Abstract
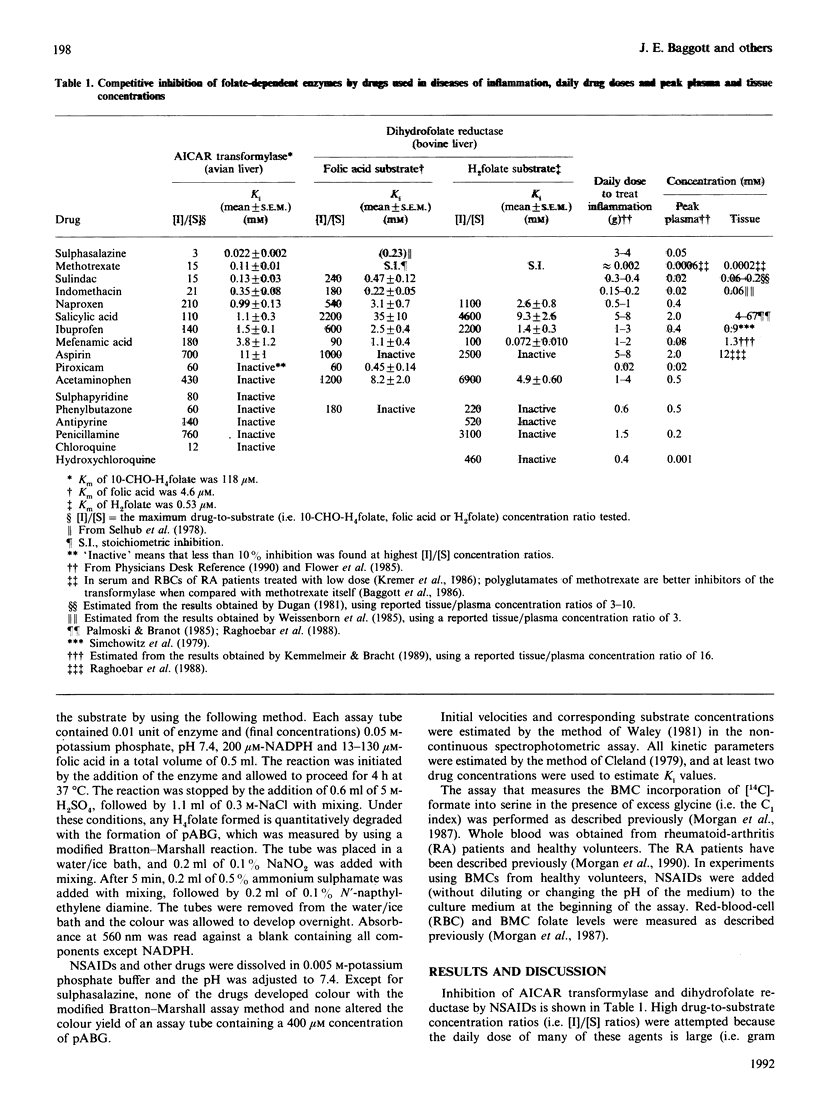
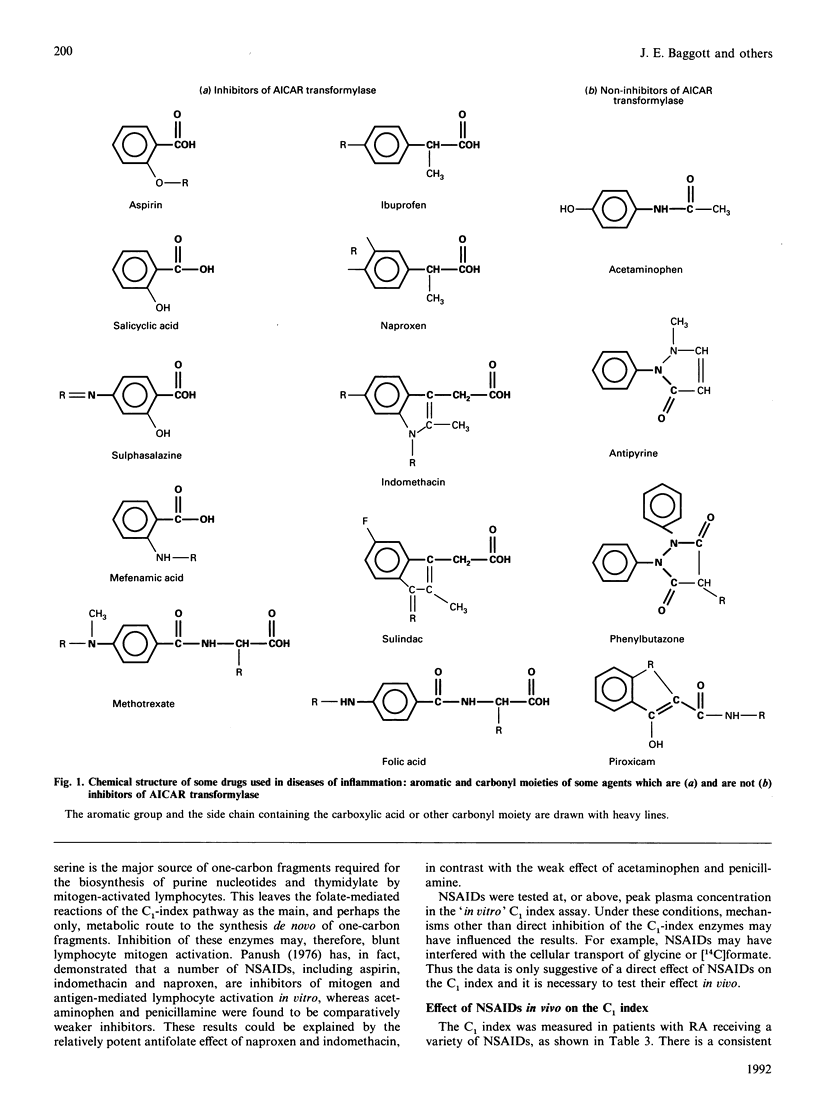
Many non-steroidal anti-inflammatory drugs (NSAIDs) (including sulphasalazine, sulindac, indomethacin, naproxen, salicylic acid, ibuprofen, piroxicam and mefenamic acid) were found to be competitive inhibitors (with respect to folate) of avian liver phosphoribosylaminoimidazolecarboxamide formyltransferase (AICAR transformylase, EC 2.1.2.3) and bovine liver dihydrofolate reductase (EC 1.5.1.3). In contrast, aspirin and the antipyretic-analgesic drugs acetaminophen and antipyrine were weak inhibitors of these enzymes. Structure-activity correlation suggests that an aromatic ring with a side chain containing a carboxylic acid is a requirement for competitive inhibition of the transformylase. The above-listed NSAIDs also inhibited the folate-coenzyme-mediated biosynthesis of serine from glycine and formate (i.e., the C1 index) by human blood mononuclear cells (BMCs) in experiments where the drug was added to a culture of BMCs. Acetaminophen had a weak inhibitory effect on the C1 index. Consistent with the results obtained in vitro is the observation that the C1 index of BMCs from rheumatoid-arthritis patients treated with drugs which possess little antifolate activity (e.g. acetaminophen) is higher than the C1 index of BMCs from rheumatoid-arthritis patients treated with NSAIDs possessing more potent antifolate activity (e.g. sulindac, sulphasalazine, naproxen and ibuprofen). The mean activity of the transformylase in BMCs taken from healthy humans was 1.98 nmol of product/h per 10(6) cells and the activity was positively correlated with BMC folate levels. These results are consistent with the hypothesis that (1) the antifolate activity of NSAIDs, and hence cytostatic consequences, are important factors in producing anti-inflammatory activity and (2) aspirin exerts its anti-inflammatory effects after its conversion into salicylic acid, which possesses greater antifolate activity than its parent compound.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. B., Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1989 Jan;32(1):1–9. doi: 10.1002/anr.1780320102. [DOI] [PubMed] [Google Scholar]
