Abstract
Background
In recent years, the mechanistic interaction between the brain and heart has been explored in detail, which explains the effects of brain injuries on the heart and those of cardiac dysfunction on the brain. Brain injuries are the predominant cause of post-stroke deaths, and cardiac dysfunction is the second leading cause of mortality after stroke onset.
Summary
Several studies have reported the association between brain injuries and cardiac dysfunction. Therefore, it is necessary to study the influence on the heart post-stroke to understand the underlying mechanisms of stroke and cardiac dysfunction. This review focuses on the mechanisms and the effects of cardiac dysfunction after the onset of stroke (ischemic or hemorrhagic stroke).
Key Messages
The role of the site of stroke and the underlying mechanisms of the brain-heart axis after stroke onset, including the hypothalamic-pituitary-adrenal axis, inflammatory and immune responses, brain-multi-organ axis, are discussed.
Keywords: Stroke, Brain-heart axis, Inflammation, Cardiac dysfunction
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in China and worldwide. CVD is defined as the first diagnosis of stroke, unstable angina, non-fatal acute myocardial infarction, heart failure, or death from a cardiovascular event [1]. It is a common event of cardiac injury in patients with cerebrovascular disease, especially in ischemic or hemorrhagic stroke [2, 3]. Electrocardiographic abnormalities, myocardial injury and arrhythmias frequently occur in patients with acute stroke, even in the absence of primary heart disease, indicating the close interaction between brain and heart [4]. Some meta-analyses have reported that cardiac dysfunction positively correlates with high risk of delayed cerebral ischemia (DCI) and death after stroke [5, 6]. Moreover, clinical evidence has revealed a relationship between brain events and heart dysfunction [7]. Due to the complex interaction between cerebrovascular and cardiovascular conditions, it is imperative to determine whether cardiac dysfunction is induced by stroke and understand the underlying mechanisms. The occurrence of post-stroke cardiac dysfunction is positively correlated with its severity, exacerbating the patient’s poor prognosis and risk of complications such as vasospasm, DCI, and pulmonary edema [5, 8–10]. This suggests that severe cardiac dysfunction may result from systemic dysfunction or inflammatory response [11]. Understanding the mechanisms of the influence on the heart post-stroke is essential for clinical management.
As early as the 1940s, there were studies reporting that cerebrovascular disease can cause cardiac injury [12]. It was experimentally confirmed through animal experiments that the injection of saline or ions into the cerebral ventricular system could induce cardiac arrhythmias and cardiac pathology [13]. At the same time, a clinical study found that over 60% of patients with ischemic stroke had electrocardiogram changes within the first 24 h, which was positively correlated with the severity of stroke [14]. This illustrates that stroke events can directly impact cardiac function. The interaction between the brain and the heart has attracted significant attention in the current decade. Neurocardiology refers to the interplay between the brain and the heart [15]. Studies have revealed the underlying pathophysiological and physiological interplay between the central nervous system (CNS) and the cardiovascular system. Neurocardiogenic events, such as post-stroke cardiovascular complications, are responsible for over 1.5 million deaths globally [16, 17]. Despite a better understanding of neurocardiogenic syndrome in the last decade, there are limited effective therapeutic strategies focusing on the brain-heart axis to attenuate cardiovascular complications or post-stroke death. In this review, we have summarized the anatomical and functional aspects of the brain-heart axis and described the underlying mechanisms and biomarkers for post-stroke cardiovascular dysfunction.
A wide range of brain injuries, including stroke, traumatic brain injury (TBI), brain tumors can result in cardiac dysfunction, arrhythmias, and even heart failure (HF). These brain disorder-related heart injuries are considered brain-heart syndrome, for which accurate diagnosis and prediction of injury progression are important for clinical management. Therefore, this review focused on the influence on the heart post-stroke after a stroke. The site of brain damage, clinical manifestations, and the underlying mechanisms of the brain-heart axis after stroke have also been discussed systematically. A variety of cardiac complications, such as heart attack, arrhythmia, cardiac arrest or heart failure, can occur immediately following a stroke [18]. Risk factors, such as diabetes mellitus, hypertension and age, can exacerbate cardiac damage after the onset of stroke. Therefore, severe cardiac dysfunction is more likely due to systemic dysfunction or inflammatory responses [19]. A clinical trial that compared patients with stroke with or without primary heart disease revealed that a history of cardiovascular disease increased the incidence of electrocardiographic abnormalities [20].
The following search terms were used in this study: brain-heart axis, inflammatory response, cardiac dysfunction and stroke. After identifying the target literature, we continued our search in the similar articles section of PubMed, and 134 eligible studies were finally included. In this present review, we screened studies in English and summarized the underlying influence on the heart post-stroke.
Neurological Disorders and Cardiac Dysfunction
Cardiovascular complications are considered the second leading cause of post-stroke death [21]. While the occurrence of cardiac dysfunction has been well documented in patients with subarachnoid hemorrhage (SAH), it has also been reported after the onset of other neurological disorders, including infection, ischemic stroke, ICH, TBI and epilepsy [20, 22–25]. Consequent to a stroke, brain damage-induced cardiac injury may further result in mild recoverable damage (neurogenic stress cardiomyopathy [NSC] and Takotsubo cardiomyopathy), heart failure and fatality [26]. Due to the resolution of cardiac dysfunction with an improvement in neurological function, stabilizing patients with stroke takes precedence over the management of cardiac manifestations. Reduced left ventricular (LV) ejection fraction and elevated serum cardiac enzymes are the predominant manifestations of NSC [27]. The following section summarizes the myocardial injury after brain damage due to stroke.
Clinical Manifestations of Cardiac Injury
Stroke also induces heart problems in ≤70% of patients, with clinical manifestations such as electrocardiographic changes, reduced LV ejection fraction, ventricular wall motion abnormalities, and increases in serum cardiac enzymes [5]. However, there is a paucity of studies investigating the mechanisms of stroke-induced cardiac dysfunction in existing studies. It has been reported that acute ischemic stroke and transient ischemic attack (TIA) are companied with electrocardiographic abnormalities in up to 75% of cases, with atrial fibrillation (AF) being the most frequent arrhythmia [28]. Therefore, AF can be served as an acute complication of the stroke. The predominant clinical manifestations of cardiac injury primarily include Electrocardiography (ECG) abnormalities and elevated cardiac enzymes. Currently, uncertainty remains about heart disorder screening after a stroke, with AF screening drawing more attention recently. Therefore, it is necessary to summarize the clinical manifestations of cardiac injury.
ECG abnormalities and cardiac arrhythmias are common complications following stroke [29, 30]. Previous studies have reported abnormal ECG findings in up to 75% of ischemic stroke patients within 24 h of symptom onset, even in the absence of pre-existing heart disease [31]. The most frequent ECG changes include T-wave inversion, ST-segment depression, prolonged QT interval, and U-wave abnormalities [32]. Post-stroke arrhythmias vary in severity from benign sinus tachycardia to life-threatening ventricular fibrillation [30, 33]. The risk and types of cardiac arrhythmias appear to correlate with clinical factors such as large infarct size, older age, and prior cardiovascular history. The underlying mechanisms involve an overwhelmed sympathetic nervous system response, myocarditis from systemic inflammation, and ischemic injury to cardiac conduction tissue. ECG monitoring is therefore recommended for all stroke patients, as arrhythmias are associated with worse functional outcomes, prolonged hospital stay, and increased mortality risk. Further research is still warranted to identify patients at highest risk and develop arrhythmia prevention strategies.
