Abstract
Introduction
In critically ill patients undergoing continuous renal replacement therapy (CRRT), a positive fluid balance (FB) is associated with adverse outcomes. However, current FB management practices in CRRT patients are poorly understood. We aimed to study FB and its components in British and Australian CRRT patients to inform future trials.
Methods
We obtained detailed electronic health record data on all fluid-related variables during CRRT and hourly FB for the first 7 days of treatment.
Results
We studied 1,616 patients from three tertiary intensive care units (ICUs) in two countries. After the start of CRRT, the mean cumulative FB became negative at 31 h and remained negative over 7 days to a mean nadir of −4.1 L (95% confidence interval (CI) of −4.6 to −3.5). The net ultrafiltration (NUF) rate was the dominant fluid variable (−67.7 mL/h; standard deviation (SD): 75.7); however, residual urine output (−34.7 mL/h; SD: 54.5), crystalloid administration (48.1 mL/h; SD: 44.6), and nutritional input (36.4 mL/h; SD: 29.7) significantly contributed to FB. Patients with a positive FB after 72 h of CRRT were more severely ill, required high-dose vasopressors, and had high lactate concentrations (5.0 mmol/L; interquartile range: 2.3–10.5). A positive FB was independently associated with increased hospital mortality (odds ratio: 1.70; 95% CI; p = 0.004).
Conclusion
In the study ICUs, most CRRT patients achieved a predominantly NUF-dependent negative FB. Patients with a positive FB at 72 h had greater illness severity and haemodynamic instability. Achieving equipoise for conducting trials that target a negative early FB in such patients may be difficult.
Keywords: Critical illness, Acute kidney injury, Water and electrolyte imbalance, Fluid management, Renal replacement therapy
Introduction
Acute kidney injury (AKI) occurs in 35–50% of patients in the intensive care unit (ICU) [1, 2]. The most severe cases of AKI are treated with continuous renal replacement therapy (CRRT) [3], with its use ranging from 3 to 8% [1, 2]. The need for CRRT in the ICU is associated with a high risk of mortality, with rates ranging from 30 to 70% within 90 days [2, 4, 5]. Among survivors, 10–30% will require long-term dialysis [6].
Observational evidence suggests that patients treated with CRRT may experience fluid overload. Patients with a significant increase in weight due to fluid overload when beginning CRRT are more likely to be dialysis dependent 1 year later [7]. In addition, a post hoc analysis of patients in the Randomized Evaluation of Normal versus Augmented Level (RENAL) study [8] found that, even after adjustment, a positive cumulative fluid balance (FB) after CRRT initiation was associated with increased mortality [9]. Finally, in patients who received CRRT, achieving a net negative FB within the first 72 h of RRT was independently linked to lower hospital mortality [3, 10].
Despite the above observations linking fluid and FB management with mortality in critically ill adult patients on RRT, a recent scoping review found limited evidence regarding its nature, trajectory, and extent [11]. Moreover, most of the research so far has concentrated on improving fluid removal, specifically by net ultrafiltration (NUF), and studying the timing of RRT initiation. In addition, such research used data that are almost 15 years old and assumed little change in practice since then [12]. Such assumptions may be incorrect. Yet, no studies have examined the characteristics and outcomes of current FB management practices.
To inform future prospective studies, we aimed to test the primary hypothesis that the cumulative FB of patients requiring CRRT would be positive before CRRT and rapidly decrease after the commencement of CRRT. In addition, we hypothesized that various pre-CRRT factors would affect the overall FB. Lastly, in an exploratory hypothesis, we aimed to identify which patients remained in a positive FB after CRRT and to examine whether a positive FB was associated with an increased risk of mortality.
Methods
Study Design
We performed a multicentre, retrospective cohort study of granular, routinely collected, electronic health record (EHR)-based clinical data.
Study Sites
The study sites were three closed-model, tertiary ICUs, two in Brisbane, QLD, Australia, and one in London, the UK. We evaluated all adult patients admitted between 1 January 2015 and 31 December 2021. All patients were eligible if their electronic medical records were retrievable, and they required CRRT during their ICU admission. We excluded patients with advanced kidney disease requiring chronic dialysis and patients with an ICU length of stay of less than 72 h. A length of stay greater than 72 h was chosen to allow sufficient time to assess our hypotheses. All study sites exclusively utilized CRRT for RRT and did not perform intermittent haemodialysis for acute RRT.
