Abstract
Purpose:
This study aims to minimize monitor units (MUs) of intensity-modulated treatments in the Monaco treatment planning system while preserving plan quality by optimizing the “Minimum Segment Width” (MSW) and “Fluence Smoothing” parameters.
Materials and Methods:
We retrospectively analyzed 30 prostate, 30 gynecological, 15 breast cancer, 10 head and neck tumor, 11 radiosurgery, and 10 hypo-fractionated plans. Original prostate plans employed “Fluence Smoothing” = Off and were reoptimized with Low, Medium, and High settings. The remaining pathologies initially used MSW = 0.5 cm and were reoptimized with MSW = 1.0 cm. Plan quality, including total MU, delivery time, and dosimetric constraints, was statistically analyzed with a paired t-test.
Results:
Prostate plans exhibited the highest MU variation when changing “Fluence Smoothing” from Off to High (average ΔMU = −5.1%; P < 0.001). However, a High setting may increase overall MU when MSW = 0.5 cm. Gynecological plans changed substantially when MSW increased from 0.5 cm to 1.0 cm (average ΔMU = −29%; P < 0.001). Organs at risk sparing and planning target volumes remained within 1.2% differences. Replanning other pathologies with MSW = 1.0 cm affected breast and head and neck tumor plans (average ΔMU = −168.38, average Δt = −11.74 s, and average ΔMU = −256.56, average Δt = −15.05 s, respectively; all with P < 0.004). Radiosurgery and hypofractioned highly modulated plans did not yield statistically significant results.
Conclusions:
In breast, pelvis, head and neck, and prostate plans, starting with MSW = 1.0 cm optimally reduces MU and treatment time without compromising plan quality. MSW has a greater impact on MU than the “Fluence Smoothing” parameter. Plans with high modulation might present divergent behavior, requiring a case-specific analysis with MSW values higher than 0.5 cm.
Keywords: Minimum segment width, monitor units, radiosurgery, volumetric-modulated arc therapy
INTRODUCTION
The development of intensity-modulated radiotherapy techniques has enhanced absorbed dose sparing and improved dose conformity, although the number of monitor units (MUs) has also increased compared to traditional three-dimensional (3D) techniques.[1,2,3] The dose rate (MU/min) and the number of total MU relate the prescribed dose to the delivery time in radiotherapy. At dose rate, an increase in MU leads to a longer irradiation time. Involuntary patient movements and secondary radiation due to a longer delivery time escalated the risk of developing long-term secondary cancers.[4,5,6,7,8,9] Moreover, the prolonged treatment duration results in a reduced number of patients that can be treated per day.
In the past few years, there has been an increase in the survival rate of patients with primary cancer. Due to the latency period associated with tumors spanning several years, there is a growing concern about mitigating the risk of long-term secondary cancers by minimizing both the number of MU and the delivery time during radiation therapy. This concern becomes particularly significant when addressing pediatric patients, who are more susceptible to the adverse effects of radiation exposure.[4,5,6,7,8,9]
Intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) techniques rely on inverse planning algorithms. The introduction of a greater number of small dynamic fields in intensity-modulated techniques implies additional investigation to mitigate the MU rising and overall delivery time, provided that the plan quality is not altered.[1,2,3] Primarily, total MU, delivery time, quality indices, and dosimetric criteria of planning target volume (PTV) and of organs at risks (OARs) define plan quality in radiotherapy treatment.[10]
When using inverse planning algorithms, the total MUs do not follow a linear relationship with the prescribed dose. Factors such as the use of a beam flattening filter (megavoltage (MV) energy) or the presence of the multileaf collimator (MLC) might significantly impact the overall MU of the plan. Employing advanced technological flattening filter-free (FFF) energy typically leads to a reduction in MU.[11,12]
The algorithm utilized by Monaco® treatment planning system (TPS) v. 5.5.1 (Elekta CMS, Maryland Heights, MO, USA) is grounded in an iterative Monte Carlo method. Within the “Planning” section, the user encounters the subsections “Calculation Properties” and “Sequencing Parameters.” The parameters “Fluence Smoothing” and “Minimum Segment Width (cm)” (MSW) are tools within the subsection “Sequencing Parameters,” employed for the calculating of the plan after optimizing the dosimetric constraints. Allowed values for “Fluence Smoothing” parameter are Off, Low, Medium, or High. The MSW parameter values range from 0.01 cm to 2.00 cm.
This work aims to find a practical method of reducing MU in intensity-modulated plans primarily utilizing 6 MV energy, while simultaneously preserving plan quality through the utilization of the “Fluence Smoothing” and “Minimum Segment Width” Monaco tools. The impact of these tools on delivery time is also investigated. Retrospective analysis was conducted on plans employing IMRT and VMAT techniques in different pathological sites to achieve a global, practical, and comprehensive method of reducing MU.