Release of cardiac enzymes such as troponin and creatine kinase are also frequently observed post-stroke [34, 35]. Release of these intracellular proteins into the bloodstream occurs when there is disruption to the myocardial cell membrane from injury or stress. Myocardial injury following stroke can be detected by elevated cardiac enzyme levels, which serve as biochemical markers of cardiac necrosis [36]. Common enzymes monitored include troponin and creatine kinase. Elevated levels of these biomarkers in the bloodstream indicate myocardial cell death and correlate with the degree of cardiac dysfunction [37]. Troponin in particular has been associated with larger infarct sizes and worse neurological outcomes [38]. Multiple studies have found higher troponin I and T concentrations correlated with larger infarct volumes and worse neurologic impairment [37]. Elevated troponin also predicts poorer functional outcomes, with each incremental increase in levels corresponding to a stepwise decrease in Glasgow Coma Scale scores and motor function [39]. The mechanisms behind stroke-induced myocardial damage and cardiac enzyme elevation are multifactorial. An overwhelming sympathetic response activates the hypothalamic-pituitary-adrenal axis and causes a catecholamine surge, predisposing to arrhythmias and ischemic injury. Systemic inflammation triggered by the stroke spreads oxidative and metabolic stress to the heart. Disruption of neurovascular autoregulation further compromises coronary blood flow [32]. While the extent of cardiac enzyme release depends on individual factors like age, sex, and premorbid health status, universal screening aids early identification of patients at higher risk of cardiogenic complications. Assays for troponin and creatine kinase therefore form an important part of the standard cardiovascular workup for all acute stroke admissions [40]. Myocardial enzymes and biomarkers of cardiac dysfunction after SAH and ischemic stroke (IS) are summarized in Table 1.
Table 1.
Summary of biomarkers of cardiac dysfunction after stroke in patients
| Ischemic stroke | Hemorrhagic stroke | |
|---|---|---|
| CK-MB | Modest and progressive increase in CK-MB levels, which may not be of cardiac origin [41] | CK-MB level is associated with poor outcome in patients with SAH [42]. Serum levels of both CK-MB and cTnT were higher in patients with hemorrhagic stroke than those with ischemic stroke, but this difference was not significant [43] |
| cTn | Elevated cTns can be observed in 5–8% of IS patients. Serum cTnT expression is associated with myocardial injury, ischemic lesion volume and the risk of death in patients with acute IS [44, 45] | cTnI level peaks within 24–36 h after SAH. High cTnT levels correlated with increased risk of LVDD, in-hospital morbidity, and mortality [46, 47] |
| CRP | Elevated CRP level is an independent predictor of future stroke and transient ischemic attack [48] | Increased plasma CRP level within 72 h of stroke onset battle high mortality because of cardiovascular complications [49]. Elevated CRP levels in the very early phase of hemorrhagic stroke were associated with poor clinical outcomes and prognosis [50] |
| NT-proBNP | NT-proBNP levels are increased after ischemic stroke and is an independent risk factor for stroke and cardioembolic cause [44, 51] | NT-proBNP increases the risk of death and delayed cerebral ischemia [44] |
Communication between the Brain and the Heart after Stroke
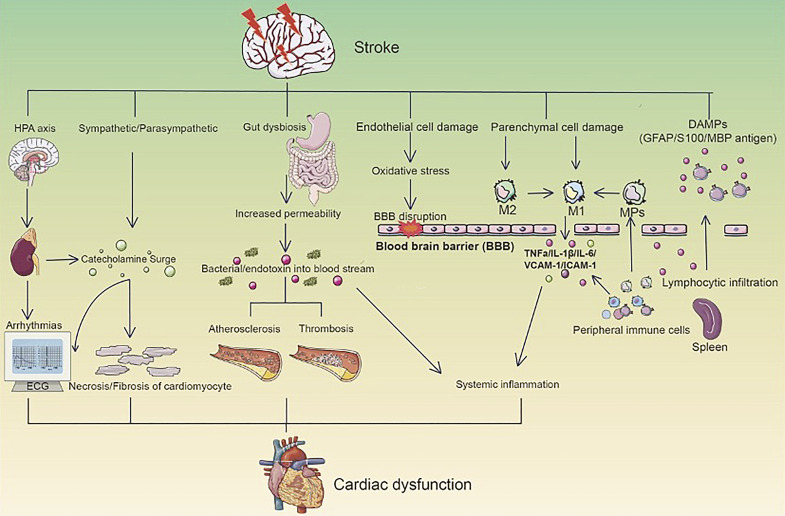
The underlying mechanisms and communication between the brain and the heart leading to cardiac dysfunction is summarized in the following section. As shown in Figure 1, stroke-induced cardiac dysfunction involves the activation of the hypothalamic-pituitary-adrenal (HPA) axis, sympathetic/parasympathetic regulation, gut dysbiosis, and inflammatory responses.
Fig. 1.
Mechanisms of brain-heart communication after stroke. A stroke event can activate bidirectional interactions through various pathways such as the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system, and gut-heart microbial interactions, leading to cardiac dysfunction. Activation of the HPA axis after stroke promotes glucocorticoid elevation and subsequent inflammatory responses and oxidative stress in cardiomyocytes. Autonomic dysfunction results in abnormal catecholamine levels that further impact cardiac electrophysiology. Additionally, gut microbiota imbalance following stroke may damage heart health via the gut-heart axis. Overall, a stroke elicits multi-level regulatory effects and network coupling to form the brain-heart axis, thereby inducing or exacerbating cardiac injury.
HPA Axis
Activation of the hypothalamic-pituitary-adrenal (HPA) axis plays a pivotal role in mediating stroke-induced cardiac complications [5]. The HPA axis is a key component of the neuroendocrine stress response system, regulating hormone production and release in response to stressors. At its core are the hypothalamus, pituitary gland, and adrenal glands [52]. The paraventricular nucleus (PVN) of the hypothalamus acts as the primary integrator that modulates HPA axis activity [53]. Upon stress detection, the PVN secretes corticotropin-releasing factor (CRF), which stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH) into circulation. ACTH then signals the adrenal cortex to secrete glucocorticoids, primarily cortisol in humans [54]. Elevated cortisol levels provide metabolic support during acute stress, but prolonged elevation can induce cardiac pathology if left unchecked [55]. Clinical and experimental evidence suggests stroke triggers a neuroendocrine storm mediated by the HPA axis that contributes to myocardial injury. Several studies have reported increased plasma cortisol concentrations post-stroke that positively correlate with infarct severity. Persistently high cortisol levels beyond the acute phase are also linked to worse functional outcomes and increased mortality risk [56]. At the cellular level, cortisol promotes inflammation and oxidative stress, impairing cardiomyocyte integrity [57, 58]. Concurrently, the PVN receives cardiac afferent input and projects to key brain regions modulating autonomic tone such as the rostral ventrolateral medulla. Animal experiments revealed PVN stimulation augments sympathetic outflow, precipitating arrhythmias [59, 60]. Ischemic stroke models demonstrate significant PVN activation that exacerbates cardiac dysfunction partly through N-methyl-D-aspartate receptor overstimulation [60, 61]. While the precise downstream mechanisms warrant more exploration, concerted evidence supports that stroke activates the HPA axis and sympathetic nervous system, predisposing the heart to insults like inflammation, ischemia, and ionic disturbances via neuroendocrine and autonomic pathways [62]. Developing therapeutic strategies targeting aberrant HPA axis signaling post-stroke may prove clinically beneficial for mitigating resultant cardiac risks.