Data and Identification of CRRT Patients
Routinely collected data were obtained from QLD-based ICUs using the eCritical MetaVision™ (iMDsoft, Boston, USA) clinical information systems and the Australia and New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD) [13–16]. For the UK cohort, ICU data were extracted from the Intellivue Clinical Information Portfolio (ICIP) data management system. Data included information on baseline demographics, admission diagnosis, the severity of illness, and outcomes, as well as daily laboratory data and hourly FB data. We used the term “Emergency Admission” to indicate an unplanned as opposed to a planned admission. The source of admission indicated the location from which the patient was admitted, and if this was from the operating theatre, the patient may have had an emergency admission or a planned admission. We applied Acute Physiology and Chronic Health Evaluation (APACHE) rather than SAPS scores to the assessment of illness severity because they were the illness severity scores collected in the study centres. Data from the three centres were combined. However, for admission diagnoses and medical co-morbidities, a direct comparison was not possible, and categorization was required (online suppl. Tables S1, S2; for all online suppl. material, see https://doi.org/10.1159/000538421). Furthermore, the dosage of vasopressor was converted to norepinephrine equivalent and vasoactive inotrope score (online suppl. Tables S3, S4) [17].
FB Data
Fluid input was categorized by the sum of all crystalloids (including 5% dextrose and fluids for medication administration), colloids (4% and 20% albumin), nutrition (enteral and parenteral nutrition), blood products (except albumin), and other inputs (oral intake and, at some sites, IV medication flushes). Fluid output was separated into urine output (UO), CRRT output (NUF rate), drain output (all drains including intercostal catheters and surgical drains), gastrointestinal output (nasogastric aspirates and bowel losses), and other outputs (miscellaneous fluid loss). Furthermore, crystalloid fluid rates >20 mL/h were used to indicate fluid administration beyond the maintenance of catheter patency [18, 19].
Outcomes
The primary outcome was the mean hourly cumulative FB after CRRT commencement. The secondary outcomes were individual components of FB, such as fluid input, UO, and CRRT factors, after CRRT commencement, as well as mortality. The exploratory outcome was the early identification of which patients had a positive FB after CRRT commencement and the assessment of which characteristics were associated with the development of such a positive FB.
Statistical Analysis
Descriptive statistics were expressed as frequencies and proportions for categorical variables and medians with interquartile ranges (IQRs) or means with standard deviations for continuous variables depending on their distribution. Pearson’s χ2 test or Fisher’s exact test was used to compare categorical variables. The Wilcoxon rank-sum test was used to compare continuous variables. Mixed-effect logistic regression models, including hospitals as a random effect, were developed to examine factors associated with a positive FB, as well as ICU and hospital mortality. For a positive FB, variables used for bivariate analysis were determined a priori and all variables with p < 0.1 were included in multivariable analysis. For mortality outcomes, the variables used for analysis were determined a priori and reflected the clinical utility of the available data. The results of the multivariable analyses were reported as odds ratios (ORs) with 95% confidence intervals (95% CIs). Given the large dataset, a two-sided p value of <0.01 was considered statistically significant. Statistical analyses were performed using R v.4.0.3.
Results
Incidence of CRRT
During the study period, 45,002 patients without dialysis-dependent chronic kidney disease were admitted to the participating ICUs. Among these patients, 1,918 (4.3%) received CRRT and, of these, 1,616 (84.3%) had a length of stay greater than 72 h. In total, 750 patients (46.4%) were from the UK cohort and 866 patients (53.6%) were from the QLD cohort.
Patient Characteristics
The baseline characteristics of the study patients are summarized in Table 1. For the overall cohort, the median age was 61 (IQR: 48–71), and 35% (571) were female. Patients were admitted from multiple sources, most commonly from the ward (31%, 501), emergency department (23%; 367), and operating theatre (21%; 338).
Table 1.