MATERIALS AND METHODS
Plans of different tumor sites were retrospectively selected, ranging from the initial stages of this work to the last calculated plans (October 2021–January 2023). They were planned in the TPS Monaco® v. 5.5.1 (Elekta CMS, Maryland Heights, MO, USA) and administered in the Versa HD LINAC with Agility MLC, previously commissioned and of clinical use at our hospital.
First, the original plan optimizations were reviewed by our physics team. Dosimetric constraints and beam arrangements, including table or collimator angulations, were optimally selected in consensus for each plan to achieve consistent results applicable to different forms of optimization when modifications are introduced.
Throughout the optimization process, Monaco TPS requires the assessment of “Sequencing Parameters” and “Calculation Properties” tools. The “Calculation Properties” parameters considered for geometry and beam arrangement were the voxel size, 2 mm for stereotactic body radiotherapy (SBRT) and radiosurgery and 3 mm in the rest of the pathologies. For VMAT, the maximum number of control points was set at 180. The statistical uncertainty of calculation was 3% per control point in IMRT and 1% per calculation point in VMAT. VMAT plans were optimized with two arcs. Absorbed dose deposition calculation was in Medium. “Fluence Smoothing” and MSW are “Sequencing Parameters” tools and they are the focal points of the investigation in this study.
Prescribed doses and OARs constraints goals are from our hospital’s internal radiation oncology quality assurance program [Table 1]. For prostate plans, the prostate plus the seminal vesicles are embedded in one PTV, and an internal SBRT hypofractioned protocol is employed. In gynecological plans, the PTV englobes the pelvis, lymph nodes, parametrical tissue, and tumor bed after uterine removal. In breast tumor plans, two PTVs are defined, the mammary gland PTV and the tumor bed PTV; and two dose fractionations are distinguished, hypofractionation and SBRT or extreme hypo-fractionation. For head-and-neck locations (H and N) tumors, PTVs cover regions such as paranasal sinuses, nasopharynx, oropharynx, hypopharynx, oral cavity, tongues, and salivary glands. Three prescription dose levels are categorized based on tumor volume, affected nodes, and nonaffected nodes, and two dose fractionations are defined. OARs dosimetric goals are the same for both prescription doses. SBRT plans include small pulmonary, cranial, and vertebral PTVs, for a number of sessions higher than one. Radiosurgery (stereotactic radiosurgery [SRS]) plans, defined with a single ablative session, are tumors of small brain metastases (V mean = 5 cm3; range [2.1–11] cm3) from lung adenocarcinomas. OARs dosimetric constraints are stated in Table 1 for each prescription dose.
Table 1.
Features of patient cohorts, prescription doses, organs at risk and dosimetric goals. In breast tumors, each dosimetric goals line corresponds to its respective dose prescription line
| Sites | ntot | n6MV-n6FFF | Technique | Number PTVs | Dose prescriptions | OARs | Constraint goals |
|---|---|---|---|---|---|---|---|
| Prostate SBRT | 30 | 306MV | IMRT and VMAT | 1 | 35Gy; 5fx | Bladder | V32Gy <40%, Dmean <37.5Gy |
| Rectum | V28Gy <40%, V33Gy <32% | ||||||
| Femoral heads | D2% <50Gy | ||||||
| Small bowel | V40Gy <120cm3 | ||||||
| Gynecological | 30 | 306MV | IMRT and VMAT | 1 | 46Gy; 23fx | Bladder | V37.5Gy <60%, Dmean <42.6Gy |
| Rectum | V37.5 <35%, V45Gy <60%, Dmean <42.6Gy | ||||||
| Femoral heads | V32Gy <12%, D2% <50Gy | ||||||
| Small bowel | V40Gy <150 cm3 | ||||||
| Breast | 15 | 156MV | VMAT | 2 | 48Gy, 40.5Gy; 15fx 29Gy, 26Gy; 5fx | Heart | V16Gy <5%, V8Gy <30%, Dmean <2.5Gy |
| Ipsi-lateral lung | V1.5Gy <15%, V7Gy <15%, Dmean <2.5Gy | ||||||
| Contra-lateral lung | V16Gy <15%, V8% <35%, V4Gy <50% | ||||||
| Contra-lateral breast | V8Gy <15% V4Gy <10%, Dmean <6.5Gy | ||||||
| V4Gy <10%, Dmean <6.5Gy | |||||||
| V4Gy <30%, V10Gy <2% | |||||||
| V4Gy <30%, V10Gy <2% | |||||||
| H&N | 10 | 106MV | VMAT | 3 | 66Gy, 60Gy, 54Gy; 30fx 69.96Gy, 59.4Gy, 54.12Gy; 33fx | Spinal cord | <45Gy |
| Parotids | Dmean <26Gy, V30Gy <50% | ||||||
| Larynx | Dmean <40Gy, V50Gy <60% | ||||||
| Thyroid | Dmean <40Gy, V45Gy <67% | ||||||
