Abstract
Introduction
Despite the known benefits of deep brain stimulation (DBS), the cost of the procedure can limit access and can vary widely. Our aim was to conduct a systematic review of the reported costs associated with DBS, as well as the variability in reporting cost-associated factors to ultimately increase patient access to this therapy.
Methods
A systematic review of the literature for cost of DBS treatment was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed and Embase databases were queried. Olsen & Associates (OANDA) was used to convert all reported rates to USD. Cost was corrected for inflation using the US Bureau of Labor Statistics Inflation Calculator, correcting to April 2022.
Results
Twenty-six articles on the cost of DBS surgery from 2001 to 2021 were included. The median number of patients across studies was 193, the mean reported age was 60.5 ± 5.6 years, and median female prevalence was 38.9%. The inflation- and currency-adjusted mean cost of the DBS device was USD 21,496.07 ± USD 8,944.16, the cost of surgery alone was USD 14,685.22 ± USD 8,479.66, the total cost of surgery was USD 40,942.85 ± USD 17,987.43, and the total cost of treatment until 1 year of follow-up was USD 47,632.27 ± USD 23,067.08. There were no differences in costs observed across surgical indication or country.
Conclusion
Our report describes the large variation in DBS costs and the manner of reporting costs. The current lack of standardization impedes productive discourse as comparisons are hindered by both geographic and chronological variations. Emphasis should be put on standardized reporting and analysis of reimbursement costs to better assess the variability of DBS-associated costs in order to make this procedure more cost-effective and address areas for improvement to increase patient access to DBS.
Keywords: Deep brain stimulation, Cost, Economic analysis, Price, Insurance
Introduction
Deep brain stimulation (DBS) is a surgical therapy for movement disorders that involves implantation of an electrode system to modulate neuronal function and improve movement disorder symptoms [1]. Since its popularization in the treatment of essential tremor (ET) in 1987 [2], DBS has revolutionized treatment for other movement disorders such as Parkinson’s disease, ET, and dystonia [3]. DBS for other indications such as pain, epilepsy, addiction, and psychiatric disorders is currently under study [4]. While the effects of DBS are profound and can significantly improve well-being for individuals with these conditions [5], the cost of this treatment may be an important consideration for patient accessibility and hospital reimbursement.
DBS cost can include several factors, such as the initial consultation, pre-operative testing, implants and other materials, surgery, and post-operative care and the reporting of these contributary costs in the current literature is inconsistent. In light of the non-standardized reporting of DBS cost, it appears that the cost of this procedure varies widely both within and between countries [6]. In the USA, the reported hospital cost of DBS ranges from US dollars (USD) 25,651 to USD100,041.11 and varies based on the location of the surgery, the medical center in which it was performed, and the insurance coverage of the patient [7, 8]. Importantly, insurance coverage can vary widely, with some policies fully covering the procedure and others covering only some portions [9]. Along with this, insurance coverage for DBS depends on the indication for surgery, with some insurance agencies not covering more recently approved indications for DBS, such as obsessive-compulsive disorder (OCD) or epilepsy, requiring many patients to cover the total cost of the procedure out of pocket [10].
In this study, we report findings from a systematic review of the published literature on costs associated with DBS. While DBS is a highly effective treatment for movement disorders, its cost may limit access, especially for patients without adequate insurance coverage [11–14]. Standardized reporting of cost elements and cost-reporting frameworks may facilitate analysis of hospital or practice cost points and the ability to effectively tailor price to optimize national DBS patient coverage. Our aim was to collect and analyze the reported costs of DBS in the present literature in order to assess cost variation and cost-reporting frameworks and better understand ways in which DBS may be made more affordable and accessible to patients. In doing so, a standardized approach is proposed for breaking down and reporting costs, emphasizing key categories such as surgical implants/materials, pre-operative workup, and post-operative care.
Methods
Search Criteria and Study Selection
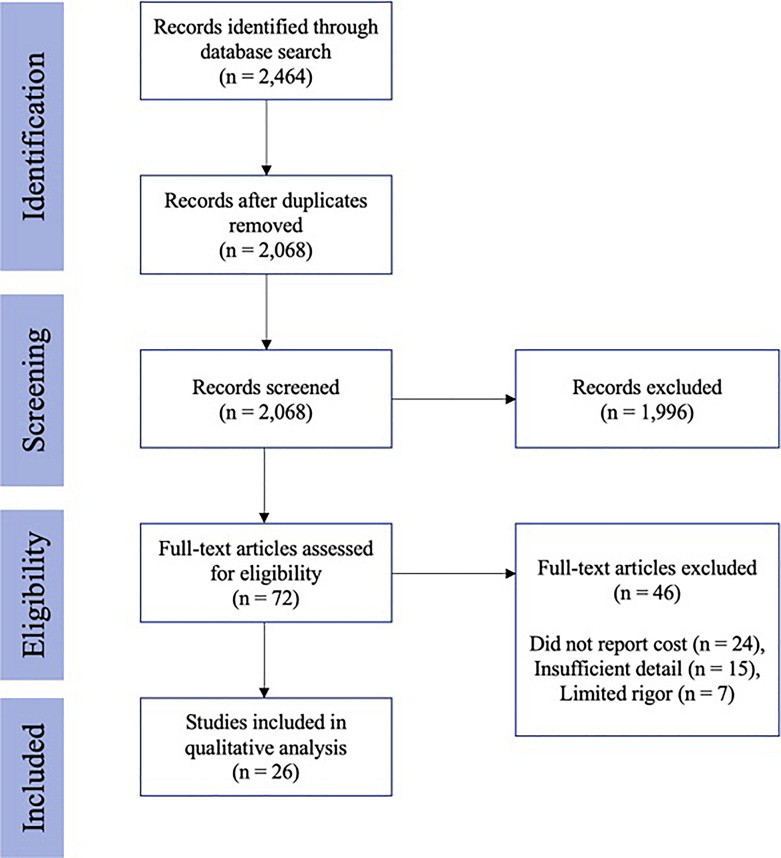
A PRISMA-guided systematic review of the literature was performed on November 2022 to investigate the costs associated with DBS surgery [15]. The terms “deep brain stimulation” and “cost” were utilized as search terms, with additional variation of cost-reporting terms included. Both PubMed and Embase databases were searched, and the complete search syntax included (“deep brain stimulation [MeSH Terms] OR “DBS” OR “brain pacemaker” OR “brain implant” OR “neurostimulation” OR “neuromodulation”) AND (“costs and cost analysis” [MeSH Terms] OR “cost-benefit analysis” [MeSH Terms] OR “health care costs” [MeSH Terms OR “health expenditures” [MeSH Terms]). Data analysis and extraction were performed by two independent reviewers (A.E.B. and A.T.L.), while confirmation of article or data inclusion was settled by a third author (S.W.K.). Studies that presented original research describing treatment-related cost information from 1990 to present day were considered. Letters to the editor, commentaries, and articles of editorials were excluded from review. The PRISMA search process included screening the title, abstract, and full-text article. Papers that did not specifically report cost of DBS were excluded in the full-text screening. Studies from all countries were considered; however, an English version of the article was required in order to be included in the final qualitative and quantitative analysis. A PRISMA flow diagram outlining the number of papers excluded at each stage of the review is provided in Figure 1. This study was deemed exempt from review by the Vanderbilt University Medical Center Institutional Review Board (IRB).
Fig. 1.
PRISMA flow diagram outlining the steps taken during study selection, inclusion, and exclusion.
Inflation and Cost Adjustment
To ensure consistency, historical currency exchange rates from Olsen & Associates (OANDA) were utilized as the primary source to convert various currencies to a common denominational currency, USD [16]. To calculate the exchange rate, we determined the average rate for the month(s), aligning with the data collection period specified in the studies. For instances in which the article provided native exchange rates, those rates were used instead of OANDA-derived rates. Additionally, the cost data were corrected for inflation using the Producer Price Index for General Medical and Surgical Hospitals over the entire year of the reported study cost year, correcting to April 2022 [17]. This approach allowed for accurate and standardized comparison of costs across different countries and time periods.