Baseline characteristics
| Variable | N = 1,6161 |
|---|---|
| Age, years | 61 (48, 71) |
| Female | 571 (35.3) |
| Body mass index | 27.7 (24.2, 32.7) |
| Source of ICU admission | |
| Emergency department | 367 (22.7) |
| Operating theatre | 338 (20.9) |
| Other hospital | 258 (16.0) |
| Other ICU | 147 (9.1) |
| Unknown | 5 (0.3) |
| Ward | 501 (31.0) |
| Admission diagnosis group | |
| Cardiovascular | 348 (21.5) |
| Gastrointestinal | 224 (13.9) |
| Genitourinary | 122 (7.5) |
| Haematological | 31 (1.9) |
| Metabolic | 74 (4.6) |
| Musculoskeletal | 3 (0.2) |
| Neurological | 46 (2.8) |
| Other | 32 (2.0) |
| Respiratory | 149 (9.2) |
| Sepsis | 520 (32.2) |
| Trauma | 67 (4.1) |
| Admission circumstances | |
| Surgical admission | 333 (20.6) |
| Emergency admission | 1,466 (90.8) |
| Severity of illness | |
| APACHE 2 score | 24 (19, 30) |
| Day of admission | |
| Any ventilation | 1,193 (76.1) |
| Urine output, mL/kg/h | 0.4 (0.1, 0.7) |
| Diuretics | 166 (10.6) |
| Maximum creatinine, µmol/L | 215 (137, 339) |
| CRRT | 477 (30.4) |
| Any vasopressors | 938 (59.9) |
| NEE score, µg/kg/min* | 0.1 (0.0, 0.2) |
| VIS, if any | 10.2 (4.3, 22.2) |
| Mean MAP, mm Hg | 74.7 (69.0, 82.8) |
| Mean CVP, mm Hg | 13.2 (10.0, 17.0) |
| Maximum SOFA score | 9.0 (7.0, 12.0) |
| Maximum lactate, mmol/L | 3.2 (1.7, 6.9) |
ICU, intensive care unit; NEE, norepinephrine equivalent; VIS, vasoactive inotrope score; MAP, mean arterial pressure; CVP, central venous pressure; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation; CRRT, continuous renal replacement therapy.
*NEE was calculated only for those patients treated with vasopressors.
1Median (IQR), or n (%).
Most patients had an admission diagnosis of sepsis, followed by cardiovascular and gastrointestinal disorders. Most patients were emergency admissions (91%; 1466), as opposed to planned admission, four-fifths were medical, and they had a median APACHE 2 score of 24 (IQR: 19–30). On the day of admission, most patients were ventilated and were receiving vasopressors. Only one in 10 patients received diuretics.
Timing and Characteristics at CRRT Initiation
Most patients commenced CRRT on day two of their ICU admission (IQR: 1–3), with a median time from ICU admission to commencement of 19 h (IQR: 6–56) (Table 2). At the time of CRRT commencement, the median serum creatinine was 272 μmol/L (IQR: 178–391), the median serum lactate was 2.6 mmol/L (IQR: 1.6–5.3), and the median base excess was –6.3 (IQR: 10.3−2.5). The median total FB from ICU admission to CRRT commencement was +1301 mL (IQR: 39–3,756) for the entire cohort.
Table 2.
Characteristics at CRRT commencement
| Variable | N = 1,6161 |
|---|---|
| ICU day CRRT commenced | 2 (1, 3) |
| Time from ICU admission to CRRT, h | 19 (6, 56) |
| SOFA score | 10 (7, 12) |
| Any vasoactive drugs | 1,124 (70) |
| VIS, if any | 11 (4, 24) |
| NEE score, if any, μg/kg/min | 0.11 (0.04, 0.24) |
| MAP, mm Hg | 74 (70, 83) |
| Ventilation | 1,315 (81) |
| PF ratio | 170 (111, 253) |
| Maximum creatinine, µmol/L | 272 (178, 391) |
| Furosemide, mg | 361 (22) |
| Urine output, mL | 405 (113, 1,026) |
| Urea, mmol/L | 18 (12, 28) |
| Potassium, mmol/L | 5.00 (4.50, 5.70) |
| Lactate, mmol/L | 2.6 (1.6, 5.3) |
| pH | 7.27 (7.19, 7.34) |
| Bicarbonate, mmol/L | 20.0 (16.5, 22.5) |
| Base excess | −6.3 (−10.3, −2.5) |
| FB pre-CRRT, mL | 1,301 (39, 3,756) |
ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; VIS, vasoactive inotrope score; NEE, norepinephrine equivalent; MAP, mean arterial pressure; PF ratio, PaO2/FiO2 ratio; CRRT, continuous renal replacement therapy.
1Median (IQR), or n (%).
Overall FB
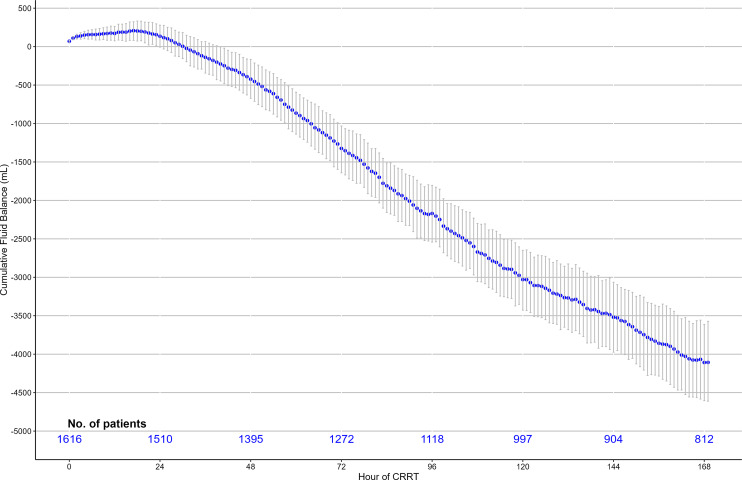
The mean cumulative FB began to decrease within 17 h of CCRT start, was less than zero at 30 h from CRRT start, and continued to decline to a median nadir of –4,158 mL (95% CI: −4,699 to −3,638) at 168 h (Fig. 1) and a mean FB of −320 mL/day (95% CI: −363 to −275 mL/day). Consistent with such a mean cumulative FB trend, the median hourly FB began to decrease immediately after CRRT commencement and was negative within 24 h (online suppl. Fig. S1).