Autonomic Nervous System
The autonomic nervous system (ANS), comprising sympathetic and parasympathetic branches, is critical for maintaining normal cardiac function and rhythm, and plays a pivotal role in mediating the influence on the heart post-stroke [63]. Several brain regions implicated in ANS control are susceptible to injury from both hemorrhagic and ischemic strokes. Strokes involving the insular lobe are frequently companied with both bradycardia and heart blocks (including third-degree block), as well as tachyarrhythmias with either atrial (supraventricular tachycardia, atrial flutter/fibrillation) or ventricular (ventricular tachycardia/fibrillation and torsade de pointes) origin [64].
The insular cortex, a key node in cognitive-autonomic integration, is particularly vulnerable. Insular stroke disrupts cardiovascular regulation and predicts poorer cardiac outcomes [65]. Ischemic insular lesions increase sympathetic predominance, leading to arrhythmias and myocardial injury. Compared with left cerebral infarction, the incidence of right cerebral infarction and arrhythmia is higher. In addition, damage to the right insular lobe is associated with more complex arrhythmias than any other site [66]. The right hemisphere primarily controls sympathetic activity, while the left hemisphere controls parasympathetic activity. Lateralization of insular function also impacts cardiac consequences – a right insular lesion leads to a decrease in sympathetic activity accompanied by parasympathetic overactivity [67]. Comparatively, left insular strokes confer less cardiac risk due to preserved parasympathetic balance [68]. In addition, infarctions in the right hemisphere are associated with a lower risk of adverse cardiac outcomes and increased long-term mortality [69]. Activation of the sympathetic nervous system can be observed in patients in the acute phase of SAH, which further induces myocardial injuries and leads to the progression of cardiac dysfunction. Thus, intensive monitoring of heart function is crucial for patients with injuries in the insular cortex. Due to the insular cortex's primary role, evaluating regional stroke location aids prognostication. Intensive hemodynamic support and autonomic modulation may help optimize cardiac outcomes, especially in patients with injuries to sites governing ANS control. Further research addressing region-specific therapies guided by advanced neuroimaging could impact secondary prevention strategies.
Catecholamine Surge
The catecholamine surge hypothesis is one of the predominant mechanisms of brain-heart interaction, which links cardiac dysfunction to physical or emotional stressors [60]. Contraction-band necrosis (CBN) and mitochondrial dysfunction are the outcomes of the catecholamine storm at the level of the myocardium, which can be observed after neurological events. A study reported that CBN was observed in approximately 26% of patients with non-cerebral death, and the proportion increased to 89% in patients with SAH and 52% with ischemic stroke [70]. Activation of the HPA axis can be observed after the onset of ischemic stroke, which induces a significant increase in catecholamines, leading to cardiac hypertrophy or myocardial ischemia [61]. The release of catecholamines is predominantly regulated by the autonomic nervous system, which can be induced to additionally secrete the neurotransmitter [55]. Neurological events can lead to excessive catecholamine secretion in the circulatory system, especially in the myocardial nerve endings, which leads to myocardial injury. The sympathetic nervous system can directly secrete catecholamines and lead to long-term elevation of serum catecholamine levels, which may trigger cardiotoxicity, inflammation and necrosis. Increased levels of catecholamines can also be observed after ischemic stroke, accounting for the occurrence of myocardial lesions and injuries. In addition, elevated levels of catecholamines following SAH are associated with QT-interval prolongation and myocardial injury. Catecholamines regulate cardiac function via β-receptors and play a critical role in enhancing myocardial strength and contraction [71]. The β-receptors activate adenylyl cyclase and increase cytosolic cAMP, which further binds to protein kinase A and phosphorylates sarcolemmal L-type Ca2+ channels [58]. Mitochondrial Ca2+ overload induces oxidative stress and the subsequent opening of its inner membrane permeability transition pore along with osmotic swelling. In addition, loss of ATP synthesis results in cell death. Administering β-blockers after Takotsubo cardiomyopathy can attenuate myocardial lesions, and some studies have reported a lack of significant effect of β-blockers on the recurrence rate of Takotsubo syndrome [58]. While the predominant source is neurogenic release from the sympathetic nervous system, the hypothalamic-pituitary-adrenal axis likely augments the storm through adrenal medullary epinephrine/norepinephrine secretion [72]. Further clarifying relative contributions may refine management strategies. Overall, studies consistently demonstrate that neuronal injury acutely increases catecholamine levels, which directly stress the myocardium through β-adrenergic mechanisms. Blocking this pathway shows promise in mitigating post-stroke cardiac complications.
Blood-Brain Barrier Disruption
The neurovascular unit is consistent with the interplay among glial, neuronal, and vascular components within the brain [73]. The blood-brain barrier (BBB) is the core of the neurovascular unit, based on a basement membrane and contains a wide range of cells, which is considered a dynamic interface between the peripheral circulation and the brain [74–76]. It has been demonstrated that the BBB can prevent brain damage due to variations in blood composition, while disruption of the BBB is the main step in the pathophysiological cascade after stroke and results in secondary tissue damage [77]. Furthermore, the invasion of inflammatory factors into the brain is facilitated via a highly permeable BBB and results in neuronal injuries, which further increases the permeability of the barrier [78]. It has been reported that increased BBB permeability can be observed in more than 50% of patients who undergo cardiac surgery, even in the absence of a stroke [79]. In addition, BBB disruption induces the entry of brain-derived substances into the blood and regulates peripheral immune cells. Disruption of the BBB induces the entry of peripheral immune cells and inflammatory factors into the brain, which further increases the permeability of BBB, thus forming a positive loop.
Immune and Inflammatory Responses
Activation of the immune system plays a critical role in the progression of stroke [80, 81]. A complex interaction exists between local and systemic inflammatory responses after stroke onset [82–84]. A wide range of immune cells and circulating signals are involved in this progression. In this section, we will describe the immune and inflammatory responses that mediate the brain-heart axis after stroke onset. Endothelial cell damage induces oxidative stress and leads to the disruption of the BBB after stroke onset. Damage to the parenchymal cells (neurons, astrocytes and oligodendrocytes) can promote polarization of the M1 phenotype of resident microglia and infiltrated macrophages. Systemic inflammation is predominantly induced by a variety of cytokines and chemokines, sympathetic/parasympathetic regulation and peripheral immune cells. In addition, stroke-caused gut dysbiosis results in the entry of bacteria and endotoxins into the bloodstream, leading to systemic inflammation.
Endothelial Cell Damage
Endothelial cells play an important role in the regulation of proinflammatory reactions and induction of cell damage [85]. In the acute period after stroke, local immune and inflammatory responses involving cytolysis and cytokine release are activated and induce the activation of endothelial cells [86]. Endothelial cell damage can be observed after stroke onset via the release of reactive oxygen species (ROS), which further induces oxidative stress and BBB disruption [87, 88]. Moreover, damaged endothelial cells release a variety of substances (especially extracellular vesicles), cross the BBB and enter the blood. Endothelial apoptosis and necrosis following oxidative bombardment release danger-associated molecular patterns such as mitochondria-derived extracellular vesicles into circulation [89]. After reaching various organs, an acute innate immune response is activated that mediates the entry of peripheral immune cells into the damaged brain sites [90].