Data Extraction
Data were extracted by the two independent reviewers (A.E.B., A.T.L.) into a common, secure Excel database. Qualitative measures such as demographic characteristics of the patient population, country of procedure, indication for DBS, single versus multi-center study, and length of follow-up were recorded. Data regarding the measures included in the study were collected, including the number of stages (i.e., separate surgeries for electrode implantation and generator installment), pre- or post-operative imaging, battery replacement, replacement surgery, complications, intraoperative testing, manufacturing company of the electrode, and single- or dual-channel electrodes. Costs were separated into one of four categories: DBS device cost, the cost of DBS surgery, total cost of surgery, and total cost of treatment. The DBS device cost was defined as the cost of the electrode, lead, and IPG only. The cost of DBS surgery was defined as the costs associated with surgery (from initial hospital visit to discharge), not including the cost of the DBS device. The total cost of surgery was defined as the cost of surgery and the cost of the device. Finally, the total cost of treatment was defined as the costs incurred for all components of DBS care up to 1 year of follow-up, including clinic appointments, pre- and post-operative imaging, complications, and drug costs. Although some studies reported costs up to 20 years of follow-up, our analysis was limited to costs up to 1 year. Due to the variation in total cost reporting, study characteristics and a breakdown of included costs for each study are provided in Tables 1 and 2, respectively.
Table 1.
Study characteristics, cost breakdown, and RBA
| Author | Pts, n | Age of Pts | % female | Indication | Country | Type of cost analysis | Year | Cost of DBS device | Cost of surgery | Total cost of surgery | Total cost of treatment | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akazawa et al. [18] | 519 | 62.7 | 57.6 | PD, T, D | Japan | Cost minimization analysis | 2018 | USD 2,587.17 | – | USD 6,032.61 | USD 6,582.67 | 3 |
| Dams et al. [19] | – | 52±6.6 | 47.4 | PD | Germany | Markov model | 2013 | – | – | USD 48,722.95 | USD 51,394.86 | 4 |
| Fann et al. [20] | – | – | – | PD | China | Cost-effectiveness analysis | 2020 | – | – | USD 63,526.92 | USD 65,300.81 | 1 |
| Gomez-Inhiesto et al. [21] | 105 | – | – | PD | Spain | Simple cost | 2020 | USD 21,437.86 | – | – | USD 42,875.72 | 4 |
| Hooper and Whittle [22] | 15 | – | – | PD | UK | Simple cost | 2003 | USD 14,527.88 | USD 28,787.68 | USD 43,315.55 | – | 2 |
| Igarashi et al. [23] | – | – | – | T | Japan | Cost minimization model | 2019 | – | – | USD 22,695.42 | USD 24,999.34 | 1 |
| Jacob et al. [24] | 211 | 65±9 | 38.86 | PD, T | USA | Cost analysis | 2016 | USD 28,257.12 | USD 17,818.54 | USD 46,075.66 | – | 4 |
| Kawamoto et al. [25] | – | – | – | PD | Japan | Markov model | 2016 | USD 13,907.69 | USD 6,825.29 | USD 20,732.98 | USD 24,905.05 | 1 |
| Lad et al. [8] | 4,200 | – | 42.6 | PD, D | USA | Simple cost | 2010 | – | – | – | USD 100,041.11 | 3 |
| Li et al. [26] | – | 71.0 | 32 | T | Canada | Cost-utility analysis | 2019 | – | – | USD 31,515.07 | USD 32,738.51 | 1 |
| Mahajan et al. [27] | – | – | – | O | USA | QALY | 2022 | – | – | USD 30,405.43 | USD 32,009.62 | 3 |
| McClelland and Jaboin [28] | – | – | – | T | USA | Cost analysis | 2017 | – | USD 20,695.67 | – | – | 4 |
| McIntosh et al. [29] | 183 | – | – | PD | UK | Cost-utility analysis | 2016 | USD 32,695.29 | USD 25,407.43 | USD 58,102.72 | USD 61,028.98 | 1 |
| Meissner et al. [30] | 46 | 58.6±1.0 | – | PD | Germany | Cost analysis | 2005 | USD 15,460.32 | USD 6,611.82 | USD 22,072.13 | USD 33,469.84 | 1 |
| Moon et al. [31] | – | – | – | OCD | Korea | QALY | 2017 | USD 24,385.06 | USD 8,923.57 | USD 33,308.64 | USD 39,146.58 | 1 |
| Pietzsch et al. [32] | – | 60.5 (50–70) | 36 | PD | USA | Markov model | 2016 | – | – | USD 39,211.15 | – | 1 |
| Rumalla et al. [7] | 3,392 | 64.8±0.4 | 36.82 | PD, T, D | USA | Simple cost | 2018 | – | – | – | USD 24,551.73 | 3 |
| Sharma et al. [33] | 2178 | – | 31.54 | PD | USA | Simple cost | 2013 | – | – | – | USD 78,233.41 | 3 |
| Spottke et al. [34] | 15 | 56.1±8.5 | 66.67 | PD | Germany | Cost-effectiveness analysis | 2002 | USD 26,740.55 | USD 11,771.91 | USD 38,512.46 | USD 66,231.47 | 3 |
| Stroupe et al. [35] | 274 | – | – | PD | USA | Cost-effectiveness analysis | 2014 | – | – | USD 82,215.79 | – | 1 |
| Tomaszewski and Holloway [36] | 193 | 55 (50–70) | – | PD | USA | QALY | 2001 | – | – | USD 68,423.27 | – | 1 |
| Valldeoriola et al. [37] | – | – | – | PD | Spain | Cost analysis | 2007 | – | – | USD 37,635.72 | USD 39,315.49 | 4 |
| Vallderoriola et al. [38] | – | – | – | PD | Spain | Cost analysis | 2013 | – | – | USD 49,884.47 | USD 69,568.28 | 1 |
| Yianni et al. [39] | 26 | – | – | D | UK | QALY | 2005 | $29,280.68 | USD 16,124.94 | USD 45,405.62 | USD 46,394.19 | 3 |
| Youngerman et al. [40] | 30,490 | 64.3±0.2 | 34.7 | D, OCD | USA | Simple cost | 2016 | – | – | – | USD 77,452.77 | 3 |
| Zhu et al. [41] | 13 | 55.5±6.2 | 46.15 | PD | China | Cost-effectiveness analysis | 2014 | USD 27,177.15 | USD 3,885.30 | USD 31,062.46 | USD 36,405.06 | 2 |
PD, Parkinson’s disease; T, essential tremor; D, dystonia; O, obesity.
Table 2.