Fig. 1.
Cumulative hourly FB in all CRRT patients.
Components of FB after CRRT
All components of fluid input and fluid output during the first 7 days after CRRT commencement are presented in Table 3. During this time, only crystalloid administration and nutritional support significantly contributed to fluid input. For all patients, CRRT output as NUF was the dominant fluid output variable to modify FB, with UO contributing a smaller volume. All other fluid variables, such as blood products and drain output, did not materially contribute to the mean FB. This is further demonstrated in the mean hourly volumes for the key fluid variables for 7 days after CRRT commencement as presented in online supplementary Figure S2. Thus, fluid removal via CRRT was the largest contributor to negative FB, though its contribution reduced over time as UO increased. Despite fluid management aimed towards a negative FB, mean crystalloid input was greater than the minimum of 20 mL/h and nutritional input continued to increase to 50 mL/h.
Table 3.
Fluid input and output variables in the 7 days after CRRT commencement
| Variable | Day 1, N = 1,6161 | Day 2, N = 1,5101 | Day 3, N = 1,3951 | Day 4, N = 1,2721 | Day 5, N = 1,1181 | Day 6, N = 9971 | Day 7, N = 9041 |
|---|---|---|---|---|---|---|---|
| Fluid input | |||||||
| Fluid input total, mL/h | 106.1 (64.6) | 94.4 (53.2) | 89.1 (46.3) | 87.3 (46.7) | 87.0 (43.1) | 87.0 (42.7) | 85.4 (45.7) |
| Crystalloid, mL/h | 72.9 (60.3) | 53.7 (45.6) | 45.9 (37.7) | 41.2 (37.1) | 36.8 (30.7) | 35.3 (30.7) | 35.4 (37.1) |
| Colloid, mL/h | 5.5 (16.0) | 3.1 (10.3) | 2.2 (8.0) | 2.3 (10.0) | 2.8 (11.7) | 2.6 (10.5) | 2.0 (6.9) |
| Nutrition, mL/h | 24.3 (27.0) | 32.3 (28.6) | 36.4 (29.4) | 39.7 (29.7) | 42.7 (29.2) | 44.3 (29.3) | 44.1 (29.2) |
| Blood products, mL/h | 13.2 (33.6) | 9.4 (26.2) | 7.6 (28.3) | 6.8 (23.8) | 5.7 (17.9) | 5.4 (17.1) | 6.3 (23.5) |
| Other input, mL/h | − | 5.3 (17.8) | 4.6 (16.4) | 4.2 (15.2) | 4.6 (15.9) | 4.8 (16.8) | 3.9 (14.3) |
| Fluid output | |||||||
| Fluid output total, mL/h | −112.9 (87.8) | −125.5 (80.8) | −128.6 (80.0) | −125.4 (79.7) | −122.7 (74.6) | −118.6 (67.9) | −114.9 (67.9) |
| Urine output, mL/h | −29.8 (59.0) | −27.3 (45.9) | −32.3 (52.8) | −37.3 (57.0) | −40.0 (54.9) | −41.8 (55.5) | −41.5 (54.3) |
| NUF rate, mL/h | −64.8 (70.5) | −78.0 (76.7) | −77.1 (81.2) | −69.6 (80.7) | −64.3 (76.4) | −57.8 (69.4) | −53.9 (68.3) |
| Drain output, mL/h | −7.1 (25.7) | −6.4 (24.4) | −6.2 (21.9) | −5.0 (19.0) | −4.7 (16.2) | −5.2 (19.2) | −5.3 (20.2) |
| GI output, mL/h | −10.9 (19.8) | −13.0 (22.7) | −12.8 (21.5) | −13.2 (22.1) | −13.6 (23.9) | −13.6 (22.1) | −13.8 (22.8) |
| Other output, mL/h | −0.2 (3.4) | −0.7 (20.3) | −0.3 (3.9) | −0.4 (3.7) | −0.1 (0.8) | −0.2 (3.4) | −0.4 (6.2) |
CRRT, continuous renal replacement therapy; NUF, net ultrafiltration; GI, gastrointestinal.
