1” for 8–10 years old. For odd ratios (ORs), estimation of exposure to BPA or DEHP, continuous variables of the detected levels, adjusted for creatinine content, or molar percentages were transformed in categorical variables with “0” if <50° percentile (median) and “1” if ≥50° percentile. To evaluate the correlation between obesity, internal exposure to BPA, and the sum of DEHP metabolites and the other explanatory covariates as questionnaires and data from food diaries, all the logistic models included as covariates the categorized variables related to BPA and DEHP levels. Only in models with statistical positive association (OR > 1) between BPA or DEHP categorical variables and obesity, the association with the other covariates was considered.
Results
Clinical Characteristics
The clinical characteristics of obese and normal weight children for each sex are reported in Table 1.
Table 1.
Clinical characteristics of obese and control children
| Control girls (n = 27) | Obese girls (n = 36) | p value | Control boys (n = 29) | Obese boys (n = 30) | p value | |
|---|---|---|---|---|---|---|
| Age, years | 7.64±1.49 | 8.41±1.27 | <0.0001 | 7.77±1.56 | 8.51±1.33 | <0.0001 |
| Weight, kg | 26.70±7.66 | 49.45±12.19 | <0.0001 | 24.50±6.16 | 50.05±10.37 | <0.0001 |
| Weight, SDS | −0.16±1.33 | 2.01±0.57 | <0.0001 | −0.34±1.38 | 2.10±0.65 | <0.0001 |
| Height, cm | 123.72±10.91 | 136.16±10.89 | <0.001 | 122.14±11.46 | 137.64±9.25 | <0.001 |
| Height, SDS | 0.064±1.43 | 1.19±0.85 | <0.0001 | −0.2±1.6 | 1.02±0.95 | <0.0001 |
| BMI kg/m2 | 17.06±2.23 | 26.27±3.58 | <0.001 | 16.18±1.66 | 26.22±3.55 | <0.001 |
| BMI, SDS | −0.09±0.89 | 2.02±0.41 | <0.0001 | −0.07±2.35 | 2.06±0.53 | <0.0001 |
Values are expressed as mean ± SD.
p value <0.05.
BPA and Phthalates Levels
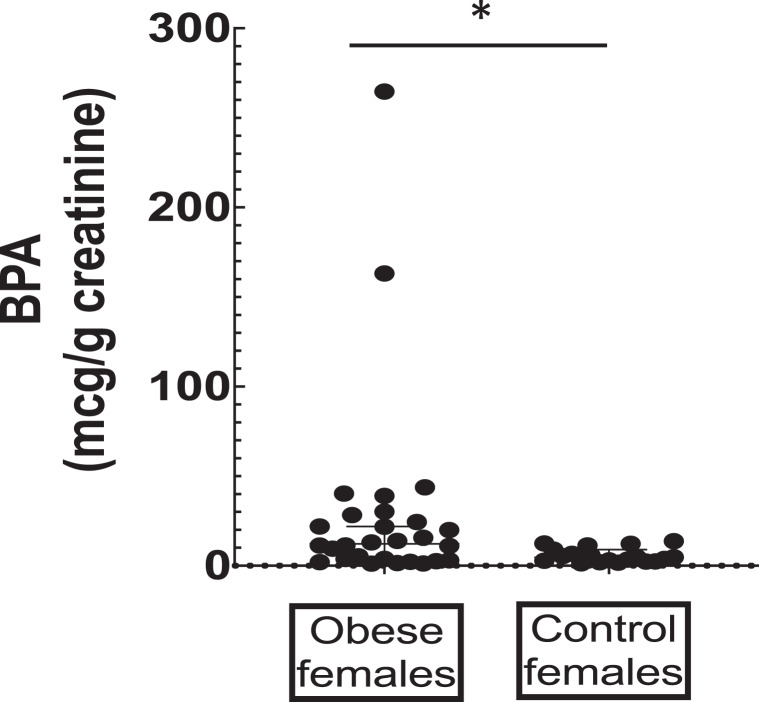
BPA and DEHP metabolites MEHP, MEHHP, and MEOHP were detected in all urine samples. The creatinine-adjusted levels, including the sum of the DEHP metabolites, stratified by sex and health condition, are summarized in Table 2. Obese girls showed significantly higher urinary BPA levels compared to normal weight girls (Fig. 1).