Infiltrated Macrophages
Infiltration of peripheral macrophages and neutrophils is facilitated by disruption of the BBB after a stroke [91–93]. After stroke onset, increased extracellular ATP levels can be observed due to the depolarization of neurons and glia, which then activate resident microglia, induce inflammatory responses and injure the plasma membrane of brain cells [94]. Microglia/macrophages are of the M1 phenotype and promote inflammatory responses by releasing proinflammatory cytokines or chemokines, including interleukins (IL-6, IL-1β), tumor necrosis factor (TNF-α), integrins, vascular cell adhesion protein (VCAM), intercellular adhesion molecule (ICAM) and chemokines [95]. These proinflammatory molecules mediate the recruitment of immune cells and the induction of systemic inflammation [96]. Additionally, CD74-positive cells (including monocytes, macrophages and dendritic cells) play an important role in the regulation of immune responses after stroke. A study reported that the number of CD74-positive cells was significantly elevated in the peripheral circulation of patients with ischemic stroke. Chronically-activated macrophages disturb the neurovascular unit through TNFα and IL-1β actions on endothelial nuclear factor-κB, worsening blood-brain barrier integrity and remote organ barrier permeability [97]. Dysregulated leukocyte trafficking disseminates pathogenic signals to distal organs like the heart through cell surface/secreted molecules initiating inflammation-induced injury cascades in susceptible tissues. Therapies modulating macrophage polarization show promise, as M2a induction lessens proinflammatory cytokines while enhancing reparative functions without immune suppression [98]. Leukocyte infiltration is a critical node linking central neuroinflammation to systemic complications through barrier disruption and protracted activation of monocytes/macrophages after stroke. Optimizing post-stroke immunity may reduce multi-organ morbidity.
Damage-Associated Molecular Patterns
Damage-associated molecular patterns (DAMPs) include a wide range of heat shock proteins released by various damaged cells after stroke. In addition, brain-derived antigens (BDA), including glial fibrillary acidic protein, S100, and myelin basic protein (MBP), are released from astrocytes, neuron or oligodendrocytes [99–102]. Both DAMPs and BDA can pass the ruptured BBB and enter the blood stream. DAMPs can promote the production of cytokines and chemokines by the activation of Toll-like receptors (TLRs) [103]. TLR4 is a member of TLR family, which is dominantly localized on cardiomyocytes [104]. It has been illustrated that both activation of TLR4 and chronic inflammation induced the progression of heart failure and also predicted the poor prognosis of patients with heart diseases. According to a previous study based on the stroke models, lymphocytic response to the MBP results in secretion of transforming growth factor-β (TGF-β) and the response of regulatory T lymphocytes [105]. However, with the influence of systemic inflammation, the immune responses are dominantly mediated by T helper cells 1 and proinflammatory cytokines [106]. TGF-β can elevate the level of IL-6 and inhibit the levels of MCP-1 and VCAM-1 and also promote the transition of fibroblasts to myofibroblasts [107, 108]. By engaging similar pattern recognition receptors on distant organ beds, circulating DAMPs/BDAs help initiate or amplify injury cascades in susceptible sites like the heart through recruitment of proinflammatory monocytes and induction of innate immune signaling pathways [109]. Blocking DAMP-TLR ligation holds therapeutic potential but requires precise targeting to mitigate inflammatory sequelae without compromising beneficial responses like debris clearance. Achieving the right context-specific balance is key. In summary, stroke-induced DAMP release into circulation forms an essential link between CNS injury detection and propagation of immunopathology to remote organs via damage alarm signaling, highlighting their critical mechanistic role.
Sympathetic Activity and Inflammatory Responses
Studies have reported the interaction between sympathetic activity and inflammatory responses in the progression of hemorrhagic and ischemic strokes [110–112]. Damaged cell-derived proinflammatory cytokines can increase the sympathetic output and catecholamine level in the bloodstream by stimulating the posterior hypothalamus. A study reported that catecholaminergic stress coincided with the influx of immune cells into the heart and induced cardiac damage in patients with SAH. Moreover, the release of catecholamines and parasympathetic dysfunction can aggravate myocardial dysfunction and induce cardiomyocyte death. In the heart, β1-adrenergic stimulation triggers oxidative stress and calcium overload, predisposing cardiomyocytes to injury. Through neuroimmune communication circuits, the autonomic nervous system propagates injury signals between the brain and body. Inhibiting sympathetic surges may dampen deleterious neurogenic immune activation and catecholamine cardiotoxicity [113]. Therefore, inflammatory responses serve as the link between brain events and cardiac injuries after stroke.
Systemic Inflammatory Responses
Systemic inflammatory responses can be dominantly enhanced by a variety of cytokines, chemokines, and sympathetic regulation [114]. The immune responses are involved in the early phases after stroke and closely related to the progression of hemorrhagic and ischemic stroke. It has been revealed that systemic inflammatory responses play a critical role in subarachnoid hemorrhage (SAH), intracranial hemorrhage (ICH), and ischemic stroke, which also carry elevated risk of various complications, including hydrocephalus, vasospasm, and systemic secondary complications [115, 116]. It has been reported that approximately 50% of patients with SAH companied with systemic inflammatory response syndrome on admission, which can be observed in about 85% of patients after admission [117]. Systemic inflammatory response syndrome is characterized by elevated leukocytes, high heart and respiratory rate, and abnormal body temperature, which further induces the occurrence of various complications [117]. It has been demonstrated that lymphocytic influx can be observed at 48 h after acute ischemic stroke. Infiltrated T lymphocytes will further enhance detrimental inflammatory cascades and lead to delayed brain injuries. Although, to some extent, the infiltration of leukocytes is beneficial to the brain after stroke, excessive leukocytes could result in elevated intracranial pressure and poor clinical outcomes, or even mortality. The spleen is the mediator of the peripheral immune responses to stroke and presents an essential role in regulating circulatory lymphocytes and proinflammatory cytokines, such as TNF-α, monocyte chemoattractant protein-1 (MCP1), interferon-γ (IFNγ), and IL-6, and exacerbating neuroinflammation in the acute phase after stroke. It has been demonstrated that splenectomy after chronic heart failure can remarkedly improve LV systolic function and reduce cardiomyocyte hypertrophy, which emphasized the role of spleen in regulation of cardiac function [114]. In the later period of stroke, splenic atrophy leads to the decreased T-lymphocytic proliferation and presented immunosuppression.
Gut Microbiome Dysbiosis and Cardiac Dysfunction
Experimental studies have revealed a close link between the gut microbiome and cardiac dysfunction (gut-heart axis), as well as the CNS (brain-gut axis) [115, 118]. The gut-blood barrier is the main form of defense from toxins and pathogenic microorganisms and regulates the absorption of water and nutrients [119]. However, in several diseases, including metabolic, gastrointestinal, cardiovascular and cerebrovascular diseases, the integrity of the gut-blood barrier can be disrupted, leading to increased permeability and permitting the entry of microbiota-derived molecules [120]. Moreover, another cause of gut dysbiosis is prophylactic broad-spectrum antibiotic treatment for pneumonia and urinary tract infection prevention after stroke [121, 122]. In particular, gut microbiome dysbiosis after stroke onset further results in the entry of bacteria and endotoxins into the bloodstream and induces systemic inflammation. In this section, we will discuss the links between the gut-heart axis and brain-gut axis in homeostasis and disease.