- Abramson S., Korchak H., Ludewig R., Edelson H., Haines K., Levin R. I., Herman R., Rider L., Kimmel S., Weissmann G. Modes of action of aspirin-like drugs. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7227–7231. doi: 10.1073/pnas.82.21.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter H. J., Zvaifler N. J., Rath C. E. Interrelationship of rheumatoid arthritis, folic acid, and aspirin. Blood. 1971 Oct;38(4):405–416. [PubMed] [Google Scholar]
- Baggott J. E., Vaughn W. H., Hudson B. B. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5'-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J. 1986 May 15;236(1):193–200. doi: 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune K., Glatt M., Graf P. Mechanisms of action of anti-inflammatory drugs. Gen Pharmacol. 1976;7(1):27–33. doi: 10.1016/0306-3623(76)90028-8. [DOI] [PubMed] [Google Scholar]
- Bökkerink J. P., Bakker M. A., Hulscher T. W., De Abreu R. R., Schretlen E. D., van Laarhoven J. P., De Bruyn C. H. Sequence-, time- and dose-dependent synergism of methotrexate and 6-mercaptopurine in malignant human T-lymphoblasts. Biochem Pharmacol. 1986 Oct 15;35(20):3549–3555. doi: 10.1016/0006-2952(86)90625-8. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Duggan D. E. Sulindac: therapeutic implications of the prodrug/pharmacophore equilibrium. Drug Metab Rev. 1981;12(2):325–337. doi: 10.3109/03602538108994035. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Flower R., Gryglewski R., Herbaczyńska-Cedro K., Vane J. R. Effects of anti-inflammatory drugs on prostaglandin biosynthesis. Nat New Biol. 1972 Jul 26;238(82):104–106. doi: 10.1038/newbio238104a0. [DOI] [PubMed] [Google Scholar]
- GOUGH K. R., MCCARTHY C., READ A. E., MOLLIN D. L., WATERS A. H. FOLIC-ACID DEFICIENCY IN RHEUMATOID ARTHRITIS. Br Med J. 1964 Jan 25;1(5377):212–217. doi: 10.1136/bmj.1.5377.