Table 2.
BPA and DEHP metabolites urinary levels adjusted for creatinine content (expressed as µg/g creatinine) in obese and control children
| Girls | Boys | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| control (N = 27) | obese (N = 36) | p value | controls (N = 29) | obese (N = 30) | p value | |||||
| GM | 95% CI | GM | 95% CI | GM | 95% CI | GM | 95% CI | |||
| BPA | 5.50 | 3.93–7.71 | 10.77 | 7.02–16.53 | 0.0151 | 9.15 | 5.79–14.47 | 9.54 | 6.63–13.75 | |
| MEHP | 8.75 | 7.08–10.83 | 8.41 | 6.18–11.43 | 6.81 | 5.36–8.66 | 4.58 | 3.75–5.59 | 0.0066 | |
| MEHHP | 13.19 | 7.04–24.73 | 23.25 | 16.29–33.17 | 21.84 | 15.89–30.00 | 16.80 | 12.50–22.57 | ||
| MEOHP | 5.20 | 4.00–6.75 | 6.42 | 4.62–8.93 | 4.88 | 3.71–6.43 | 4.57 | 3.45–6.06 | ||
| Σ phthalates | 32.52 | 24.57–43.06 | 41.04 | 29.93–26.29 | 34.99 | 26.45–46.28 | 27.08 | 20.90–35.09 | ||
| RMR1 | 2.32 | 1.65–3.26 | 3.38 | 2.55–4.49 | 0.0470 | 3.77 | 2.96–4.79 | 4.49 | 3.49–5.77 | |
| RMR2 | 3.97 | 2.40–6.57 | 2.78 | 2.44–3.17 | 2.25 | 1.86–2.72 | 2.74 | 2.35–3.20 | ||
| %MEHP | 27.90 | 22.50–34.60 | 21.34 | 17.67–25.76 | 0.0470 | 20.32 | 16.83–25.54 | 17.65 | 14.40–21.64 | |
| %MEHHP | 39.74 | 25.99–60.74 | 55.80 | 49.57–62.82 | 61.58 | 57.09–66.42 | 61.26 | 57.74–65.00 | ||
| %MEOHP | 15.77 | 13.78–18.05 | 15.51 | 13.78–17.47 | 13.86 | 12.02–15.97 | 16.78 | 14.77–19.07 | ||
RMR and molar percentages of DEHP metabolites are also shown.
Values are geometric means (GM) and 95% confidence intervals (CI).
Fig. 1.
BPA concentrations in obese girls (n = 36) and controls (n = 27). Values are expressed as means ± SD. *p < 0.05.
No significant difference was seen for the sum of DEHP metabolites. However, the first step of DEHP metabolic rate, RMR1, involving the transformation from MEHP to MEHHP, was significantly higher in obese compared to control girls. Accordingly, also the percentage of MEHP molar concentration with respect to the other metabolites was significantly lower in obese girls than in control girls with a parallel increased percentage of MEHHP fraction, although not significant (p = 0.0984).
No significant differences were observed for the levels of BPA and the sum of DEHP metabolites between obese and control boys. However, significantly lower MEHP levels were observed in boys with obesity compared to matched controls. No difference in the phthalate metabolic rates and molar percentages was observed.
The multivariable regression model, adjusted for age, showed an OR for the obese girls of having BPA above the 50° percentile 4.03 higher than the controls (95% confidence intervals [CI] = 1.32–12.29; p = 0.014). An inverse association with MEHP was observed in boys with obesity (OR = 0.1940, 95% CI = 0.056–0.6716; p = 0.010) meaning they have a risk 5.16 times higher to have MEHP levels below the median. Thus, both models confirm the results of the univariate correlation analysis.