Brain-Gut Axis
The brain-gut axis is mediated by neural and humoral pathways and a wide range of signaling molecules, including cytokines, chemokines, hormones and peptides, which play an essential role in regulating immune responses and lymphocyte populations [123, 124]. The brain-gut axis can affect the normal functioning [125] of the brain and induce the pathological cascade in neurological events, especially in stroke. Acute brain injury-induced gut dysbiosis can activate neuroinflammatory and immune responses within the brain and subsequently impair neurological function [126]. A clinical study reported that gut dysbiosis, characterized by elevated levels of opportunistic bacteria and decreased beneficial bacteria, could be observed in patients with large-artery atherosclerotic ischemic stroke or transient ischemic attack (TIA), wherein the increased [127] level of Proteobacteria in the gut was proportional to the severity of stroke. In addition, commensal gut microbiota plays a protective role against the brain damage caused by ischemic stroke, while depletion or dysbiosis can lead to mortality and affect the outcomes after stroke [128]. Intestinal paralysis-induced gut dysbiosis has been reported to alter T lymphocytic hemostasis, involving the migration of T lymphocytes from the intestine towards the brain and the activation of proinflammatory reactions, which further worsen the brain damage after stroke, leading to poor clinical prognosis. A previous study based on a mouse model of transient focal cerebral ischemia reported that intestinal T lymphocytes were trafficked into the meninges of the brain, wherein they induced neuroinflammation and the release of IL-17, which further facilitated the infiltration of immune cells into the damaged brain and promoted the secretion of proinflammatory cytokines [129]. Dysbiosis associates with post-stroke systemic inflammation, mediated partly by bacterial metabolites regulating microbiota-immune crosstalk [130]. The vagus nerve innervates the gut-brain axis, with vagal signaling maintaining intestinal barrier integrity and symbiosis [131]. Preclinical models demonstrate vagus nerve stimulation suppresses circulating TNF-α and IL-1β, mitigating neuroinflammation [132]. Clinically, vagal blocking devices show anti-inflammatory effects in rheumatoid arthritis [133]. Additionally, the hypothalamic-pituitary-adrenal axis and sympathetic activation propagate inflammation between organs via neuroendocrine circuits [134]. Catecholamines modulate T cell trafficking and cytokine production [135]. In addition, stroke onset was found to increase the permeability of the gut and facilitate the invasion of bacteria into the lymph nodes and various organs, including the liver, spleen and lung, and activate immune reactions, which were proportional to the severity and poor outcomes [136]. Furthermore, it has been revealed that gut dysbiosis and elevated bacterial counts are related to systemic inflammation in patients with ischemic stroke, while the bacterial metabolites also mediate the balance between gut microbiota and proinflammatory immune responses [137, 138]. Modulating neuro-enteric pathways may help reshape the post-stroke immune landscape. Multi-targeted therapies incorporating prebiotics, probiotics, dietary nutrients, and neurostimulation techniques show promise to curb remote organ inflammation by normalizing host-microbiota crosstalk.
Gut-Heart Axis
Increased gut permeability activates inflammatory reactions, and systemic inflammatory responses can further increase the permeability of the intestine, thereby forming a positive loop [118]. In addition, the entry of bacteria and endotoxins into the bloodstream can induce the secretion of proinflammatory cytokines and activate systemic inflammation, which further leads to the exacerbation of cardiac dysfunction [119, 120]. An independent clinical trial on a large cohort revealed that three gut microbiota metabolites (choline, trimethylamine-n-oxide, and betaine) were the predictive factors for cardiovascular disease [139]. Trimethylamine-n-oxide is released by the gut microbes and directly contributes to the hyperreaction of platelets and induces thrombosis [140]. Furthermore, the altered levels of trimethylamine-n-oxide are closely related to various forms of cardiac dysfunction [119, 140]. Murine models demonstrate antibiotic-mediated changes in gut microbiota composition attenuate cardiac inflammatory injury [141]. Inhibiting cardiotoxic bacterial species skews microbiota profiles toward cardioprotection, mitigating experimental autoimmune myocarditis [142]. Prebiotics/probiotics favorably modulate trimethylamine-n-oxide levels and generation of cardioprotective metabolites like short-chain fatty acids through directed microbiota metabolism [143]. Intestinal pretreatment with potential probiotics limits endotoxin absorption and systemic dissemination after inflammatory injuries. Neurotransmitters/hormones like serotonin and ghrelin likewise stabilize gut barriers. In summary, the gut-heart axis represents an important avenue by which remote organ dysfunction manifests following stroke. Multi-pronged restoration of gut symbiosis and barrier function through modulation of the intestinal ecosystem holds promise to mitigate cardiac sequelae.
Conclusions
In summary, cardiac dysfunction after stroke is commonly encountered in clinical practice, which affects the clinical outcomes, and even causes mortality. In addition, secondary brain injuries caused by impaired homeostasis and activated inflammatory responses are clinical challenges that require further attention. The pathophysiology of cardiac dysfunction likely involves the complicated interaction between the neuroendocrine system, catecholamine release and neuroinflammation. Although there are specific interventions for cardiac complications after stroke onset, guidelines for the management of these complications are lacking. Further studies are required to better understand the role of cardiac dysfunction on the prognosis of patients with stroke and to develop effective therapeutic strategies for neurogenic cardiac dysfunction. Furthermore, more clinical indicators should be identified to diagnose cardiac dysfunction in the early stages, thereby enabling prompt interventions that can enhance the therapeutic effects and reduce mortality. However, there are some limitations to our literature review. Studies of targeting therapeutic strategies based on brain-heart axis with possible neuroprotective effects on stroke are still in the exploratory stage, and clinical studies are yet to be conducted. Regarding basic research, the establishment of more novel therapeutic strategies to protect cardiac function and alleviate inflammatory responses warrants further investigation. Various mechanisms are summarized in this work, and although they may seem independent of each other, potential links and interconnections are likely to exist. A further comprehensive understanding of these different mechanisms is desired, with a preference for understanding them as interconnected networks.
Conflict of Interest Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding Sources
There were no sources of funding associated with this manuscript.
Author Contributions
X.C. and J.G. wrote the main manuscript text; X.C. and X.Z. prepared Figure 1. All authors reviewed the manuscript.
Funding Statement
There were no sources of funding associated with this manuscript.