Detailed included cost breakdown across studies
| Author | Stages, n | Pre-op imaging | Post-op imaging | Battery replacement | Removal surgery | Complications | Intraoperative testing | Rechargeable (1) or non-rechargeable (0) | Device company | Single (1) or dual (0) channel | Single (S) or multi (M)-center | Inflation adjusted – yes (1) or no (0) | Min-max length of follow-up, months | Bilateral (1) or unilateral (0) | Conversion rate provided | Level of inclusivity1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akazawa et al. [18] | Single | 0 | 1 | 1 | 1 | 1 | – | 1 | Medtronic, Boston Scientific, St. Jude Medical | Both | M | 1 | 6–12 | 1 | 1 | HD |
| Dams et al. [19] | Single | 0 | 0 | 1 | 0 | 1 | – | 0 | – | – | S | 1 | 12–240 | 1 | 0 | LD |
| Fann et al. [20] | Single | 0 | 0 | 1 | 0 | 1 | – | 0 | – | – | M | 1 | 36–120 | 1 | 0 | LD |
| Hooper and Whittle [22] | Multiple | -- | -- | 0 | 0 | 1 | – | – | Medtronic | – | S | 0 | 1–12 | – | – | – |
| Gomez-Inhiesto et al. [21] | Single | 1 | 1 | 1 | 0 | 1 | – | 0 | – | – | S | 0 | 60 | – | 1 | HD |
| Igarashi et al. [23] | Single | 1 | 1 | 0 | 0 | 0 | – | – | – | – | M | 1 | 24–48 | 0 | 1 | LD |
| Jacob et al. [24] | Single | 1 | 1 | 0 | 0 | 1 | – | – | – | – | S | 0 | 1 | – | 0 | – |
| Kawamoto et al. [25] | Single | – | – | 1 | 0 | 1 | – | – | – | – | M | 1 | 120 | 1 | 1 | LD |
| Lad et al. [8] | Multiple | – | – | – | – | – | – | – | – | – | M | 0 | – | Both | 0 | ND |
| Li et al. [26] | Multiple | – | – | 1 | 0 | 0 | – | – | Medtronic | – | M | 1 | – | Both | 0 | HD |
| Mahajan et al. [27] | Single | – | – | 0 | 0 | 1 | – | – | – | 0 | M | 1 | 1–60 | – | 0 | LD |
| McClelland and Jaboin [28] | Single | 0 | 0 | 0 | 0 | 0 | – | – | – | – | S | 1 | – | 0 | 0 | – |
| McIntosh et al. [29] | Multiple | 1 | 1 | 1 | 0 | 1 | 1 | 0 | – | – | M | 1 | – | – | 0 | LD |
| Meissner et al. [30] | Single | – | – | 0 | 0 | 1 | – | – | Medtronic | – | M | 1 | 12–24 | 1 | 1 | HD |
| Moon et al. [31] | Single | 1 | 1 | 1 | 0 | 1 | – | 1 | Medtronic | Both | M | 1 | – | – | 1 | HD |
| Pietzsch et al. [32] | Multiple | 1 | 1 | 1 | 1 | 1 | 1 | 0 | Medtronic | – | M | 1 | 6–120 | – | 0 | – |
| Rumalla et al. [7] | Single | 1 | 1 | 0 | 1 | 1 | – | – | – | – | M | 0 | 1–3 | – | 0 | ND |
| Sharma et al. [33] | Multiple | – | – | – | – | – | – | – | – | – | M | 0 | – | 1 | 0 | ND |
| Spottke et al. [34] | Multiple | 1 | -- | – | 0 | 0 | – | – | Medtronic | – | M | 0 | 6–12 | – | 0 | HD |
| Stroupe et al. [35] | Single | 1 | 1 | 1 | 1 | 1 | – | – | – | – | M | 1 | 6–36 | 1 | 0 | – |
| Tomaszewski and Holloway [36] | Multiple | 1 | 1 | 0 | 0 | – | 1 | 0 | Medtronic | 0 | M | 1 | 6–60 | 1 | 1 | – |
| Valldeoriola et al. [37] | Single | 1 | 1 | – | – | 1 | – | – | Medtronic | 0 | S | 0 | 3–12 | 1 | 1 | LD |
| Vallderoriola et al. [38] | Single | 1 | 1 | 1 | – | 1 | – | Both | Medtronic | 0 | M | 1 | 6–60 | 1 | 1 | HD |
| Yianni et al. [39] | Single | 1 | 1 | 1 | – | 1 | 1 | – | Medtronic | – | M | 0 | 2–24 | – | 1 | LD |
| Youngerman et al. [40] | Single | – | – | 1 | 1 | 1 | – | – | – | – | M | 0 | – | – | 1 | ND |
| Zhu et al. [41] | Single | 1 | 1 | 1 | 1 | 1 | 1 | – | – | – | S | 1 | 12–24 | 1 | 1 | HD |
1Level of inclusivity was given only for those that reported total cost of treatment. Highest detail (HD) papers were those that included pre- and post-operative assessments, imaging costs, follow-up costs, drug costs, and costs of complications. Low detail papers only reported some of what was included in the “inclusive” criteria. Papers were defined as not detail (ND) if a breakdown was not given.
Statistical Analysis
Statistical analyses of quantitative measures of DBS costs were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Data are presented as the percentage, mean, and standard deviation of inflation-corrected cost. A two-sample independent t test was used to compare the mean costs between US and non-US studies, as well as PD and non-PD studies. The assumption of unequal variances was tested using Levene’s test. If the assumption of equal variances was violated, the independent t test with the Welch correction was used. A one-way ANOVA was employed to detect any differences in costs across all countries or indications. Similarly, an independent-samples t test (cost of DBS device and surgery) and one-way ANOVA (total cost of treatment) were used to compare studies across their level of inclusivity. A univariate regression model was used to assess the predictive value of year of cost on total cost of surgery and total cost of treatment. The significance level was set at α = 0.05.
Results
Article Characteristics
From 2,464 initial studies, 26 articles [7, 8, 18–41] were included (Fig. 1), spanning years 2001 to 2021. No studies were excluded because of non-English language. Articles were included from 9 different countries: 10 (38.5%) from USA, 3 (11.5%) from UK, 3 (11.5%) from Japan, 3 (11.5%) from Germany, 3 (11.5%) from Spain, 2 (7.7%) from China, 1 (3.8%) from Canada. The risk of bias assessment (RBA), as described in online supplementary Table 1 (for all online suppl. material, see https://doi.org/10.1159/000537865), was used to establish transparency in results and findings. Scores ranged from 1 to 5, with a score of 1 indicating minimal bias and a score of 5 indicating maximal bias. Eleven (42.3%) studies had an RBA of 1, 2 (7.7%) studies had an RBA of 2, 8 (30.8%) studies had an RBA of 3, and 5 (19.2%) studies had an RBA of 4.
Among the 26 papers, six studies (23.1%) performed simple cost reporting, 5 (19.2%) performed a cost analysis, four (15.4%) performed a quality-adjusted life year assessment, four (15.4%) performed a cost-effectiveness analysis, three (11.5%) studies used a Markov simulation, two (7.7%) performed a cost-utility analysis, and two (7.7%) performed a cost-minimization analysis.
The median number of patients across studies was 193 patients (IQR 1st–3rd: 36–1,348.5). The mean reported age of study participants was 60.5 ± 5.6 years. The median female prevalence was 38.9% across all studies.
Electrode Characteristics
Electrode manufacturer information was included in only 11 (42.3%) studies. Of those studies, all included Medtronic electrodes. One study also reported using Boston Scientific and St. Jude (now Abbott Laboratories) electrodes. A total of 9 (34.6%) studies reported information regarding whether rechargeable or non-rechargeable implantable pulse generators (IPGs) were used. Two (7.7%) studies used rechargeable IPGs, 6 (23.1%) used non-rechargeable IPGs, 1 (3.8%) study used both rechargeable and non-rechargeable IPGs, while 17 (65.4%) studies did not specify. None of the studies used only single-channel systems, 4 (15.4%) studies used dual-channel systems, 2 (7.7%) studies used both single- and dual-channel systems, and 20 (76.9%) studies did not specify whether single- or dual-channel systems were used. Two (7.7%) studies performed only unilateral DBS implantation, 11 (42.3%) studies performed only bilateral, 2 (7.7%) studies included both, and 11 (42.3%) did not report whether a unilateral or bilateral procedure was performed.
DBS Surgery and Treatment Costs
DBS-related costs were extracted from all 26 included articles. Seven studies (26.9%) were single center, while 19 (73.1%) studies were multi-center. Nineteen (73.1%) studies included DBS for patients with Parkinson’s disease, five (19.2%) for tremor, three (11.5%) for dystonia, two (7.7%) for OCD, and one (3.8%) study for obesity. Multiple studies included more than one indication for DBS (Table 1). Pre-operative and post-operative imaging was included in cost by 14 studies (53.8%), battery replacement costs were included by 14 studies (53.8%), and costs of revision surgery were included by six studies (23.1%).