Metabolic Biomarkers
Table 3 summarizes the serum levels of the metabolic parameters in obese and normal children. Both girls and boys with obesity were characterized by significantly lower concentrations of HDL cholesterol and significantly increased concentrations of leptin and triglycerides/HDL ratio in comparison with sex- and age-matched controls (Table 3). Obese boys showed significantly lower concentrations of total cholesterol and adiponectin. In obese children, adiponectin/leptin (Adpn/Lep) ratio was significantly lower in comparison with healthy controls (Table 3).
Table 3.
Metabolic parameters in serum of obese and control children
| Control girls (N = 27) | Obese girls (N = 36) | p value | Control boys (N = 29) | Obese boys (N = 30) | p value | |
|---|---|---|---|---|---|---|
| Glucose, mg/dL | 82 (78–85) | 82.5 (78–88) | 84 (77–88.5) | 82.5 (77–88) | ||
| Total cholesterol, mg/mL | 148 (137–171) | 143 (132–165) | 169 (151–172) | 149 (140–163) | 0.0294 | |
| HDL cholesterol, mg/mL | 61 (52–70) | 45 (40–55) | 0.0004 | 60 (56–74) | 50 (42–54) | 0.0051 |
| LDL cholesterol, mg/mL | 85 (75–101) | 93 (77–110) | 84 (75–106) | 93 (76–107) | ||
| TGs, mg/dL | 58 (48–82) | 80 (61–99) | 52 (48–68) | 65 (50–85) | ||
| TGs/HDL ratio | 1.05 (0.56–1.35) | 1.78 (1.08–2.09) | 0.0141 | 0.83 (0.69–1.16) | 1.27 (0.94–1.71) | 0.0186 |
| Leptin, ng/mL | 4.83 (4.16–9.97) | 17.93 (10.31–31.58) | <0.0001 | 2.45 (1.82–5.07) | 14.98 (8.19–18.46) | <0.0001 |
| Adiponectin, mg/mL | 21.77 (15.95–35.02) | 19.95 (12.54–26.78) | 24.13 (16.22–30.04) | 15.48 (10.79–22.48) | 0.0165 | |
| Adpn/Lep ratio | 3.65 (1.64–7.87) | 0.89 (0.66–1.61) | <0.0001 | 7.92 (4.13–13.24) | 1.24 (0.85–1.73) | 0.0002 |
Values are median with interquartile range (25th–75th percentile).
Table 4 shows the relationship between levels of plasticizers and metabolic parameters. In obese girls, BPA levels were not associated to a worse lipid profile given that BPA correlated negatively with LDL cholesterol and DEHP metabolites correlated positively with HDL cholesterol. DEHP metabolites showed positive association with leptin and negative association with adiponectin and Adpn/Lep ratio (Table 4). In obese boys, BPA positively correlated with LDL cholesterol and negatively with Adpn/Lep ratio (Table 4).
Table 4.
Significant pairwise correlations in girls and boys with obesity
| Obese girls | Glucose | HDL | LDL | Leptin | Adiponectin | Adipo/Lep ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPA | −0.4784 | 0.0156 | ||||||||||
| MEHHP | 0.4373 | 0.0225 | 0.3723 | 0.0276 | −0.3457 | 0.0420 | ||||||
| MEOHP | 0.4841 | 0.0105 | ||||||||||
| Σ phthalates | 0.4939 | 0.0088 | 0.3588 | 0.0343 | −0.3266 | 0.0555 | ||||||
| %MEHP | 0.3266 | 0.0555 | ||||||||||
| %MEHHP | 0.3504 | 0.0422 | 0.4443 | 0.0075 | −0.4157 | 0.0130 | ||||||
| %MEOHP | 0.4274 | 0.0117 | −0.3599 | 0.0337 | ||||||||
| Obese boys | ||||||||||||
| BPA | 0.4261 | 0.0337 | 0.3714 | 0.0516 | −0.3993 | 0.0391 | ||||||
Values are rho Spearman’s coefficient and p values.
Questionnaire and Food Diary
The analysis of the association between BPA or DEHP metabolite exposure and their possible determinants, as derived by the questionnaires and food diaries, showed limited significant associations. In particular, the habit to eat food packaged in plastic was associated with the risk of obesity only when considering BPA levels above the median, having the exposed to food packaged in plastic 11.09 times (95% CI = 1.28–95.78; p < 0.05) the risk (odds) of being obese than those not exposed, irrespective of the sex. Although the association was no more significant stratifying by sex, this is probably due to the small number of subjects in each group.