References
- 1. Liu Q, Liu F, Li J, Huang K, Yang X, Chen J, et al. Sedentary behavior and risk of incident cardiovascular disease among Chinese adults. Sci Bull. 2020;65(20):1760–6. [DOI] [PubMed] [Google Scholar]
- 2. Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, et al. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66:1325–9. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Zhang A, Yu Q, Wang Z, Wang J, Xu P, et al. Single-cell RNA sequencing and spatial transcriptomics reveal pathogenesis of meningeal lymphatic dysfunction after experimental subarachnoid hemorrhage. Adv Sci. 2023;10(21):e2301428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheshire WP Jr, Saper CB. The insular cortex and cardiac response to stroke. Neurology. 2006;66(9):1296–7. [DOI] [PubMed] [Google Scholar]
- 5. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-heart interaction: cardiac complications after stroke. Circ Res. 2017;121(4):451–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Fang Y, Zhang Z, Luo Y, Zhang A, Lenahan C, et al. Ferroptosis: an emerging therapeutic target in stroke. J Neurochem. 2022;160(1):64–73. [DOI] [PubMed] [Google Scholar]
- 7. Kulaklı F, Koklu K, Ersoz M, Ozel S. Relationship between urinary dysfunction and clinical factors in patients with traumatic brain injury. Brain Inj. 2014;28(3):323–7. [DOI] [PubMed] [Google Scholar]
- 8. Yousef KM, Balzer JR, Bender CM, Hoffman LA, Poloyac SM, Ye F, et al. Cerebral perfusion pressure and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Am J Crit Care. 2015;24(4):e65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z, Zhang A, Liu Y, Hu X, Fang Y, Wang X, et al. New mechanisms and targets of subarachnoid hemorrhage: a focus on mitochondria. Curr Neuropharmacol. 2022;20(7):1278–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang A, Liu Y, Xu H, Zhang Z, Wang X, Yuan L, et al. CCL17 exerts neuroprotection through activation of CCR4/mTORC2 axis in microglia after subarachnoid haemorrhage in rats. Stroke Vasc Neurol. 2022;8(1):4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Silva-Pereira C, Albino-Teixeira A, Sousa T. Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol. 2021;12:746494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byer E, Ashman R, Toth LA. Electrocardiograms with large, upright T waves and long Q-T intervals. Am Heart J. 1947;33(6):796–806. [DOI] [PubMed] [Google Scholar]
- 13. Heavner JE. Cardiac dysrhythmias induced by infusion of local anesthetics into the lateral cerebral ventricle of cats. Anesth Analg. 1986;6(4):309–8. [PubMed] [Google Scholar]
- 14. Lavy S, Yaar I, Melamed E, Stern S. The effect of acute stroke on cardiac functions as observed in an intensive stroke care unit. Stroke. 1974;5(6):775–80. [DOI] [PubMed] [Google Scholar]
- 15. Samuels MA. The brain-heart connection. Circulation. 2007;116(1):77–84. [DOI] [PubMed] [Google Scholar]
- 16. Sposato LA, Lam M, Allen B, Richard L, Shariff SZ, Saposnik G. First-ever ischemic stroke and increased risk of incident heart disease in older adults. Neurology. 2020;94(15):e1559–70. [DOI] [PubMed] [Google Scholar]
- 17. Pelliccia F, Pasceri V, Patti G, Tanzilli G, Speciale G, Gaudio C, et al. Long-term prognosis and outcome predictors in Takotsubo syndrome: a systematic review and meta-regression study. JACC Heart Fail. 2019;7(2):143–54. [DOI] [PubMed] [Google Scholar]
- 18. Winbo A, Paterson DJ. The brain-heart connection in sympathetically triggered inherited arrhythmia syndromes. Heart Lung Circ. 2020;29(4):529–37. [DOI] [PubMed] [Google Scholar]
- 19. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. [DOI] [PubMed] [Google Scholar]
- 20. Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis. 2002;14(2):67–76. [DOI] [PubMed] [Google Scholar]
- 21. Yamasaki T, Hayashi K, Shibata Y, Furuta T, Yamamoto K, Uchimura M, et al. Takotsubo cardiomyopathy following mechanical thrombectomy for acute ischemic stroke: illustrative case. J Neurosurg Case Lessons. 2021;2(9):Case21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prathep S, Sharma D, Hallman M, Joffe A, Krishnamoorthy V, Mackensen GB, et al. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Crit Care Med. 2014;42(1):142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruggieri F, Cerri M, Beretta L. Infective rhomboencephalitis and inverted Takotsubo: neurogenic-stunned myocardium or myocarditis? Am J Emerg Med. 2014;32(2):191.e1–3. [DOI] [PubMed] [Google Scholar]
- 24. Krishnamoorthy V, Sharma D, Prathep S, Vavilala MS. Myocardial dysfunction in acute traumatic brain injury relieved by surgical decompression. Case Rep Anesthesiol. 2013;2013:482596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dombrowski K, Laskowitz D. Cardiovascular manifestations of neurologic disease. Handb Clin Neurol. 2014;119:3–17. [DOI] [PubMed] [Google Scholar]
- 26. Yoshimura S, Toyoda K, Ohara T, Nagasawa H, Ohtani N, Kuwashiro T, et al. Takotsubo cardiomyopathy in acute ischemic stroke. Ann Neurol. 2008;64(5):547–54. [DOI] [PubMed] [Google Scholar]
- 27. van der Bilt IA, Hasan D, van den Brink RB, Cramer MJ, van der Jagt M, van Kooten F, et al. Time course and risk factors for myocardial dysfunction after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2015;76(6):700–6; discussion 705–6. [DOI] [PubMed] [Google Scholar]
- 28. Escudero-Martínez I, Morales-Caba L, Segura T. Atrial fibrillation and stroke: a review and new insights. Trends Cardiovasc Med. 2023;33(1):23–9. [DOI] [PubMed] [Google Scholar]
- 29. Christensen H, Fogh Christensen A, Boysen G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci. 2005;234(1–2):99–103. [DOI] [PubMed] [Google Scholar]
- 30. Friberg L, Rosenqvist M, Lindgren A, Terént A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45(9):2599–605. [DOI] [PubMed] [Google Scholar]
- 31. Arab D, Yahia AM, Qureshi AI. Cardiovascular manifestations of acute intracranial lesions: pathophysiology, manifestations, and treatment. J Intensive Care Med. 2003;18(3):119–29. [DOI] [PubMed] [Google Scholar]
- 32. Mihalovic M, Tousek P. Myocardial injury after stroke. J Clin Med. 2021;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frontera JA, Parra A, Shimbo D, Fernandez A, Schmidt JM, Peter P, et al. Cardiac arrhythmias after subarachnoid hemorrhage: risk factors and impact on outcome. Cerebrovasc Dis. 2008;26(1):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mochmann HC, Scheitz JF, Petzold GC, Haeusler KG, Audebert HJ, Laufs U, et al. Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: the troponin elevation in acute ischemic stroke (TRELAS) study. Circulation. 2016;133(13):1264–71. [DOI] [PubMed] [Google Scholar]
- 35. Bell RD, Alexander GM, Nguyen T, Albin MS. Quantification of cerebral infarct size by creatine kinase BB isoenzyme. Stroke. 1986;17(2):254–60. [DOI] [PubMed] [Google Scholar]
- 36. Aydin S, Ugur K, Aydin S, Sahin İ, Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag. 2019;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oras J, Grivans C, Bartley A, Rydenhag B, Ricksten SE, Seeman-Lodding H. Elevated high-sensitive troponin T on admission is an indicator of poor long-term outcome in patients with subarachnoid haemorrhage: a prospective observational study. Crit Care. 2016;20:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dous GV, Grigos AC, Grodman R. Elevated troponin in patients with acute stroke: is it a true heart attack? Egypt Heart J. 2017;69(3):165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miketic JK, Hravnak M, Sereika SM, Crago EA. Elevated cardiac troponin I and functional recovery and disability in patients after aneurysmal subarachnoid hemorrhage. Am J Crit Care. 2010;19(6):522–9. quiz 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Radhakrishnan S, Moorthy S, Gadde S, Madhavan K. Role of cardiac biomarkers in the assessment of acute cerebrovascular accident. J Neurosci Rural Pract. 2021;12(1):106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ay H, Arsava EM, Sarıbaş O. Creatine kinase-MB elevation after stroke is not cardiac in origin: comparison with troponin T levels. Stroke. 2002;33(1):286–9. [DOI] [PubMed] [Google Scholar]
- 42. Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30(4):780–6. [DOI] [PubMed] [Google Scholar]