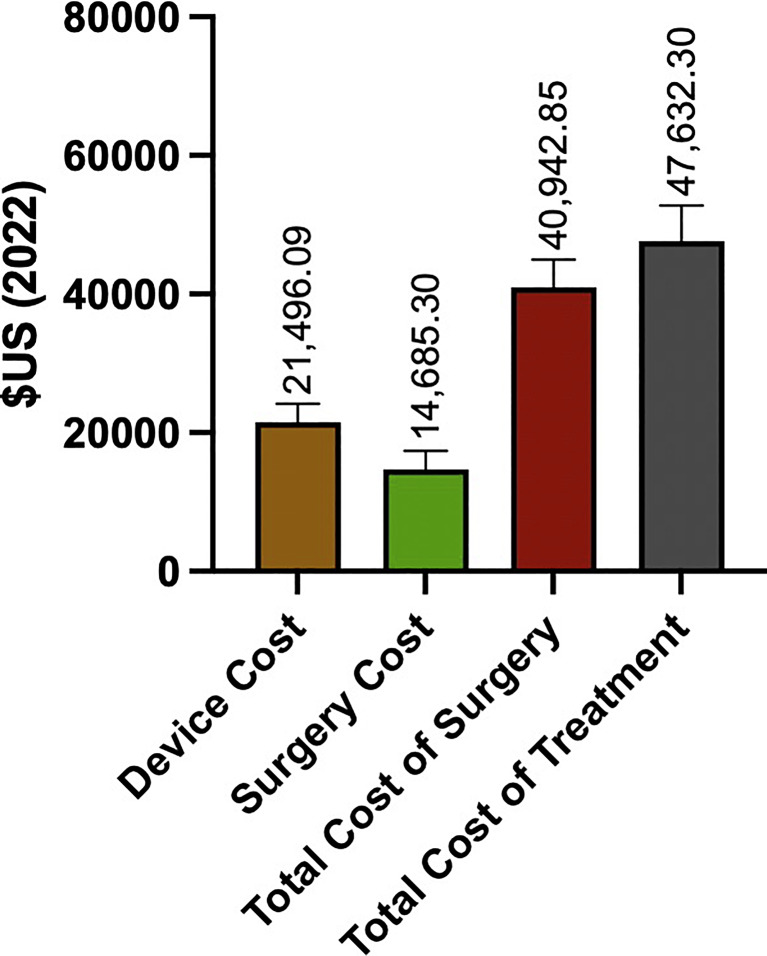
Costs associated with complications were included by 19 studies (73.1%), and five studies (19.2%) included costs of intraoperative testing. One (3.8%) study specified that the procedure was performed under general anesthesia, 1 (3.8%) study reported that it was performed under local anesthesia, 3 (11.5%) studies used both local and general anesthesia, and 21 (80.8%) studies did not specify whether the procedure was performed under local or general anesthesia. The inflation- and currency-adjusted mean cost of the DBS device was USD 21,496.07 ± USD 8,944.16, the cost of surgery alone was USD 14,685.22 ± USD 8,479.66, the total cost of surgery was USD 40,942.85 ± USD 17,987.43, and the total cost of treatment until 1 year of follow-up was USD 47,632.27 ± USD 23,067.08 (Fig. 2).
Fig. 2.
Cost components of DBS surgery. Cost of DBS device, surgery, total cost of surgery, and total cost of treatment.
Cost of Surgery and Treatment by Indication
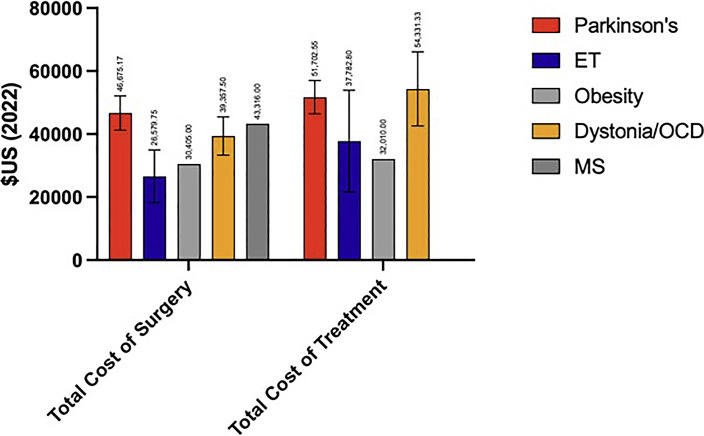
For those studies that reported PD-indicated costs (n = 14), the mean total cost of surgery was USD 43,729.38 ± USD 20,422.43 and the mean total cost of treatment was USD 49,993.18 ± USD 24,947.23. For non-PD-indicated costs, the mean total cost of surgery was USD 34,440.96 ± USD 8,521.37 and the mean total cost of treatment was USD 42,123.50 ± USD 18,755.61. The mean total cost of surgery (t = 1.062; p = 0.302) and total cost of treatment (t = 0.689, p = 0.499) were similar between PD and non-PD indications. The total cost of surgery [F(6, 19) = 0.648, p = 0.691] and total cost of treatment [F(6, 19) = 0.581, p = 0.739] were similar across all indications. Mean costs by indication are provided in Figure 3.
Fig. 3.
Total cost of surgery and treatment by indication. ET, essential tremor; OCD, obsessive-compulsive disorder; MS, multiple sclerosis.
Cost of Surgery and Treatment by Country
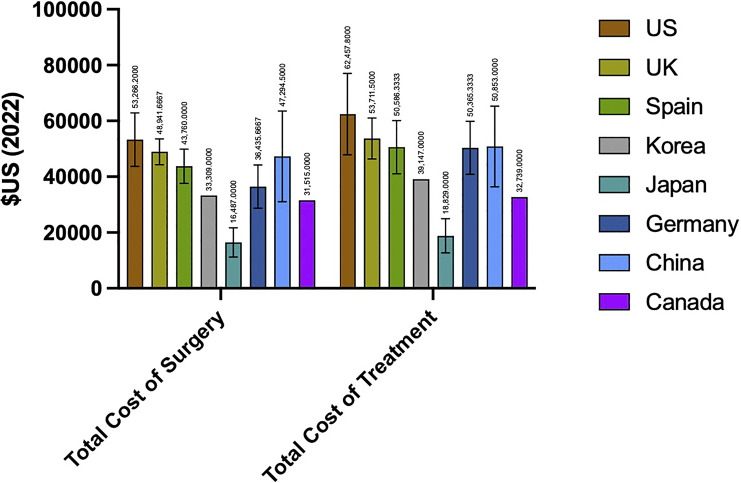
The mean cost of DBS device and surgery for US studies was USD 53,266.26 ± USD 21,455.74, while DBS device and surgery outside the USA had a mean cost of USD 36,835.05 ± USD 15,342.02. Mean cost of total treatment for US studies was USD 62,457.73 ± USD 32,569.73, while mean cost of non-US studies was USD 42,690.46 ± USD 17,716.48. The mean total cost of surgery (t = 0.236; p = 0.076) and total cost of treatment (t = 1.747; p = 0.098) were similar between US and non-US studies. Mean total cost of surgery [F(7, 19) = 1.688, p = 0.203] and mean total cost of treatment [F(7, 19) = 1.134, p = 0.404] were also similar across all countries. Mean costs by country are provided in Figure 4.
Fig. 4.
Total cost of surgery and treatment by country.
Cost of Surgery and Treatment by Year
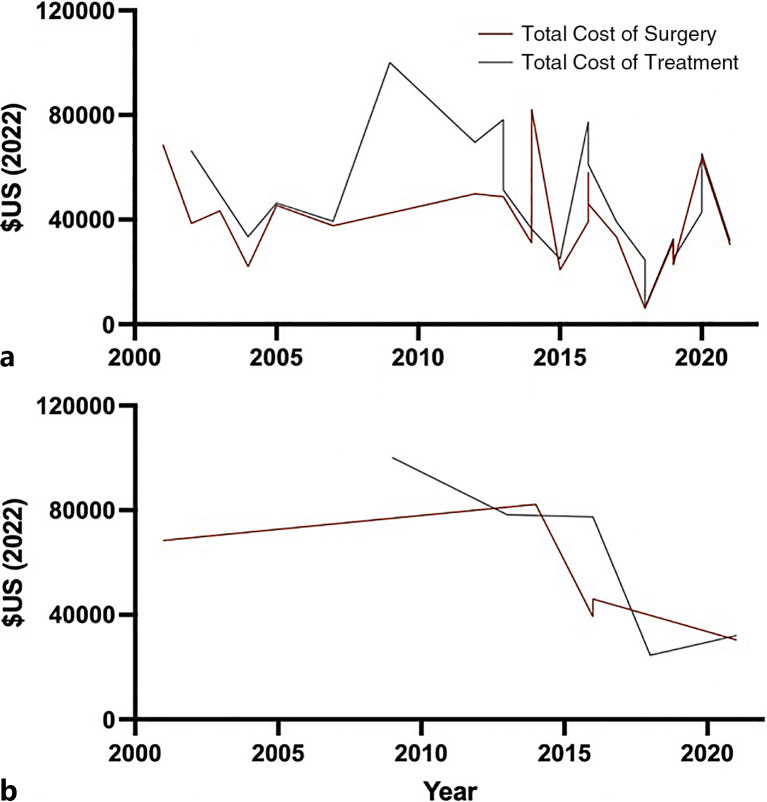
Across all studies, there was no association between year and total cost of surgery (β = −0.192, p = 0.418) or total cost of treatment (β = −0.314, p = 0.177) on univariate regression (Table 3). For US studies, the year of cost was not associated with the total cost of surgery (β = −0.621, p = 0.264), but was significantly associated with the cost of total treatment (β = −0.895, p = 0.040), such that the total cost of treatment decreased over time (Table 3). A representative graph of cost trend over time is provided in Figure 5.