Discussion
The major findings of this study are that urinary BPA concentration, expressed as creatinine-adjusted value, is significantly increased in girls with obesity, with an OR of 4.03, and that DEHP metabolism is deranged in children with obesity, especially in girls. The finding of increased BPA concentrations in obese girls is further confirmed by comparing these levels with the recent reported human biomonitoring data of Italian normal weight children matched for age and sex (reported on IPCheM: the Information Platform for Chemical Monitoring https://ipchem.jrc.ec.europa.eu/index.html#showmetadata/LIFEPERSUADED; https://ipchem.jrc.ec.europa.eu/) [22, 24]. Moreover, this finding is consistent with the results of a recent survey on 1,860 children aged 8–19 years who participated in the 2003–2006 National Health and Nutrition Examination Survey (NHANES). This cross-sectional survey had complete data on both urinary BPA concentration and body composition measured by dual-energy X-ray absorptiometry and showed that higher BPA levels were associated with increased fat mass in girls but not in boys [26].
Previous studies provided conflicting results. Trasande et al. [27], after analyzing a cohort of 2,838 children aged 6–19 years from the 2003–2008 NHANES, reported a significant positive association between urinary BPA and BMI in both girls and boys. Conversely, in a study carried out in eastern China, no difference in urinary BPA concentrations between normal weight and obese children was observed [28]. A recent meta-analysis has reported sixteen studies conducted in children and adolescents. Ten of them showed a positive association between exposure to BPA and obesity. Interestingly, three studies indicated that the association was significant in girls only [29].
We investigated the association between urinary levels of BPA and DEHP metabolites with markers of lipid metabolism (LDL and HDL cholesterol) and adipose tissue dysfunction (low adiponectin and high leptin) [30]. We observed different correlations between BPA levels and metabolic parameters in children with obesity. In particular, girls and boys displayed opposite correlations with LDL cholesterol. Interestingly, we found a significantly lower Adpn/Lep ratio in children with obesity compared with healthy controls. Both high leptin and low adiponectin have been related to increased cardiometabolic risk [30]. Thus, the Adpn/Lep ratio has been proposed as a marker of adipose tissue dysfunction. This biomarker correlates with insulin resistance better than adiponectin or leptin alone, being significantly reduced in patients with the metabolic syndrome. The Adpn/Lep ratio has been proposed as a predictive marker for the metabolic syndrome [31]: an Adpn/Lep ratio of ≥1.0 can be considered normal, between 0.5 and 1.0 suggests moderate-medium increased risk, and less than 0.5 indicates a high cardiometabolic risk. In our study population, obese girls had an Adpn/Lep ratio between 0.66 and 1.61 (interquartile range) and obese boys between 0.85 and 1.73, significantly lower than in the control normal weight groups that had a median of 3.65 and 7.92 in girls and boys, respectively (Table 3). It is noteworthy that Adpn/Lep ratio was negatively associated with the sum of DEHP metabolites in obese girls and with BPA in obese boys (Table 4).
The gender-specific effects of perinatal BPA exposure on adipose tissue deposition were previously reported in animal models, females being more susceptible to BPA effects [32–34]. However, the mechanisms by which BPA increases body weight and adipose tissue mass and its gender-specific effect have not been elucidated yet. Pu et al. [35] have recently investigated the effects of gestational BPA exposure and its analog, bisphenol S, on the adipogenic differentiation ability of fetal preadipocytes in pregnant sheep exposed from days 30 to 100 of gestation, demonstrating that BPA stimulated adipogenic differentiation of preadipocytes in female but not in male offspring. This effect was associated with up-regulation of the unfolded protein response pathway, suggestive of an increased expression of estrogen receptor and glucocorticoid receptor in female preadipocytes. These changes may be the underlying causes of BPA gender dimorphism. Indeed, the finding that urinary BPA concentrations are increased only in obese girls may be explained by the estrogen-like actions of BPA. Girls with higher BPA levels show an increased risk of early onset of puberty, which is often associated with childhood obesity [36]. However, the wide CI range in obese girls indicates a high individual variability of BPA exposure with a large group of outliers making the difference with the normal weight girls. This finding suggests that exposure to BPA may be obesogenic only in a subgroup of obese girls (genetically predisposed). Overall, our findings suggest a higher susceptibility of females to BPA and, potentially, a higher risk to develop obesity and its consequences in adulthood.