- 43. Apak I, Iltumur K, Tamam Y, Kaya N. Serum cardiac troponin T levels as an indicator of myocardial injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp Med. 2005;205(2):93–101. [DOI] [PubMed] [Google Scholar]
- 44. Cushman M, Judd SE, Howard VJ, Kissela B, Gutiérrez OM, Jenny NS, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45(6):1646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krause T, Werner K, Fiebach JB, Villringer K, Piper SK, Haeusler KG, et al. Stroke in right dorsal anterior insular cortex Is related to myocardial injury. Ann Neurol. 2017;81(4):502–11. [DOI] [PubMed] [Google Scholar]
- 46. Bruder N, Rabinstein A. Cardiovascular and pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):257–69. [DOI] [PubMed] [Google Scholar]
- 47. Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35(2):548–51. [DOI] [PubMed] [Google Scholar]
- 48. Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32(11):2575–9. [DOI] [PubMed] [Google Scholar]
- 49. Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001;32(4):917–24. [DOI] [PubMed] [Google Scholar]
- 50. Mazaheri S, Reisi E, Poorolajal J, Ghiasian M. C-reactive protein levels and clinical outcomes in stroke patients: a prospective cohort study. Arch Iran Med. 2018;21:8–12. [PubMed] [Google Scholar]
- 51. Yip HK, Sun CK, Chang LT, Chen MC, Liou CW. Time course and prognostic value of plasma levels of N-terminal pro-brain natriuretic peptide in patients after ischemic stroke. Circ J. 2006;70(4):447–52. [DOI] [PubMed] [Google Scholar]
- 52. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Herman JP, Tasker JG. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol. 2016;7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kageyama K, Iwasaki Y, Daimon M. Hypothalamic regulation of corticotropin-releasing factor under stress and stress resilience. Int J Mol Sci. 2021;22:12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. M Costa V, Carvalho F, L Bastos M, A Carvalho R, Carvalho M, Remião F. Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr Med Chem. 2011;18(15):2272–314. [DOI] [PubMed] [Google Scholar]
- 56. Barugh AJ, Gray P, Shenkin SD, MacLullich AM, Mead GE. Cortisol levels and the severity and outcomes of acute stroke: a systematic review. J Neurol. 2014;261(3):533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fioranelli M, Bottaccioli AG, Bottaccioli F, Bianchi M, Rovesti M, Roccia MG. Stress and inflammation in coronary artery disease: a review psychoneuroendocrineimmunology-based. Front Immunol. 2018;9:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saini HK, Tripathi ON, Zhang S, Elimban V, Dhalla NS. Involvement of Na+/Ca2+ exchanger in catecholamine-induced increase in intracellular calcium in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;290(1):H373–80. [DOI] [PubMed] [Google Scholar]
- 59. Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256(6 Pt 2):R1325–30. [DOI] [PubMed] [Google Scholar]
- 60. Jia S, Xia Q, Zhang B, Wang L. Involvement of the paraventricular nucleus in the occurrence of arrhythmias in middle cerebral artery occlusion rats. J Stroke Cerebrovasc Dis. 2015;24(4):844–51. [DOI] [PubMed] [Google Scholar]
- 61. Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, et al. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res. 2010;106(11):1763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bourhy L, Mazeraud A, Bozza FA, Turc G, Lledo PM, Sharshar T. Neuro-inflammatory response and brain-peripheral crosstalk in sepsis and stroke. Front Immunol. 2022;13:834649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gordan R, Gwathmey JK, Xie LH. Autonomic and endocrine control of cardiovascular function. World J Cardiol. 2015;7(4):204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Christensen H, Boysen G, Christensen AF, Johannesen HH. Insular lesions, ECG abnormalities, and outcome in acute stroke. J Neurol Neurosurg Psychiatry. 2005;76(2):269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Laredo C, Zhao Y, Rudilosso S, Renu A, Pariente JC, Chamorro A, et al. Prognostic significance of infarct size and location: the case of insular stroke. Sci Rep. 2018;8(1):9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cheung RT, Hachinski V. The insula and cerebrogenic sudden death. Arch Neurol. 2000;57(12):1685–8. [DOI] [PubMed] [Google Scholar]
- 67. Hachinski VC, Smith KE, Silver MD, Gibson CJ, Ciriello J. Acute myocardial and plasma catecholamine changes in experimental stroke. Stroke. 1986;17(3):387–90. [DOI] [PubMed] [Google Scholar]
- 68. Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004;35(9):2094–8. [DOI] [PubMed] [Google Scholar]
- 69. Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66(4):477–63; discussion 463. [DOI] [PubMed] [Google Scholar]
- 70. Taggart P, Critchley H, Lambiase PD. Heart-brain interactions in cardiac arrhythmia. Heart. 2011;97(9):698–708. [DOI] [PubMed] [Google Scholar]
- 71. Moss RL, Fitzsimons DP, Ralphe JC. Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium. Circ Res. 2015;116(1):183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Byrne CJ, Khurana S, Kumar A, Tai TC. Inflammatory signaling in hypertension: regulation of adrenal catecholamine biosynthesis. Front Endocrinol. 2018;9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yan L, Moriarty RA, Stroka KM. Recent progress and new challenges in modeling of human pluripotent stem cell-derived blood-brain barrier. Theranostics. 2021;11(20):10148–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol. 2019;4(2):78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Poon C, Pellow C, Hynynen K. Neutrophil recruitment and leukocyte response following focused ultrasound and microbubble mediated blood-brain barrier treatments. Theranostics. 2021;11(4):1655–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mamtilahun M, Jiang L, Song Y, Shi X, Liu C, Jiang Y, et al. Plasma from healthy donors protects blood-brain barrier integrity via FGF21 and improves the recovery in a mouse model of cerebral ischaemia. Stroke Vasc Neurol. 2021;6(4):561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Merino JG, Latour LL, Tso A, Lee KY, Kang DW, Davis LA, et al. Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol. 2013;34(3):518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shi K, Wood K, Shi FD, Wang X, Liu Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc Neurol. 2018;3(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc Neurol. 2019;4(2):71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liao Y, Cheng J, Kong X, Li S, Li X, Zhang M, et al. HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics. 2020;10(21):9644–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Y, Jin H, Wang Y, Yao Y, Yang C, Meng J, et al. Sult2b1 deficiency exacerbates ischemic stroke by promoting pro-inflammatory macrophage polarization in mice. Theranostics. 2021;11(20):10074–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Barca C, Wiesmann M, Calahorra J, Wachsmuth L, Döring C, Foray C, et al. Impact of hydroxytyrosol on stroke: tracking therapy response on neuroinflammation and cerebrovascular parameters using PET-MR imaging and on functional outcomes. Theranostics. 2021;11(9):4030–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Song K, Zeng X, Xie X, Zhu R, Liang J, Chen G, et al. Dl-3-n-butylphthalide attenuates brain injury caused by cortical infarction accompanied by cranial venous drainage disturbance. Stroke Vasc Neurol. 2022;7(3):222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Simats A, Liesz A. Systemic inflammation after stroke: implications for post-stroke comorbidities. EMBO Mol Med. 2022;14(9):e16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gauberti M, Montagne A, Quenault A, Vivien D. Molecular magnetic resonance imaging of brain-immune interactions. Front Cell Neurosci. 2014;8:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Doll DN, Barr TL, Simpkins JW. Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 2014;5:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Di Mambro T, Pellielo G, Agyapong ED, Carinci M, Chianese D, Giorgi C, et al. The tricky connection between extracellular vesicles and mitochondria in inflammatory-related diseases. Int J Mol Sci. 2023;24(9):8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dickens AM, Tovar-Y-Romo LB, Yoo SW, Trout AL, Bae M, Kanmogne M, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473):eaai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139(1–2):93–101. [DOI] [PubMed] [Google Scholar]