Table 3.
Univariable linear regression predicting costs by country
| Adjusted R2 | B | SE | β | t | p value | |
|---|---|---|---|---|---|---|
| Total cost of surgery – all countries | ||||||
| Year | −0.017 | −531.16 | 640.11 | −0.192 | −0.830 | 0.418 |
| Total cost of surgery – USA | ||||||
| Year | 0.385 | −1,774.24 | 1,293.72 | −0.621 | −1.371 | 0.264 |
| Total cost of treatment – all countries | ||||||
| Year | 0.049 | −1,262.83 | 899.39 | −0.314 | −1.404 | 0.177 |
| Total cost of treatment – USA | ||||||
| Year | 0.734 | −6,319.99 | 1,820.49 | −0.895 | −3.472 | 0.040 |
Fig. 5.
Total cost of surgery and treatment by year. a Total cost of surgery and treatment for all studies. b Total cost of surgery and treatment for US studies.
Cost of Surgery and Treatment by Level of Inclusion
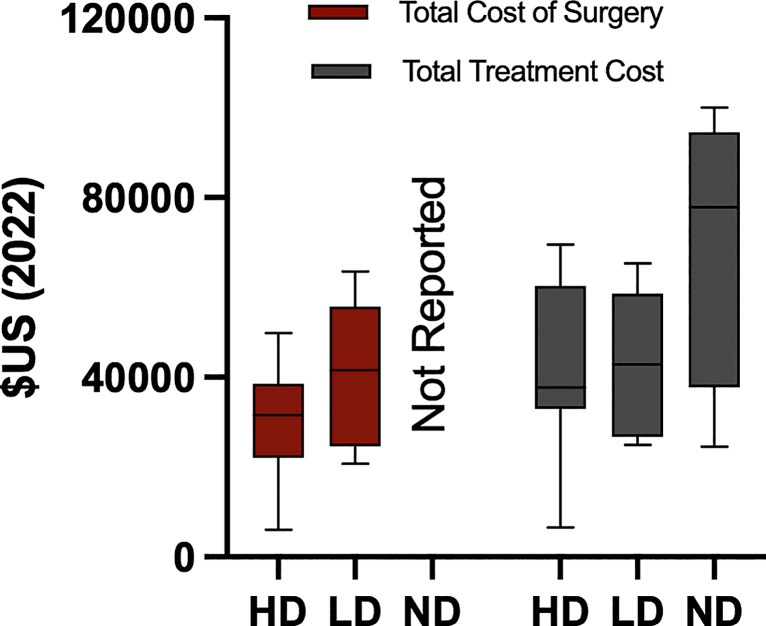
Sources of cost varied and are reported in online supplementary Table 2. Studies that reported total cost (n = 20) were grouped based on their extent of detailed data into highest detail (HD) (n = 8, 40.0%), low detail (LD) (n = 8, 40.0%), and no detail (ND) (n = 4, 20.0%). The mean cost of the device and surgery was USD 30,341.12 ± USD 13,654.66 for the HD group and USD 40,903.47 ± USD 15,803.78 for the LD group (t = −1.374, p = 0.193) (Fig. 6). No studies in the ND group included the cost of the device and surgery. The total cost of treatment in the HD group was USD 40,877.27 ± USD 19,965.07, in the LD group was USD 43,168.54 ± USD 15,537.75, and in the ND group was USD 70,069.76 ± USD 32,100.50 (Fig. 6). There were no differences in the total cost of treatment [F(2, 19) = 2.849, p = 0.086] across studies based on their level of inclusivity (Fig. 6).
Fig. 6.
Total cost of surgery and treatment by level of detailed data. HD, highest detail; LD, low detail; ND, no detail.
Discussion
This report includes an in-depth analysis detailing the costs associated with DBS for treatment of Parkinson’s disease, ET, dystonia, general movement disorders, obesity, and OCD based on the current published literature. The inflation- and currency-adjusted median cost of total DBS treatment worldwide was USD 47,632.27 with a staggering price range from USD 6,582.67 (Japan) to USD 100,041.1 (USA). This reported price variability can be attributed to a variety of factors including different cost-reporting frameworks, inclusivity of reporting, device cost, the country and healthcare system within which DBS is performed, and insurance coverage. Though non-significant, large variations in reported total cost of treatment exist across surgical indications for DBS. Understanding current cost-reporting methods for DBS is a first step toward creating a standardized framework for reporting cost, with focus on elements that are most variable and subject to change, in an effort to make the procedure more cost-effective and widely accessible to more patients. As the field currently stands regarding cost reporting, there are several major hurdles, which must be overcome to facilitate productive discourse.
Variability across Countries
DBS cost data from a total of nine countries were included in this analysis. The diversity of countries included provides a broad perspective on the cost of DBS globally as can be seen in Figure 4. Although DBS cost was not statistically different across countries, there was notable international variability in cost, with Japan and Canada displaying the lowest costs and the USA demonstrating the highest. Economic disparities between countries impact the patient population capable of accessing and undergoing DBS surgery. Countries with higher per capita income tend to have higher costs for medical procedures, and, conversely, countries with lower per capita income may have lower costs due to differences in labor and overhead expenses. This is seen in the dramatic differences in cost of medications and medical devices across countries [42]. Additionally, there exists great variability centered around differences within the healthcare system itself such as models of billing, insurance reimbursement, administrative costs, physician compensation, and available facilities [43]. The USA has a reported median DBS total cost of treatment USD 19,767.27 greater than other countries, a discrepancy that aligns with the well-documented pattern of higher healthcare costs in the USA. Nevertheless, this observed variation accentuates an important area for improvement in removing inefficiencies and exploring avenues for cost reduction of DBS, especially in the USA.
The majority of the included studies originated in the USA, which corroborates the literature on the nation with the highest DBS publication frequency [44]. Factors for this could be several fold. First, the USA performs a large share of the global DBS surgeries, which may lend itself increased publication frequency [4]. The apparent bias for research from the USA could also stem from higher publication rates in the USA compared to other countries where DBS is also commonly performed [45]. The median number of patients across studies was 193, with a mean reported age of 60.5 years and 38.9% female makeup, which is comparable to the typical US demographic treated with DBS [46]. The demographic factors of DBS patients are important determinants in the overall cost-effectiveness of DBS. Policy-makers, healthcare systems, and health insurance agencies put great emphasis on the cost-effectiveness of DBS given the anticipated accessibility and long-term viability of the procedure [47, 48].
Variability in Total Cost Reporting
The majority of studies (80%) had an RBA score equal to or less than 3, indicating that these studies were either single- or multi-center studies that included a cost breakdown, and were adjusted for inflation. Despite the variety of economic evaluations used, the current analysis solely included cost data reported by these studies. Unfortunately, the quality of cost data was dependent on collection and reporting methods, with some studies performing a top-down approach (estimating costs based on national averages), while others use a bottom-up approach (calculating costs by combining costs of individual components of care, such as medication, staff time, and diagnostic tests). Importantly, many studies utilized costs directly obtained from their own institution, allowing for a more accurate and localized cost estimate, as opposed to estimating by national averages, which may inflate the reported cost variability. Studies showed large discrepancies of reported expenditures with inclusion of pre-operative and post-operative imaging in 53.8% of studies, post-operative maintenance/programming in 73.1% of studies, battery replacement in 53.8% of studies, and costs of revision surgery in 23.1% of studies. The lack of specified cost breakdowns was a large barrier in our ability to compare costs directly and points to the importance of a more standardized method of reporting costs. The current cost-reporting frameworks are non-standardized, which lends to great variation in reported numbers even within respective cost categories. This was particularly salient when assessing surgical costs. A potential cost-reporting framework is provided in Table 4.