However, only few studies reported data on metabolic markers in relation to BPA exposure in children with obesity. Menale et al. [37] reported a negative association between BPA and adiponectin gene expression in children with obesity, irrespective of the gender, whereas a positive association between BPA exposure and leptin was found in normal weight peripubertal boys [38]. LDL cholesterol was found positively associated with BPA in large adult cohorts [39, 40], whereas no association was found in both children and adults in a meta-analysis of NHANES studies [41]. However, both studies evaluated data of the general population not stratified for obesity and gender.
No significant differences in the sum of DEHP metabolites were observed between obese and normal weight children, whose levels of exposure fell into the background levels measured in Italian children population during the HBM study [22, 24]. However, we found that obese girls transform more efficiently MEHP to MEHHP compared to control girls, thus having higher RMR1 and a lower relative MEHP percentage. Similarly, boys with obesity had lower urinary MEHP levels than age-matched controls but with no concomitant imbalance of the secondary metabolites. We also observed several positive correlations between DEHP metabolites or their molar percentages and glucose, HDL cholesterol, and leptin but only in girls with obesity. A lower MEHP/MEHHP concentration ratio was observed in prepubertal girls with obesity [11], with a parallel association between higher %MEHHP and increased BMI, waist circumference, body fat percentage, and insulin resistance index (HOMA-IR). The results were further confirmed in a recent report showing that lower MEHP levels and higher secondary metabolites levels, as well as RMR1, were associated with obesity in both girls and boys [42]. Positive associations between MEHHP and MEOHP metabolites and markers of glucose metabolism or HOMA-IR were observed also in the general population and children [43, 44]. Our results confirm that the imbalance in the first step of DEHP metabolism, with a shift toward secondary metabolites, is associated with obesity [42].
Many experimental studies carried out on animal models showed an association between the exposure to phthalates and increased adiposity. However, human studies on the impact of the exposure to phthalates on obesity are limited and mainly focused on fetal exposure and subsequent risk of obesity in childhood [2, 10, 45]. More recently, a study conducted on 2,372 children (NHANES 2005–2010) has shown that monoethyl phthalate and mono-isobutyl phthalate were positively associated with obesity (OR 1.28 and 1.42, respectively) [46]. In the above-cited meta-analysis reporting seven studies conducted in children, an overall positive association between exposure to phthalates and obesity was found [29].
The multivariable analysis on the determinants of exposure and the risk of obesity showed that the only significant association was between BPA and the children habit to eat food packaged in plastic. However, this association was not observed analyzing data by gender. In students aged 3–24 years, those drinking in plastic bottle or cups had higher BPA levels than those drinking in ceramic cups [47]. In two dietary interventions in families where food packaged in plastic was eliminated by the diet for 3 days, or 2 months, 66% and 100% reduction of BPA urine levels were observed, respectively [48, 49]. In a study of duplicated diet, the urinary level of BPA in the group of individuals following a diet based on canned foodstuffs was remarkably higher than those following a BPA-free diet, made of fresh foodstuffs and food products packed in glass containers and other BPA-free materials [50]. Our data [51] support the concept that canned foodstuffs and food packed in plastics may represent one of the most relevant sources of dietary BPA exposure.
In conclusion, higher urinary BPA concentrations and altered DEHP metabolism was found in obese girls compared to girls with normal weight, but not in obese boys, indicating a sex-specific effect. High urine BPA and DEHP levels may be considered as possible biomarkers of effect in the exposure-health impact relationships, thus deserving further investigations. Prospective longitudinal studies are needed to clarify the clinical relevance of exposure to these plasticizers during childhood as risk factor for obesity.