1Mean (SD).
FB Quartiles
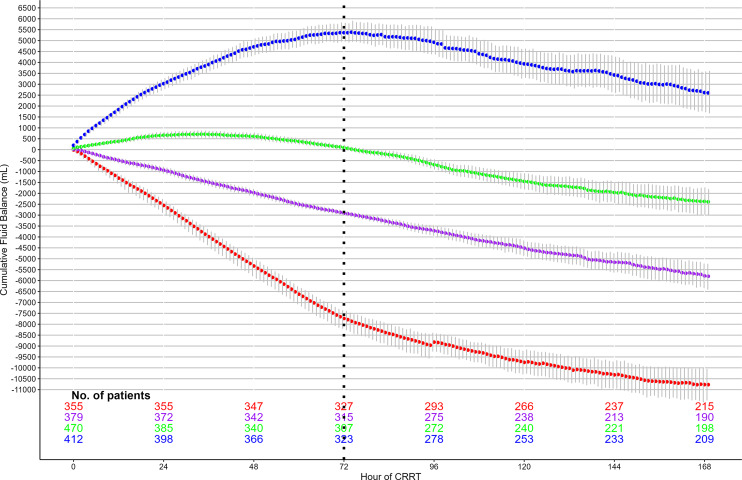
We separated patients into quartiles based on their cumulative FB after 72 h of CRRT (quartile 1: < – 4,498 mL, quartile 2: ≥ −4,498 mL and <−1,353 mL, quartile 3: ≥−1.353 mL and <1,677 mL, and quartile 4: ≥1,677 mL) (Fig. 2). Patients with a more positive FB, quartile 4 patients, were more severely ill with significantly higher rates of vasopressor usage and organ dysfunction as measured by the Sequential Organ Failure Assessment (SOFA) score (online suppl. Table S5). At the time of CRRT commencement, these patients required high-dose vasopressors (norepinephrine equivalent score 0.19 μg/kg/min; IQR: 0.08–0.37), were oliguric (278 mL/24 h; IQR: 72–748), and had a median lactate concentration of 5.0 mmol/L (2.3–10.5) (online suppl. Table S6). The more positive FB in quartile 4 patients was a consequence of greater crystalloid fluid administration and lower NUF rates (online suppl. Table S7).
Fig. 2.
Cumulative hourly FB in CRRT patients by FB category.
A logistic regression was performed to determine which pre-CRRT patient characteristics and measures of disease severity were associated with quartile 4 patients, who had a positive FB ≥1677 mL 72 h after RRT commencement (online suppl. Tables S8, S9). A higher APACHE 2 score and a higher cardiac SOFA score on the day of CRRT start, as well as a more positive FB, a higher lactate, and a lower creatinine before CRRT, were all associated with the development of a positive FB in the 72 h after the start of CRRT.
Clinical Outcomes
For the entire cohort, the median length of stay in the ICU was 241 h (IQR: 144–458). Overall, 26% (428) and 37% (583) of patients died in the ICU and hospital, respectively. When comparing patients with different FB at 72 h, those with a more positive FB had higher ICU mortality (quartile 1: 19% (68), quartile 2: 24% (92), quartile 3: 23% (109), and quartile 4: 39% (159); p < 0.001) and hospital mortality (quartile 1: 28% (99), quartile 2: 33% (123), quartile 3: 34% (160), and quartile 4: 49% (201); p < 0.001) (online suppl. Table S10).
After adjustment for patient characteristics and disease severity, quartile 4, compared to quartile 1, was associated with an increased risk of ICU (OR: 1.79; 95% CI: 1.21–2.63; p = 0.003) and hospital (OR: 1.74; 95% CI: 1.22–2.48; p = 0.002) mortality (online suppl. Tables S11, S12). In addition, APACHE 2 score, liver SOFA score, maximum lactate, maximum creatinine, and maximum urea were also associated with an increased risk of ICU and hospital mortality. A sensitivity analysis was performed after excluding patients with chronic liver disease. It demonstrated that compared to quartile 1, quartile 4 was still associated with an increased risk of ICU mortality (OR: 2.13; 95% CI: 1.41–3.21; p < 0.001) (online suppl. Table S13).