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T., Morgan S. L., Vaughn W. H., Eto I., Baggott J. E. Detection of inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase by thioinosinic acid and azathioprine by a new colorimetric assay. Biochem J. 1990 Dec 1;272(2):339–342. doi: 10.1042/bj2720339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine R. J., Everson M. P., Hardin J. M., Morgan S. L., Alarcòn G. S., Baggott J. E., Koopman W. J., Krumdieck C. L. Methotrexate therapy in rheumatoid arthritis patients diminishes lectin-induced mononuclear cell proliferation. Rheumatol Int. 1990;10(4):165–169. doi: 10.1007/BF02274842. [DOI] [PubMed] [Google Scholar]
- Kemmelmeier F. S., Bracht A. Effects of the nonsteroidal anti-inflammatory drug mefenamic acid on energy metabolism in the perfused rat liver. Biochem Pharmacol. 1989 Mar 1;38(5):823–830. doi: 10.1016/0006-2952(89)90237-2. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Galivan J., Streckfuss A., Kamen B. Methotrexate metabolism analysis in blood and liver of rheumatoid arthritis patients. Association with hepatic folate deficiency and formation of polyglutamates. Arthritis Rheum. 1986 Jul;29(7):832–835. doi: 10.1002/art.1780290703. [DOI] [PubMed] [Google Scholar]
- McCairns E., Fahey D., Sauer D., Rowe P. B. De novo purine synthesis in human lymphocytes. Partial co-purification of the enzymes and some properties of the pathway. J Biol Chem. 1983 Feb 10;258(3):1851–1856. [PubMed] [Google Scholar]
- Morgan S. L., Baggott J. E., Altz-Smith M. Folate status of rheumatoid arthritis patients receiving long-term, low-dose methotrexate therapy. Arthritis Rheum. 1987 Dec;30(12):1348–1356. doi: 10.1002/art.1780301205. [DOI] [PubMed] [Google Scholar]
- Morgan S. L., Baggott J. E., Vaughn W. H., Young P. K., Austin J. V., Krumdieck C. L., Alarcón G. S. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 1990 Jan;33(1):9–18. doi: 10.1002/art.1780330102. [DOI] [PubMed] [Google Scholar]
- Omer A., Mowat A. G. Nature of anaemia in rheumatoid arthritis. IX. Folate metabolism in patients with rheumatoid arthritis. Ann Rheum Dis. 1968 Sep;27(5):414–424. doi: 10.1136/ard.27.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Correction of data on salicylate and indomethacin concentrations in cartilage. Arthritis Rheum. 1985 Feb;28(2):237–237. doi: 10.1002/art.1780280226. [DOI] [PubMed] [Google Scholar]