Acknowledgments
LIFE PERSUADED project group: Istituto Superiore di Sanità, Rome, Italy: Cinzia La Rocca, Luca Busani, Lucia Coppola, Gabriele Lori, Francesca Maranghi, Laura Narciso, Annalisa Silenzi, Sabrina Tait, and Roberta Tassinari (Center for Gender-Specific Medicine); Roberta Urciuoli (Department of Infectious Diseases); Antonio Di Virgilio, Andrea Martinelli, and Mauro Valeri (Centro Nazionale Sperimentale Benessere Animale); French Institute of Health and Medical Research: Francesca Romana Mancini; Dipartimento Pediatrico Universitario Ospedaliero “Bambino Gesu,” Rome, Italy: Stefano Cianfarani, Giorgia Bottaro, Annalisa Deodati, Romana Marini, Giuseppe Scirè, and Gian Luigi Spadoni; Tor Vergata University, Rome, Italy: Stefano Cianfarani and Daniela Germani; Istituto di Fisiologia Clinica, CNR, Pisa, Italy: Amalia Gastaldelli, Graziano Barsotti, Emma Buzzigoli, Fabrizia Carli, Demetrio Ciociaro, Raffaele Conte, Veronica Della Latta, Graziella Distante, Melania Gaggini, Patrizia Landi, Anna Paola Pala, and Chiara Saponaro; and Network of Italian pediatricians: https://lifp.iss.it/?p%20=%2073 (February 10, 2021).
Statement of Ethics
All procedures were approved by local Institutional Review Boards (IRCCS “Bambino Gesù” Children’s Hospital) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from participants (or their legal guardian in the case of minors) prior to enrollment for publication of the details of their medical case. All data generated or analyzed during this study are included in this article.
Conflict of Interest Statement
The authors have no conflicts of interest to declare, except Stefano Cianfarani who is the Editor-in-Chief of Hormone Research in Pediatrics.
Funding Sources
This work was funded by the European Commission Directorate General for Environment, LIFE Programme, Environment Policy, and Governance (Grant No. LIFE13 ENV/IT/000482).
Author Contributions
Annalisa Deodati, Giorgia Bottaro, Daniela Germani, Fabrizia Carli, Sabrina Tait, Luca Busani, Veronica Della Latta, Anna Paola Pala, Francesca Maranghi, Roberta Tassinari, Amalia Gastaldelli, Cinzia La Rocca, and Stefano Cianfarani wrote the article and designed and performed experimental data. Annalisa Deodati, Cinzia La Rocca, Amalia Gastaldelli, and Stefano Cianfarani critically reviewed the article.
Funding Statement
This work was funded by the European Commission Directorate General for Environment, LIFE Programme, Environment Policy, and Governance (Grant No. LIFE13 ENV/IT/000482).
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Caprio S, Santoro N, Weiss R. Childhood obesity and the associated rise in cardiometabolic complications. Nat Metab. 2020;2(3):223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11:653–61. [DOI] [PubMed] [Google Scholar]
- 3. Heindel JJ, Blumberg B. Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol. 2019;59:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endo. 2020;8:703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1(3):130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alonso-Magdalena P, Ropero AB, Soriano S, García-Arévalo M, Ripoll C, Fuentes E, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355(2):201–7. [DOI] [PubMed] [Google Scholar]
- 8. Ma Y, Liu H, Wu J, Yuan L, Wang Y, Du X, et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ Res. 2019;176:108575. [DOI] [PubMed] [Google Scholar]
- 9. Boucher JG, Boudreau A, Atlas E. Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr Diabetes. 2014;4(1):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veiga-Lopez A, Pu Y, Gingrich J, Padmanabhan V. Obesogenic endocrine disrupting chemicals: identifying knowledge gaps. Trends Endocrin Met. 2018;29(9):607–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim KY, Lee E, Kim Y. The association between bisphenol A exposure and obesity in children-a systematic review with meta-analysis. Int J Environ Res PublicHealth. 2019;16(14):2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Zhu HK, Kannan K. A review of biomonitoring of phthalate exposures. Toxics. 2019;7(2):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Envir Heal. 2007;210(5):623–34. [DOI] [PubMed] [Google Scholar]