Discussion
Key Findings
Utilizing granular EHR data, we conducted a binational study from three academic ICUs to describe fluid input and output trajectories in a contemporary population of 1,616 critically ill patients receiving CRRT and to determine if contemporary FB management has the potential for modifications to achieve a less positive FB. In this regard, first, we first found that mean hourly FB and subsequent cumulative FB decreased within 24 h of CRRT commencement. Second, cumulative FB consistently continued to decrease over 7 days to a nadir of approximately 4 L of negative FB and a main daily negative FB by approximately a third of a litre. Third, while crystalloid administration and nutrition contributed towards a positive FB and residual UO attenuated it, fluid removal by NUF via CRRT was the dominant fluid control variable. Fourth, despite persistent crystalloid administration and increasing nutrition volumes, high NUF volumes resulted in a net negative FB. Fifth, patients who continued to accumulate fluid after CRRT commencement had markers of ongoing haemodynamic instability and multiorgan failure. Finally, after adjustment, a more positive FB was associated with an increased risk of mortality.
Relationship to Previous Studies
Available knowledge on FB management in critically ill patients receiving CRRT is limited in scope and detail [11]. Most research has indirectly examined FB while focusing on the timing of CRRT initiation [2, 20–23] and NUF [12, 24, 25]. Only one study has investigated the manipulation of fluid interventions in patients on CRRT to achieve a more negative FB [26]. The Early Dry Cohort study involved 87 patients and was a before-and-after cohort study. It aimed to investigate the effectiveness of a restrictive fluid protocol combined with perfusion-based adjustment of ultrafiltration in critically ill patients receiving RRT who had more than 5% fluid overload. On day 5, the intervention led to a difference of 4,292 mL in cumulative FB. However, even though the intervention aimed to limit the amount of fluid intake, there was no significant difference in fluid input between the groups, and the difference in FB was exclusively due to greater NUF.
Aligned with our findings, a retrospective single-centre study examined hourly FB after the commencement of RRT in 350 critically ill Australian patients receiving CRRT [27] and demonstrated that mean hourly FB became negative after 20 h [27]. However, this study did not examine the individual fluid variables that contribute to FB, nor did it identify the characteristics of patients who had a positive FB. Of note, the study demonstrated a decreased risk of mortality with an hourly FB between 18.5 and −33 mL/h, which is consistent with the findings in our study. Patients in the RENAL trial [8], and its subsequent post hoc analysis examining NUF [12], found a negative mean daily FB of approximately 20 mL/day for the entire CRRT treatment. In contrast, in our study, over the first 7 days of CRRT, patients had a mean FB of >300 mL, suggesting that contemporary FB management of CRRT patients is significantly different from the historical RENAL cohort.
A post hoc analysis of the STARRT-AKI trial examined the cumulative FB in 2,738 patients treated with either CRRT or intermittent haemodialysis [21]. The study demonstrated that at 14 days, accelerated RRT initiation resulted in lower cumulative FB (+4,509 mL) than standard initiation (+5,646 mL). Relevant to our study, the day seven mean cumulative FB seen in the STARRT-AKI differed from ours by over 8 L, demonstrating significant variation in FB practice across different ICUs. These major differences in FB management philosophy and practice were confirmed by significant variability in fluid removal targets as reported by clinicians in a recent international survey [28].
Implications
The results from our study imply that, in some ICUs, current FB management may predominantly achieve an early and ongoing negative FB after the commencement of CRRT. Thus, in such ICUs and such patients, there may be limited scope for manipulation of FB to achieve a neutral to negative daily FB. Moreover, our study suggests that in the subgroup of patients with a positive FB, 72 h after CRRT commencement, the use of high-dose vasopressors and high illness severity may signal a lack of clinical equipoise in pursuing an early negative FB. In addition, the independent increase in mortality associated with a positive FB at 72 h suggests the need for further investigation to determine whether, in such patients, there is scope to achieve a less positive FB after the hyperacute phase has abated.
Finally, in our study cohort, most patients had a negative FB and those patients who did not have a negative FB (i.e., had a positive FB) had a very high severity of illness and were shocked, making targeting a negative FB in such patients a difficult proposition. Logically, then, a clinical trial intervention aimed at achieving a negative FB would need to be performed in ICUs where standard practice is to target a positive FB. This is in contrast to our study cohort where standard practice was clearly to target a negative FB. Accordingly, taken in conjunction with the literature, our findings imply that any clinical trial intervention aimed at achieving a negative FB in critically ill patients receiving CRRT would need to be performed in ICUs where standard practice is to target a positive FB.
Strengths and Limitations
Our study has several strengths. First, we assessed very granular routinely collected data from the EHR which allowed an unprecedented, detailed understanding of FB after CRRT commencement. Second, for the first time, we were able to dynamically analyse the individual components of FB and determine their contribution to cumulative FB. Third, for the first time, we identified different groups of patients with different FB management and trajectories. Fourth, we demonstrated that patients with a positive FB after CRRT had plausible clinical reasons for the avoidance of an early negative FB. Finally, we included sites from two geographical locations, which enhances the potential generalizability of our findings to other resource-rich centres that treat AKI with CRRT.