Table 4.
Proposed cost-reporting framework
| Cost of device | DBS device | Hardware cost of the DBS device itself |
| Lead wires | Cost of the electrodes that connect the DBS device to the brain | |
| Battery replacement | Cost of replacement batteries for the DBS device (if applicable) | |
| Cost of surgery | Pre-operative assessment | Neurological and medical evaluations before surgery. Imaging studies (MRI, CT scans) |
| Surgical team fees | Surgeon, anesthesia, or neurologist fees | |
| Hospitalization costs | Room charges, nursing care, operating room charges, intensive care unit (ICU) charges (if applicable) | |
| Medical supplies | Surgical instruments and supplies | |
| Medications | Cost of medications administered during and after surgery | |
| Post-operative imaging | Follow-up imaging studies to assess the placement and effectiveness of the DBS device | |
| Rehabilitation therapy | Physical therapy or occupational therapy if required | |
| Complications | Additional costs associated with managing potential complications, emergency room visits | |
| Cost of treatment | Programming system | Cost of the equipment and software used for programming and adjusting the DBS settings |
| Medications | Cost of medications prescribed for PD or ET management, adjustments to medication dosages | |
| Follow-up appointments | Neurologist visits, programming sessions for DBS adjustments | |
| Imaging studies | Follow-up imaging studies to monitor the progression of the disease and assess the DBS device | |
| Physical therapy/occupational therapy | Continued rehabilitation therapy sessions | |
| Complications management | Costs associated with managing any complications that arise post-surgery | |
| Psychological support | Counseling or psychological support if required for the patient and their family |
Contributing Factors of Outliers
In scrutinizing the aggregate treatment costs documented in various papers, a discernible pattern emerges, with the majority converging closely around the median. Noteworthy outliers, namely, Lad et al. [8], Sharma et al. [33], and Youngerman et al. [40], report conspicuously elevated costs, all drawing on data from the HCUP-NIS database. This clustering suggests a potential bias inherent within this data source, which may include more comprehensive costs that are omitted in other studies. It is important to acknowledge that the data acquisition for these three studies predates the 2012 NIS redesign, which worked to make prices lower, potentially driving the observed escalation in costs within the USA. Conversely, Akazawa et al. [18] report the most modest cost, a metric significantly shaped by the presence of universal health coverage in Japan. Kawamoto et al. [25], also situated in Japan, report a relatively low cost, albeit not as pronounced. The variance between these studies may be attributed to the cost of the device, with Kawamoto et al. [25] reporting a device cost more than that of Akazawa et al. [18] by USD 11,320.52. Despite these intricacies, Akazawa et al. [18] provides substantive insights into the overarching variability of DBS costs based on country and serves as a potential model to optimize cost efficiency for DBS.
Bundling Procedures and Cost Reporting
One important consideration for the cost of DBS is that multiple stages of the procedure, including pre-operative evaluation, surgery, post-operative monitoring, and programming, are often bundled together into one cost in terms of hospital billing. Bundling services may obscure the true costs of individual stages of the DBS procedure due to cost averaging and a lack of cost evaluation and reporting [49], making it difficult to identify areas where cost reductions could be made or opportunities to standardize cost reporting and make bundling more efficient [50]. Bundling has the potential to create a conflict of interest for healthcare administrators, who may prioritize cost savings over optimal care [51]. However, cost bundling can also yield a more predictable cost schema for the patient as they are offered one fixed price for the entire procedure and related care [52]. Bundling may also incentivize healthcare administrators to be more efficient and coordinated [51]. From a cost analysis perspective, it may simplify comparisons across administrators or hospitals as they are using a standardized pricing model. However, many studies included in our report used de-bundled costs and did not have a standardized method of reporting these costs. It is important to note that the studies included in our report did not compare the cost of DBS to the reimbursement received; therefore, an assessment of the true profitability of DBS is difficult to ascertain. However, DBS was deemed cost-effective in comparison to best medical therapies, supporting its value from a patient-centered cost analysis perspective [53, 54].
Improving the Cost-Effectiveness of DBS
Avansino et al. [55] previously reported that variations in procedure-related cost exist based on variable operative equipment and materials, with standardization of this equipment resulting in significant cost reductions without impacting quality or delivery of care. Interestingly, the current study exhibits homogeneity in DBS devices used, as all studies utilized Medtronic electrodes, though the cost of the device still varied between studies. This trend was observed both between and within countries, indicating a lack of consistency in market value of the technology or transparency in negotiated prices. Furthermore, intraoperative differences in cost were difficult to identify due to partial lack of itemized reporting, though factors such as imaging, anesthesia, and testing all likely contributed to variations in cost and are all areas of target to improve the cost-effectiveness of DBS.
Beyond the operating room, other systemic inefficiencies could also be targeted to improve cost effectiveness, ultimately benefiting patients. Analysis of follow-up length showed great heterogeneity between studies, with the follow-up interval extending from as short as 1 month to 20 years. As many of the studies used projections to estimate follow-up costs, these projected follow-up costs were omitted from our analysis and only charges procured from billing records were included. Nevertheless, understanding the long-term economic burden of DBS is imperative and should continue to be pursued in future studies. Since optimal electrode settings may be found after several iterations of trials and often require adjustments [56], many clinic visits may be necessary, each with additional cost. Streamlined programming could eliminate redundant visits and decrease overall costs associated with post-operative DBS care, resulting in a more patient-centered approach. Importantly, more standardized cost reporting will lend itself to accurate interpretation of the impact of certain variables such as device programing and length of follow-up, in order to more accurately determine methods of cost improvement. Furthermore, decisions regarding resource allocation could be informed by a better understanding of individual components of multidisciplinary DBS care. Standardized cost reporting can additionally pave the way toward greater transparency and shed light on broader systemic areas for improvement in the interest of patient-centered care. With improved cost reporting and analysis, hospitals will gain a more comprehensive perspective of expenses related to DBS, allowing them to better negotiate reimbursement rates. Insurance companies will be able to track costs more directly and could reimburse cost more accurately for procedures. Through identification and elimination of inefficiencies, DBS-related costs can be further modified with the goal of maximizing procedure reimbursement for hospital systems and minimizing patient-facing costs. As cost reporting is standardized, data can be more easily compared across organizations, offering the possibility for broader systemic areas of improvement.
Limitations
There are several limitations to our study. First, despite converting currency and controlling for inflation, the cost of medical treatment has changed significantly over the last two decades. As a result, comparing older studies to newer ones poses an inevitable challenge in that inflation over time may not be properly estimated, potentially falsely elevating cost variability. Second, though our study observed a decrease in total cost of treatment in the USA over time, these results should be interpreted cautiously as high initial costs may likely be due to data collection sources presenting inflated costs. Importantly, extensive variation in currency conversion rate exists over time. Though attempts to mitigate these discrepancies were implemented, it is possible that fluctuations in the global market limited the accuracy of the data. Conversion rates provided in individual studies were used whenever available. When absent, the month(s) of data collection reported in the studies were the basis for currency conversion. Even with these precautions, it is likely that some variation in currency exchange rates could not be fully accounted for. Another limitation was the difficulty in identifying the specific domains or subcategories primarily responsible for this difference, due to the manner in which the data were reported. Similarly, some costs were not reported directly from the institution. Consequently, some studies likely included factors in their overall expenditure that were not accounted for by other researchers. Without a detailed cost analysis, it can be difficult to identify what exactly comprised each set of costs. This was further confounded by the variability in follow-up periods and associated costs. Ideally, the cost of a procedure should be calculated over the course of a lifetime; however, due to the limited research regarding long-term costs of DBS, efforts were focused on the first year of treatment. Finally, variations in cost due to the battery type (rechargeable vs. non-rechargeable) are invariably present. However, only two studies confirmed using rechargeable batteries, resulting in a sample size too small to draw significant conclusions. As reporting becomes more standardized, future studies should investigate cost differences arising from the specific battery used.