- 14. Hao C, Cheng X, Guo J, Xia H, Ma X. Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Front Biosci. 2013;5(2):725–33. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ Health Perspect. 2012;120(8):1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radke EG, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int. 2019;132:104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol Cell Endocrinol. 2009;304(1–2):43–8. [DOI] [PubMed] [Google Scholar]
- 18. La Rocca C, Maranghi F, Tait S, Tassinari R, Baldi F, Bottaro G, et al. The LIFE PERSUADED project approach on phthalates and bisphenol A biomonitoring in Italian mother-child pairs linking exposure and juvenile diseases. Environ Sci Pollut Res Int. 2018;25:25618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 year). J Endocrinol Invest. 2006;29(7):581–93. [DOI] [PubMed] [Google Scholar]
- 20. Becker K, Seiwert M, Casteleyn L, Joas R, Joas A, Biot P, et al. A systematic approach for designing a HBM Pilot Study for Europe. Int J Hyg Envir Heal. 2014;217(2–3):312–22. [DOI] [PubMed] [Google Scholar]
- 21. Marshall WA, Tanner JM. Variations in pat- tern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tait S, Carli F, Busani L, Buzzigoli E, Della Latta V, Deodati A, et al. Biomonitoring of Bis(2-ethylhexyl)phthalate (DEHP) in Italian children and adolescents: data from LIFE PERSUADED project. Environ Res. 2020;185:109428. [DOI] [PubMed] [Google Scholar]
- 23. Carli F, Ciociaro D, Gastaldelli A. Assessment of exposure to di-(2-ethylhexyl) phthalate (DEHP) metabolites and bisphenol A (BPA) and its importance for the prevention of cardiometabolic diseases. Metabolites. 2022;12(2):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tait S, Carli F, Busani L, Ciociaro D, Della Latta V, Deodati A, et al. Italian children exposure to bisphenol A: biomonitoring data from the LIFE PERSUADED project. Int J Environ Res Public Health. 2021;18(22):11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. EPA . Title 40: protection of environment; Part 136—guidelines establishing test procedures for the analysis of pollutants; appendix B to Part 136—definition and procedure for the determination of the method detection limit—revision 2. Code federal regulat. 2017. p. 343–6. Available from: https://www.govinfo.gov/content/pkg/CFR-2012-title40-vol24/pdf/CFR-2012-title40-vol24-part136-appB.pdf(2021). [Google Scholar]
- 26. Li J, Lai H, Chen SG, Zhu H, Lai SH. Gender differences in the associations between urinary bisphenol A and body composition among American children: the National Health and Nutrition Examination Survey, 2003–2006. J Epidemiol. 2017;27(5):228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308(11):1113–21. [DOI] [PubMed] [Google Scholar]
- 28. Wang B, Wang H, Zhou W, He Y, Zhou Y, Chen Y, et al. Exposure to bisphenol A among school children in eastern China: a multicenter cross-sectional study. J Expo Sci Env Epid. 2014;24(6):657–64. [DOI] [PubMed] [Google Scholar]
- 29. Ribeiro CM, Beserra BTS, Silva NG, Lima CL, Rocha PRS, Coelho MS, et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ Open. 2020;10(6):e033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis. 2010;20(7):481–90. [DOI] [PubMed] [Google Scholar]
- 31. Frühbeck G, Catalán V, Rodríguez A, Ramírez B, Becerril S, Salvador J, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation inflammation. Nutrients. 2019;11(2):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Persp. 2001;109(7):675–80. https://doi.org/10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, et al. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ Health Persp. 2009;117(10):1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rubin BS, Paranjpe M, DaFonte T, Schaeberle C, Soto AM, Obin M, et al. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: the addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod Toxicol. 