We acknowledge some limitations. First, there was no FB data before ICU admission; therefore, the analysis could not account for the impact of prior fluid administration on fluid management in the ICU. Second, the data on FB may be independent of interstitial fluid accumulation; however, FB is a well-recognized surrogate of such fluid accumulation as used in research [29, 30]. Third, while intravenous fluid administration is reported, it was not possible to determine the indication for fluid, such as maintenance fluids or medication administration. Fourth, we could not account for insensible fluid losses related to fever. However, they are likely to have been limited in amount when compared to the other components of FB. Fifth, we did not include the change in weight over time in our manuscript because regular weights are not performed in the study units. Furthermore, the accuracy and feasibility of regular weights in critically ill patients seem to be poor [31, 32]. Additionally, our analysis assessed hourly changes in FB, which is a level of granularity that weights would not be able to assess. Fifth, we did not have data on baseline chronic kidney disease (CKD). As such, we could not stratify patients on CKD status and, given the unique nature of AKI in CKD, such patients may have had different FB characteristics. Lastly, our data did not include information on clinical decision-making or FB prescriptions; therefore, they could not be adjusted for such a clinician-based assessment.
Conclusion
In a large multicentre international study of critically ill patients treated with CRRT, analysis of current fluid management suggests that there are limited opportunities for interventions directed at achieving a less positive early FB in such settings. In this regard, cumulative FB decreased within 24 h after AKI diagnosis and such a decrease was driven by NUF despite persistent crystalloid fluid administration and increasing nutritional fluid intake. In the quartile of patients with a positive FB, the degree of illness severity was so high that clinicians chose not to pursue a negative FB, suggesting likely limited equipoise for the pursuit of a negative fluid removal at such time.
Statement of Ethics
This study was approved on 12 May 2022 by the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/2022/QMS/84927) with an individual waiver of consent granted. In the UK, the study had institutional approval. All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Study conception and design: K.W., K.L., R.B., and M.O. Data acquisition: K.W., R.H., P.C., and B.S. Analysis: K.W. and A.S. Interpretation of data, article revision for important intellectual content, and final approval of the version submitted for publication: all authors. Article drafting: K.W., K.L., M.L.G., M.O., and R.B. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: K.W., K.L., and R.B.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations. Data released for research under Sect. 280 of the Public Health Act 2005 requires an application to the Director General of Queensland Health (PHA@health.qld.gov.au). Further enquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Tolwani A. Continuous renal-replacement therapy for acute kidney injury. N Engl J Med. 2012;367(26):2505–14. [DOI] [PubMed] [Google Scholar]
- 2. STARRT-AKI InvestigatorsCanadian Critical Care Trials GroupAustralian and New Zealand Intensive Care Society Clinical Trials GroupUnited Kingdom Critical Care Research GroupCanadian Nephrology Trials NetworkIrish Critical Care Trials Group, et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383(3):240–51. [DOI] [PubMed] [Google Scholar]
- 3. Uusalo P, Hellman T, Löyttyniemi E, Peltoniemi J, Järvisalo MJ. Early restrictive fluid balance is associated with lower hospital mortality independent of acute disease severity in critically ill patients on CRRT. Sci Rep. 2021;11(1):18216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu C. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tandukar S, Palevsky PM. Renal replacement therapy: who, when, why, and how. Chest. 2019;155(3):626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang C, Hiremath S, Sikora L, Sood MM, Kong J, Clark E. Outpatient kidney recovery after acute kidney injury requiring dialysis: a systematic review protocol. Syst Rev. 2019;8(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012;27(3):956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–38. [DOI] [PubMed] [Google Scholar]
- 9. RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med. 2012;40(6):1753–60. [DOI] [PubMed] [Google Scholar]