- Panush R. S. Effects of certain antirheumatic drugs on normal human peripheral blood lymphocytes. Inhibition of mitogen- and antigen-stimulated incorporation of tritiated thymidine. Arthritis Rheum. 1976 Sep-Oct;19(5):907–917. doi: 10.1002/art.1780190512. [DOI] [PubMed] [Google Scholar]
- Paulus H. E. Aspirin versus nonacetylated salicylate. J Rheumatol. 1989 Mar;16(3):264–265. [PubMed] [Google Scholar]
- Raghoebar M., Van den Berg W. B., Van Ginneken C. A. Mechanisms of cell association of some non-steroidal anti-inflammatory drugs with isolated leucocytes. Biochem Pharmacol. 1988 Apr 1;37(7):1245–1250. doi: 10.1016/0006-2952(88)90777-0. [DOI] [PubMed] [Google Scholar]
- Rowe P. B., Sauer D., Fahey D., Craig G., McCairns E. One-carbon metabolism in lectin-activated human lymphocytes. Arch Biochem Biophys. 1985 Jan;236(1):277–288. doi: 10.1016/0003-9861(85)90627-7. [DOI] [PubMed] [Google Scholar]
- Scheufler E. Improved enzymatic assay for methotrexate: shape of standard curve, stability of reagents, sensitivity. Clin Chim Acta. 1981 Mar 19;111(1):113–116. doi: 10.1016/0009-8981(81)90429-0. [DOI] [PubMed] [Google Scholar]
- Selhub J., Dhar G. J., Rosenberg I. H. Inhibition of folate enzymes by sulfasalazine. J Clin Invest. 1978 Jan;61(1):221–224. doi: 10.1172/JCI108921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Mehta J., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: effect of metabolic inhibitors and antiinflammatory drugs. Arthritis Rheum. 1979 Jul;22(7):755–763. doi: 10.1002/art.1780220711. [DOI] [PubMed] [Google Scholar]
- Smith M. J. Prostaglandins and aspirin: an alternative view. Agents Actions. 1975 Oct;5(4):315–317. doi: 10.1007/BF02205237. [DOI] [PubMed] [Google Scholar]
- Vane J. R. The mode of action of aspirin-like drugs. Agents Actions. 1978 Jun;8(4):430–431. doi: 10.1007/BF01968671. [DOI] [PubMed] [Google Scholar]
- Waley S. G. An easy method for the determination of initial rates. Biochem J. 1981 Mar 1;193(3):1009–1012. doi: 10.1042/bj1931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn U., Maedge S., Buettner D., Sewing K. F. Indometacin-induced gastrointestinal lesions in relation to tissue concentration, food intake and bacterial invasion in the rat. Pharmacology. 1985;30(1):32–39. doi: 10.1159/000138047. [DOI] [PubMed] [Google Scholar]