- 92. Wang D, Liu F, Zhu L, Lin P, Han F, Wang X, et al. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J Neuroinflammation. 2020;17(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fang W, Zhai X, Han D, Xiong X, Wang T, Zeng X, et al. CCR2-dependent monocytes/macrophages exacerbate acute brain injury but promote functional recovery after ischemic stroke in mice. Theranostics. 2018;8(13):3530–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liesz A, Zhou W, Mracskó É, Karcher S, Bauer H, Schwarting S, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134(Pt 3):704–20. [DOI] [PubMed] [Google Scholar]
- 96. Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12(2):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang Y, Leak RK, Cao G. Microglia-mediated neuroinflammation and neuroplasticity after stroke. Front Cell Neurosci. 2022;16:980722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yuwen Y, Wang X, Liu J, Liu Z, Zhu H. Delta- like ligand 4- expressing macrophages and human diseases: insights into pathophysiology and therapeutic opportunities. Heliyon. 2023;9(10):e20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18(6):911–7. [DOI] [PubMed] [Google Scholar]
- 100. Becker K. Autoimmune responses to brain following stroke. Transl Stroke Res. 2012;3:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jin W, Wu Y, Chen N, Wang Q, Wang Y, Li Y, et al. Early administration of MPC-n(IVIg) selectively accumulates in ischemic areas to protect inflammation-induced brain damage from ischemic stroke. Theranostics. 2021;11(17):8197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xu X, Shi Y, Luan P, Kou W, Li B, Zhai M, et al. The subcellular redistribution of NLRC5 promotes angiogenesis via interacting with STAT3 in endothelial cells. Theranostics. 2021;11(9):4483–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158(3):1007–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pradillo JM, Fernández-López D, García-Yébenes I, Sobrado M, Hurtado O, Moro MA, et al. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem. 2009;109(1):287–94. [DOI] [PubMed] [Google Scholar]
- 105. Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25(12):1634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dabbagh K, Lewis DB. Toll-like receptors and T-helper-1/T-helper-2 responses. Curr Opin Infect Dis. 2003;16(3):199–204. [DOI] [PubMed] [Google Scholar]
- 107. Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW, et al. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Boen JRA, Gevaert AB, De Keulenaer GW, Van Craenenbroeck EM, Segers VFM. The role of endothelial miRNAs in myocardial biology and disease. J Mol Cell Cardiol. 2020;138:75–87. [DOI] [PubMed] [Google Scholar]
- 109. Roth S, Wernsdorf SR, Liesz A. The role of circulating cell-free DNA as an inflammatory mediator after stroke. Semin Immunopathol. 2023;45(3):411–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Winklewski PJ, Radkowski M, Demkow U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflammation. 2014;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. van der Bilt IA, Vendeville JP, van de Hoef TP, Begieneman MP, Lagrand WK, Kros JM, et al. Myocarditis in patients with subarachnoid hemorrhage: a histopathologic study. J Crit Care. 2016;32:196–200. [DOI] [PubMed] [Google Scholar]
- 112. Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135(4):1295–307. [DOI] [PubMed] [Google Scholar]
- 113. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve: an integrative interface between two supersystems – the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 114. Vahidy FS, Parsha KN, Rahbar MH, Lee M, Bui TT, Nguyen C, et al. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J Cereb Blood Flow Metab. 2016;36(6):1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bao Z, Zhang Z, Zhou G, Zhang A, Shao A, Zhou F. Novel mechanisms and therapeutic targets for ischemic stroke: a focus on gut microbiota. Front Cell Neurosci. 2022;16:871720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang A, Zhang Z, Zhang WB, Wang X, Lenahan C, Fang Y, et al. Development of a nomogram for predicting clinical outcome in patients with angiogram-negative subarachnoid hemorrhage. CNS Neurosci Ther. 2021;27(11):1339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care. 2008;8(3):404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nagatomo Y, Tang WH. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail. 2015;21(12):973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Adrie C, Parlato M, Salmi L, Adib-Conquy M, Bical O, Deleuze P, et al. Bacterial translocation and plasma cytokines during transcatheter and open-heart aortic valve implantation. Shock. 2015;43(1):62–7. [DOI] [PubMed] [Google Scholar]
- 121. Lucas SE, Walton SL, Mirabito Colafella KM, Mileto SJ, Lyras D, Denton KM. Antihypertensives and antibiotics: impact on intestinal dysfunction and hypertension. Hypertens (Dallas, Tex. 2023;80(7):1393–402. [DOI] [PubMed] [Google Scholar]
- 122. Liang P, Shan W, Zuo Z. Perioperative use of cefazolin ameliorates postoperative cognitive dysfunction but induces gut inflammation in mice. J Neuroinflammation. 2018;15(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–8. [DOI] [PubMed] [Google Scholar]
- 124. Zhang S, Kong C, Yang Y, Cai S, Li X, Cai G, et al. Human oral microbiome dysbiosis as a novel non-invasive biomarker in detection of colorectal cancer. Theranostics. 2020;10(25):11595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dinan TG, Cryan JF. The microbiome-gut-brain Axis in health and disease. Gastroenterol Clin North Am. 2017;46(1):77–89. [DOI] [PubMed] [Google Scholar]
- 126. Mörkl S, Butler MI, Holl A, Cryan JF, Dinan TG. Probiotics and the microbiota-gut-brain Axis: focus on psychiatry. Curr Nutr Rep. 2020;9(3):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. 2016;47(5):1354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22(5):516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Huang W, Zhu L, Song W, Zhang M, Teng L, Wu M. Crosstalk between the gut and brain in ischemic stroke: mechanistic insights and therapeutic options. Mediators Inflamm. 2022;2022:6508046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kanai T, Teratani T. Role of the vagus nerve in the gut-brain Axis: development and maintenance of gut regulatory T cells via the liver-brain-gut vago-vagal reflex. Brain Nerve. 2022;74(8):971–7. [DOI] [PubMed] [Google Scholar]
- 132. Fang YT, Lin YT, Tseng WL, Tseng P, Hua GL, Chao YJ, et al. Neuroimmunomodulation of vagus nerve stimulation and the therapeutic implications. Front Aging Neurosci. 2023;15:1173987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kanashiro A, Shimizu Bassi G, de Queiróz Cunha F, Ulloa L. From neuroimunomodulation to bioelectronic treatment of rheumatoid arthritis. Bioelectron Med (Lond). 2018;1(2):151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jiang J, Zhou D, Zhang A, Yu W, Du L, Yuan H, et al. Thermogenic adipocyte-derived zinc promotes sympathetic innervation in male mice. Nat Metab. 2023;5(3):481–94. [DOI] [PubMed] [Google Scholar]
- 135. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Crapser J, Ritzel R, Verma R, Venna VR, Liu F, Chauhan A, et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging. 2016;8(5):1049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12(2):e0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Liu Y, Chen LJ, Li XW, Yang JZ, Liu JL, Zhang KK, et al. Gut microbiota contribute to Methamphetamine-induced cardiotoxicity in mouse model. Chem Biol Interact. 2023;379:110512. [DOI] [PubMed] [Google Scholar]
- 142. Wang J, Zhang X, Yang X, Yu H, Bu M, Fu J, et al. Revitalizing myocarditis treatment through gut microbiota modulation: unveiling a promising therapeutic avenue. Front Cell Infect Microbiol. 2023;13:1191936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhen J, Zhou Z, He M, Han HX, Lv EH, Wen PB, et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol. 2023;14:1085041. [DOI] [PMC free article] [PubMed] [Google Scholar]