- 10. Hall A, Crichton S, Dixon A, Skorniakov I, Kellum JA, Ostermann M. Fluid removal associates with better outcomes in critically ill patients receiving continuous renal replacement therapy: a cohort study. Crit Care. 2020;24(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. White KC, Nasser A, Gatton ML, Laupland KB. Current management of fluid balance in critically ill patients with acute kidney injury: a scoping review. Crit Care Resusc. 2023;25(3):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murugan R, Kerti S, Chang C, Gallagher M, Clermont G, Palevsky P, et al. Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the randomized evaluation of normal vs augmented level (RENAL) of renal replacement therapy trial. Jama Netw Open. 2019;2(6):e195418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committe . A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transpl. 2008;23(5):1569–74. [DOI] [PubMed] [Google Scholar]
- 14. Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. Jama. 2017;317(3):290–300. [DOI] [PubMed] [Google Scholar]
- 15. Corrigan C, Duke G, Millar J, Paul E, Butt W, Gordon M, et al. Admissions of children and adolescents with deliberate self-harm to intensive care during the SARS-CoV-2 outbreak in Australia. Jama Netw Open. 2022;5(5):e2211692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. New Engl J Med. 2015;372(17):1629–38. [DOI] [PubMed] [Google Scholar]
- 17. Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419–30. [DOI] [PubMed] [Google Scholar]
- 18. Sakuraya M, Yoshihiro S, Onozuka K, Takaba A, Yasuda H, Shime N, et al. A burden of fluid, sodium, and chloride due to intravenous fluid therapy in patients with respiratory support: a post-hoc analysis of a multicenter cohort study. Ann Intensive Care. 2022;12(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doyle B, Kelsey L, Carr PJ, Bulmer A, Keogh S. Determining an appropriate to-keep-vein-open (TKVO) infusion rate for peripheral intravenous catheter usage. J Assoc Vasc Access. 2021;26(2):13–20. [Google Scholar]
- 20. Gaudry S, Hajage D, Martin-Lefevre L, Lebbah S, Louis G, Moschietto S, et al. Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): a multicentre, open-label, randomised, controlled trial. Lancet. 2021;397(10281):1293–300. [DOI] [PubMed] [Google Scholar]
- 21. Wald R, Kirkham B, daCosta BR, Ghamarian E, Adhikari NKJ, Beaubien-Souligny W, et al. Fluid balance and renal replacement therapy initiation strategy: a secondary analysis of the STARRT-AKI trial. Crit Care. 2022;26(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wald R, Adhikari NKJ, Smith OM, Weir MA, Pope K, Cohen A, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88(4):897–904. [DOI] [PubMed] [Google Scholar]
- 23. Xing ZQ, Liu DW, Wang XT, Long Y, Zhang HM, Pan P, et al. Early initiation renal replacement therapy for fluid management to reduce central venous pressure is more conducive to renal function recovery in patients with acute kidney injury. Chin Med J. 2019;132(11):1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu B, Shen Y, Peng Y, Xing C, Mao H. The association of an early net ultrafiltration rate and 28-day mortality in patients receiving continuous kidney replacement therapy. Front Med. 2021;8:766557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naorungroj T, Neto AS, Zwakman-Hessels L, Yanase F, Eastwood G, Murugan R, et al. Early net ultrafiltration rate and mortality in critically ill patients receiving continuous renal replacement therapy. Nephrol Dial Transpl. 2021;36(6):1112–9. [DOI] [PubMed] [Google Scholar]
- 26. Ruste M, Sghaier R, Chesnel D, Didier L, Fellahi JL, Jacquet-Lagrèze M. Perfusion-based deresuscitation during continuous renal replacement therapy: a before-after pilot study (The early dry Cohort). J Crit Care. 2022;72:154169. [DOI] [PubMed] [Google Scholar]
- 27. Naorungroj T, Neto AS, Zwakman-Hessels L, Yanase F, Eastwood G, Bellomo R. Hourly fluid balance in patients receiving continuous renal replacement therapy. Blood Purif. 2020;49(1–2):93–101. [DOI] [PubMed] [Google Scholar]
- 28. Ledoux-Hutchinson L, Wald R, Malbrain MLNG, Carrier FM, Bagshaw SM, Bellomo R, et al. Fluid management for critically ill patients with acute kidney injury receiving kidney replacement therapy: an international survey. Clin J Am Soc Nephrol. 2023;18(6):705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang N, Jiang L, Zhu B, Wen Y, Xi XM, Beijing Acute Kidney Injury Trial BAKIT Workgroup . Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015;19(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ostermann M, Straaten HMO, Forni LG. Fluid overload and acute kidney injury: cause or consequence? Crit Care. 2015;19(1):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider AG, Baldwin I, Freitag E, Glassford N, Bellomo R. Estimation of fluid status changes in critically ill patients: fluid balance chart or electronic bed weight? J Crit Care. 2012;27(6):745.e7–12. [DOI] [PubMed] [Google Scholar]
- 32. Schneider AG, Thorpe C, Dellbridge K, Matalanis G, Bellomo R. Electronic bed weighing vs daily fluid balance changes after cardiac surgery. J Crit Care. 2013;28(6):1113.e1–1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations. Data released for research under Sect. 280 of the Public Health Act 2005 requires an application to the Director General of Queensland Health (PHA@health.qld.gov.au). Further enquiries can be directed to the corresponding author.