Future Direction
Not only does this work highlight the large variation in reported DBS cost but also the inconsistent manner in which the many factors contributing to DBS cost are reported. Future efforts should be applied to standardize the reporting of cost for DBS procedures and supplies, with subcategories of cost reporting as suggested in the framework described (Table 4). This groundwork would allow more efficacious analysis of quality-of-life improvement controlled for standardized cost. Due to the limited quantity of data from individual countries, we were unable to provide a more granular analysis of DBS costs by country. With an expanded data set, work could be done to examine discrepancies of cost between more nations than were included in this analysis. While we focused primarily on the overall cost of DBS, we reported no information on reimbursement. Considering that reimbursement is the primary determining driver of cost-effectiveness decision-making, more attention should be directed to this aspect of the financial model. Reimbursement likely varies based on the indication for DBS such as PD, tremor, dystonia, and psychiatric disorders. More effort could be directed toward comparing the cost-effectiveness of DBS and its reimbursements for these different conditions.
Conclusion
Our findings highlight the substantial variation in costs associated with DBS, as well as the diverse ways in which these costs are reported. With such large differences in reporting, it is difficult to compare the true expenses of DBS as it fluctuates both geographically and chronologically. This lack of transparency must be demystified to make effective comparison of DBS cost more attainable. By reporting various cost elements and employing effective cost-reporting frameworks, healthcare providers can gain a better understanding of the specific cost factors involved in DBS treatment in an effort to optimize national DBS accessibility and cost transparency. DBS studies have sought to make cost comparative studies with heterogenous reporting and results. While the results demonstrate a general range of costs for key procedure and hospital stay elements, future research is needed to elucidate how to consider and configure costing in a uniform fashion.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Conception and design: A.E.B., S.W.K., D.P., C.L., R.J.D., and L.B.C. Acquisition of data, analysis and interpretation of data, and drafting the article: A.E.B., A.T.L., and S.W.K.
Critically revising the article: A.E.B., A.T.L., S.W.K., D.P., T.J.B., S.K.B., D.J.E., and L.B.C. Statistical analysis: A.E.B. Administrative/technical/material support: D.P., C.L., R.J.D., M.J.F., T.J.B., S.K.B., D.J.E., and L.B.C. Study supervision: L.B.C. All authors approved the final manuscript.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Pycroft L, Stein J, Aziz T. Deep brain stimulation: an overview of history, methods, and future developments. Brain Neurosci Adv. 2018;2:2398212818816017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50(1–6):344–6. [DOI] [PubMed] [Google Scholar]
- 3. Groiss SJ, Wojtecki L, Südmeyer M, Schnitzler A. Deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord. 2009;2(6):20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez-Oroz MC, Zamarbide I, Guridi J, Palmero MR, Obeso JA. Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson’s disease 4 years after surgery: double blind and open label evaluation. J Neurol Neurosurg Psychiatry. 2004;75(10):1382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dang TTH, Rowell D, Connelly LB. Cost-effectiveness of deep brain stimulation with movement disorders: a systematic review. Mov Disord Clin Pract. 2019;6(5):348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rumalla K, Smith KA, Follett KA, Nazzaro JM, Arnold PM. Rates, causes, risk factors, and outcomes of readmission following deep brain stimulation for movement disorders: analysis of the U.S. Nationwide Readmissions Database. Clin Neurol Neurosurg. 2018;171:129–34. [DOI] [PubMed] [Google Scholar]
- 8. Lad SP, Kalanithi PS, Patil CG, Itthimathin P, Batya S, Bronte-Stewart H, et al. Socioeconomic trends in deep brain stimulation (DBS) surgery. Neuromodulation. 2010;13(3):182–6. [DOI] [PubMed] [Google Scholar]
- 9. Kusyk DM, Costa G, Schirmer CM, Whiting AC, Rosenow JM. Cross-sectional analysis of US health insurance payer policies for humanitarian device exemption indications for deep brain stimulation. Stereotact Funct Neurosurg. 2022;100(4):244–7. [DOI] [PubMed] [Google Scholar]
- 10. Davis RA, Giordano J, Hufford DB, Sheth SA, Warnke P, Widge AS, et al. Restriction of access to deep brain stimulation for refractory OCD: failure to apply the federal parity act. Front Psychiatry. 2021;12:706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Willis AW, Schootman M, Kung N, Wang X-Y, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. 2014;82(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crispo JAG, Lam M, Le B, Richard L, Shariff SZ, Ansell DR, et al. Disparities in deep brain stimulation use for Parkinson’s disease in ontario, Canada. Can J Neurol Sci. 2020;47(5):642–55. [DOI] [PubMed] [Google Scholar]
- 13. Skelton HM, Grogan DP, Laxpati NG, Miocinovic S, Gross RE, Yong NA. Identifying the sources of racial disparity in the treatment of Parkinson’s disease with deep brain stimulation. Neurosurgery. 2023;92(6):1163–1170. [DOI] [PubMed] [Google Scholar]
- 14. Cramer SW, Do TH, Palzer EF, Naik A, Rice AL, Novy SG, et al. Persistent racial disparities in deep brain stimulation for Parkinson’s disease. Ann Neurol. 2022;92(2):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. “Currency converter | foreign exchange rates | OANDA.” [Online]. Available from: https://www.oanda.com/currency-converter/en/ [accessed April 5, 2023].
- 17. “Producer Price Index by Industry: General Medical and Surgical Hospitals (PCU622110622110) | FRED | St. Louis Fed.” [Online]. Available from: https://fred.stlouisfed.org/series/PCU622110622110 [accessed April 5, 2023].