2017;68:130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pu Y, Gingrich JD, Steibel JP, Veiga-Lopez A. Sex-specific modulation of fetal adipogenesis by gestational bisphenol A and bisphenol S exposure. Endocrinology. 2017;158(11):3844–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Durmaz E, Aşçı A, Erkekoğlu P, Akçurin S, Gümüşel BK, Bircan I. Urinary bisphenol A levels in girls with idiopathic central precocious puberty. J Clin Res Pediatr E. 2014;6(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menale C, Grandone A, Nicolucci C, Cirillo G, Crispi S, Di Sessa A, et al. Bisphenol A is associated with insulin resistance and modulates adiponectin and resistin gene expression in obese children. Pediatr Obes. 2017;12(5):380–7. [DOI] [PubMed] [Google Scholar]
- 38. Watkins DJ, Peterson KE, Ferguson KK, Mercado-García A, Tamayo y Ortiz M, Cantoral A, et al. Relating phthalate and BPA exposure to metabolism in peripubescence: the role of exposure timing, sex, and puberty. J Clin Endocrinol Metab. 2016;101(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li R, Yang S, Gao R, Deng Y, Liu J, Yuan C, et al. Relationship between the environmental endocrine disruptor bisphenol a and dyslipidemia: a five-year prospective study. Endocr Pract. 2020;26(4):399–406. [DOI] [PubMed] [Google Scholar]
- 40. Wang B, Wang S, Zhao Z, Chen Y, Xu Y, Li M, et al. Bisphenol A exposure in relation to altered lipid profile and dyslipidemia among Chinese adults: a repeated measures study. Environ Res. 2020;184:109382. [DOI] [PubMed] [Google Scholar]
- 41. Dunder L, Lejonklou MH, Lind PM, Lind L. Urinary bisphenol A and serum lipids: a meta-analysis of six NHANES examination cycles (2003–2014). J Epidemiol Community Health. 2019;73(11):1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tassinari R, Narciso L, Tait S, Busani L, Martinelli A, Di Virgilio A, et al. Juvenile toxicity rodent model to study toxicological effects of bisphenol A (BPA) at dose levels derived from Italian children biomonitoring study. Toxicol Sci. 2020;173(2):387–401. [DOI] [PubMed] [Google Scholar]
- 43. On J, Kim SH, Lee J, Park MJ, Lee SW, Pyo H. Urinary di(2-ethylhexyl)phthalate metabolite ratios in obese children of South Korea. Environ Sci Pollut Res Int. 2021;28(23):29590–600. [DOI] [PubMed] [Google Scholar]
- 44. Dales RE, Kauri LM, Cakmak S. The associations between phthalate exposure and insulin resistance, β-cell function and blood glucose control in a population-based sample. Sci Total Environ. 2018;612:1287–92. [DOI] [PubMed] [Google Scholar]
- 45. Han H, Lee HA, Park B, Park B, Hong YS, Ha EH, et al. Associations of phthalate exposure with lipid levels and insulin sensitivity index in children: a prospective cohort study. Sci Total Environ. 2019;662:714–21. [DOI] [PubMed] [Google Scholar]
- 46. Schaedlich K, Gebauer S, Hunger L, Beier LS, Koch HM, Wabitsch M, et al. DEHP deregulates adipokine levels and impairs fatty acid storage in human SGBS-adipocytes. Sci Rep. 2018;8(1):3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Philips EM, Jaddoe VWV, Deierlein A, Asimakopoulos AG, Kannan K, Steegers EAP, et al. Exposures to phthalates and bisphenols in pregnancy and postpartum weight gain in a population-based longitudinal birth cohort. Environ Int. 2020;144:106002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li X, Ying GG, Zhao JL, Chen ZF, Lai HJ, Su HC. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ Int. 2013;52:81–6. [DOI] [PubMed] [Google Scholar]
- 49. Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hutter HP, Kundi M, Hohenblum P, Scharf S, Shelton JF, Piegler K, et al. Life without plastic: a family experiment and biomonitoring study. Environ Res. 2016;150:639–44. [DOI] [PubMed] [Google Scholar]
- 51. González N, Marquès M, Cunha SC, Fernandes JO, Domingo JL, Nadal M. Biomonitoring of co-exposure to bisphenols by consumers of canned foodstuffs. Environ Int. 2020;140:105760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.