- 18. Akazawa M, Konomura K, Shiroiwa T. Cost-minimization analysis of deep-brain stimulation using national database of Japanese health insurance claims. Neuromodulation. 2018;21(6):548–52. [DOI] [PubMed] [Google Scholar]
- 19. Dams J, Siebert U, Bornschein B, Volkmann J, Deuschl G, Oertel WH, et al. Cost-effectiveness of deep brain stimulation in patients with Parkinson’s disease. Mov Disord. 2013;28(6):763–71. [DOI] [PubMed] [Google Scholar]
- 20. Fann JC-Y, Chang KC, Yen AMF, Chen SLS, Chiu SYH, Chen HH, et al. Cost-effectiveness analysis of deep brain stimulation for Parkinson disease in Taiwan. World Neurosurg. 2020;138:e459–68. [DOI] [PubMed] [Google Scholar]
- 21. Gomez-Inhiesto E, Acaiturri-Ayesta MT, Ustarroz-Aguirre I, Camahuali D, Urtaran-Laresgoiti M, Basabe-Aldecoa M, et al. Direct cost of Parkinson’s disease: a real-world data study of second-line therapies. Parkinsons Dis. 2020;2020:9106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hooper J, Whittle IR. Costs of thalamic deep brain stimulation for movement disorders in patients with multiple sclerosis. Br J Neurosurg. 2003;17(1):40–5. [PubMed] [Google Scholar]
- 23. Igarashi A, Tanaka M, Abe K, Richard L, Peirce V, Yamada K. Cost-minimisation model of magnetic resonance-guided focussed ultrasound therapy compared to unilateral deep brain stimulation for essential tremor treatment in Japan. PLoS One. 2019;14(7):e0219929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacob RL, Geddes J, McCartney S, Burchiel KJ. Cost analysis of awake versus asleep deep brain stimulation: a single academic health center experience. JNS. 2016;124(5):1517–23. [DOI] [PubMed] [Google Scholar]
- 25. Kawamoto Y, Mouri M, Taira T, Iseki H, Masamune K. Cost-effectiveness analysis of deep brain stimulation in patients with Parkinsonʼs disease in Japan. World Neurosurg. 2016;89:628–35.e1. [DOI] [PubMed] [Google Scholar]
- 26. Li C, Gajic-Veljanoski O, Schaink AK, Higgins C, Fasano A, Sikich N, et al. Cost-effectiveness of magnetic resonance-guided focused ultrasound for essential tremor. Mov Disord. 2019;34(5):735–43. [DOI] [PubMed] [Google Scholar]
- 27. Mahajan UV, Ojukwu DI, Azagury DE, Safer DL, Cunningham T, Halpern CH. Can responsive deep brain stimulation be a cost-effective treatment for severe obesity? Obesity. 2022;30(2):338–46. [DOI] [PubMed] [Google Scholar]
- 28. McClelland S, Jaboin JJ. Treatment of the ventral intermediate nucleus for medically refractory tremor: a cost-analysis of stereotactic radiosurgery versus deep brain stimulation. Radiother Oncol. 2017;125(1):136–9. [DOI] [PubMed] [Google Scholar]
- 29. McIntosh E, Gray A, Daniels J, Gill S, Ives N, Jenkinson C, et al. Cost-utility analysis of deep brain stimulation surgery plus best medical therapy versus best medical therapy in patients with Parkinson’s: economic evaluation alongside the PD SURG trial. Mov Disord. 2016;31(8):1173–82. [DOI] [PubMed] [Google Scholar]
- 30. Meissner W, Schreiter D, Volkmann J, Trottenberg T, Schneider GH, Sturm V, et al. Deep brain stimulation in late stage Parkinson’s disease: a retrospective cost analysis in Germany. J Neurol. 2005;252(2):218–23. [DOI] [PubMed] [Google Scholar]
- 31. Moon W, Kim SN, Park S, Paek SH, Kwon JS. The cost-effectiveness of deep brain stimulation for patients with treatment-resistant obsessive-compulsive disorder. Medicine. 2017;96(27):e7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pietzsch JB, Garner AM, Marks WJ. Cost-effectiveness of deep brain stimulation for advanced Parkinson’s disease in the United States. Neuromodulation. 2016;19(7):689–97. [DOI] [PubMed] [Google Scholar]
- 33. Sharma M, Ambekar S, Guthikonda B, Wilden J, Nanda A. Regional trends and the impact of various patient and hospital factors on outcomes and costs of hospitalization between academic and nonacademic centers after deep brain stimulation surgery for Parkinson’s disease: a United States Nationwide Inpatient Sample analysis from 2006 to 2010. Neurosurg Focus. 2013;35(5):E2. [DOI] [PubMed] [Google Scholar]
- 34. Spottke EA, Volkmann J, Lorenz D, Krack P, Smala AM, Sturm V, et al. Evaluation of healthcare utilization and health status of patients with Parkinson’s disease treated with deep brain stimulation of the subthalamic nucleus. J Neurol. 2002;249(6):759–66. [DOI] [PubMed] [Google Scholar]
- 35. Stroupe KT, Weaver FM, Cao L, Ippolito D, Barton BR, Burnett-Zeigler IE, et al. Cost of deep brain stimulation for the treatment of Parkinson’s disease by surgical stimulation sites. Mov Disord. 2014;29(13):1666–74. [DOI] [PubMed] [Google Scholar]
- 36. Tomaszewski KJ, Holloway RG. Deep brain stimulation in the treatment of Parkinson’s disease: a cost-effectiveness analysis. Neurology. 2001;57(4):663–71. [DOI] [PubMed] [Google Scholar]
- 37. Valldeoriola F, Morsi O, Tolosa E, Rumià J, Martí MJ, Martínez-Martín P. Prospective comparative study on cost-effectiveness of subthalamic stimulation and best medical treatment in advanced Parkinson’s disease. Mov Disord. 2007;22(15):2183–91. [DOI] [PubMed] [Google Scholar]
- 38. Valldeoriola F, Puig-Junoy J, Puig-Peiró R; Workgroup of the SCOPE study . Cost analysis of the treatments for patients with advanced Parkinson’s disease: SCOPE study. J Med Econ. 2013;16(2):191–201. [DOI] [PubMed] [Google Scholar]
- 39. Yianni J, Green AL, McIntosh E, Bittar RG, Joint C, Scott R, et al. The costs and benefits of deep brain stimulation surgery for patients with dystonia: an initial exploration. Neuromodulation. 2005;8(3):155–61. [DOI] [PubMed] [Google Scholar]
- 40. Youngerman BE, Chan AK, Mikell CB, McKhann GM, Sheth SA. A decade of emerging indications: deep brain stimulation in the United States. J Neurosurg. 2016;125(2):461–71. [DOI] [PubMed] [Google Scholar]
- 41. Zhu XL, Chan DTM, Lau CKY, Poon WS, Mok VCT, Chan AYY, et al. Cost-effectiveness of subthalmic nucleus deep brain stimulation for the treatment of advanced Parkinson disease in Hong Kong: a prospective study. World Neurosurg. 2014;82(6):987–93. [DOI] [PubMed] [Google Scholar]
- 42. Baicker K, Chandra A. Challenges in understanding differences in health care spending between the United States and other high-income countries. JAMA. 2018;319(10):986–7. [DOI] [PubMed] [Google Scholar]
- 43. Cutler DM, Ly DP. The (paper) work of medicine: understanding international medical costs. J Econ Perspect. 2011;25(2):3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harmsen IE, Wolff Fernandes F, Krauss JK, Lozano AM. Where are we with deep brain stimulation? A review of scientific publications and ongoing research. Stereotact Funct Neurosurg. 2022;100(3):184–97. [DOI] [PubMed] [Google Scholar]
- 45. Koester S, Bishay AE, Batista S, Bertani R, Naik A, Haizel-Cobbina J, et al. The current state of global contribution to open access publishing in neurosurgery: a bibliometric analysis. Brain Spine. 2023;3:101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hariz G-M, Nakajima T, Limousin P, Foltynie T, Zrinzo L, Jahanshahi M, et al. Gender distribution of patients with Parkinson’s disease treated with subthalamic deep brain stimulation; a review of the 2000–2009 literature. Parkinsonism Relat Disord. 2011;17(3):146–9. [DOI] [PubMed] [Google Scholar]
- 47. “NCA: deep brain stimulation for Parkinson’s disease (CAG-00124N) – decision memo.” [Online]. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=21 [accessed April 5, 2023].
- 48. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed.Oxford University Press; 2015. [Google Scholar]
- 49. Stead SW, Merrick SK. Bundled payments and hidden costs. Anesthesiol Clin. 2018;36(2):241–58. [DOI] [PubMed] [Google Scholar]
- 50. Shih T, Chen LM, Nallamothu BK. Will bundled payments change health care?: examining the evidence thus far in cardiovascular care. Circulation. 2015;131(24):2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Antonova E, Boye ME, Sen N, O’Sullivan AK, Burge R. Can bundled payment improve quality and efficiency of care for patients with hip fractures? J Aging Soc Policy. 2015;27(1):1–20. [DOI] [PubMed] [Google Scholar]
- 52. Yee CA, Pizer SD, Frakt A. Medicare’s bundled payment initiatives for hospital-initiated episodes: evidence and evolution. Milbank Q. 2020;98(3):908–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guo X, Feng C, Pu J, Jiang H, Zhu Z, Zheng Z, et al. Deep brain stimulation for advanced Parkinson disease in developing countries: a cost-effectiveness study from China. Neurosurgery. 2023;92(4):812–9. [DOI] [PubMed] [Google Scholar]
- 54. Ooms P, Blankers M, Figee M, Bergfeld IO, van den Munckhof P, Schuurman PR, et al. Cost-effectiveness of deep brain stimulation versus treatment as usual for obsessive-compulsive disorder. Brain Stimul. 2017;10(4):836–42. [DOI] [PubMed] [Google Scholar]
- 55. Avansino JR, Goldin AB, Risley R, Waldhausen JHT, Sawin RS. Standardization of operative equipment reduces cost. J Pediatr Surg. 2013;48(9):1843–9. [DOI] [PubMed] [Google Scholar]
- 56. McIntyre CC, Butson CR, Maks CB, Noecker AM. “Optimizing deep brain stimulation parameter selection with detailed models of the electrode-tissue interface,” in 2006 international conference of the IEEE engineering in medicine and biology society. New York, NY: IEEE; 2006. p. 893–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its online supplementary material. Further inquiries can be directed to the corresponding author.