| submaxillary glands | Dmean <32Gy | ||||||
| Oral cavity | Dmean <30Gy or 40Gy | ||||||
| Cochleae | Dmean <36Gy, V5Gy <55% | ||||||
| Brainstem | <56Gy, V30Gy <60% | ||||||
| Jaws | <70Gy | ||||||
| SBRTs | 3 vertebral 6 lung | 46MV-66FFF | VMAT | 1 | 16Gy; 2fx 50Gy; 10fx 60Gy; 10fx 60Gy, 8fx 30Gy; 5fx |
Spinal cord | <14Gy |
| MRI spinal cord | V10Gy <1% | ||||||
| Small bowel | <28.5Gy, V20.7Gy <30cm3, 19Gy <5cm3 <22.5Gy <50Gy <40Gy, D4 cm3 <18Gy | ||||||
| Sacral plexus | D0.5cm3 <39Gy or V40Gy <120 cm3 <55Gy | ||||||
| Aorta artery | V12.5Gy <20%, V40Gy <5% | ||||||
| Trachea | D10% <20Gy, D15% <12.5Gy <35Gy, V36.6 <15 cm3 | ||||||
| 1 cranial | Ribs | ||||||
| Large vessels | |||||||
| Ipsi-lateral lung | |||||||
| Contra-lateral lung | |||||||
| Heart | |||||||
| SRS | 11 | 86MV-36FF | VMAT | 1 | 18Gy; 1fx 21Gy; 1fx | Healthy brain | V12Gy <10cm3, V4Gy <23 cm3, <18Gy <10Gy <8Gy <7Gy, Dmean <6.5Gy <3Gy |
| Brainstem | |||||||
| Chiasm and optic nerves | |||||||
| Cochleae | |||||||
| Crystalline lens |
n: Number of patients, MRI: Magnetic resonance image, VMAT: Volumetric-modulated arc therapy, SBRT: Stereotactic body radiation therapy, SRS: Stereotactic radiosurgery, IMRT: Intensity-modulated radiation therapy, PTVs: Planning target volumes, OAR: Organs at risks
For all PTVs, homogeneity was analyzed with V95%, V107%; and coverage with D2%, D99%, and V100%. In the original plans, dose normalizations were V95%=95% for prostate and breast plans; and V95%=97% for H and N, SBRT, and SRS plans.
Prostate plans and gynecologic plans were calculated with sliding windows IMRT, with 7 or 9 gantry angles equally spaced over 360°, respectively. With VMAT, fractioned dose plans were calculated with one beam, and SRS employed from 2 to 5 noncoplanar beams. Every beam was optimized with two complete arcs, outgoing and returning.
Regarding IMRT prostate plans treated at our Hospital, “Fluence Smoothing” value is Off. They were replanned three times with the following values: Low, Medium, and High. In each reoptimization, the MSW value, the absorbed dose constraints, and all components of the whole dosimetric cost functions and beam arrangement remained unaltered to evaluate solely the influence of the “Fluence Smoothing” parameter. Prostate SBRT plans, characterized by a higher dose per fraction, were selected to evaluate any possible correlation between the “Fluence Smoothing” and total MU. In light of the obtained results, the focus of the investigation was reoriented toward the examination of the MSW parameter. This parameter appears to exert a more significant influence on the number of MU than the “Fluence Smoothing” parameter. The investigation of the MSW parameter was specifically focused on the gynecological anatomical site, characterized by heightened complexity. Consequently, a greater variability in the MU was expected, attributable to the greater modulation requirements. Original IMRT and VMAT pelvic plans were calculated with MSW = 0.5 cm, and replanned with MSW = 1.0 cm, regardless of the “Fluence Smoothing” value. On confirming the dependency of the MSW parameter on both MU and irradiation time, the examination of MSW values was extended to encompass additional tumor sites.
Differences in number of MU, delivery time, and the cumulative dose-volume histogram (DVH) were analyzed with a t-test of paired samples (α = 0.005) in normally distributed samples, and with the Wilcoxon test (α = 0.005) in nonnormally distributed samples. Previously, an assessment of the normality of sample distributions was carried out through both quantitative and qualitative analyses. The quantitative assessment involved the application of the Shapiro–Wilk test (α = 0.005), while qualitative assessment utilized the quantile–quantile plot (Q-Q plot). Shapiro–Wilk test determined whether the Student’s t-test (P > 0.005) or the Wilcoxon test (P < 0.005) is applied.
Relative differences were normalized to the original plan calculated using MSW = 0.5 cm. Assuming a rate of four patients per hour with a workload of 55 patients treated daily, and considering the average difference in delivery time per patient obtained when introducing the MSW variation, an estimation is made of the additional number of patients that might be treated. Delivery time is an output of Monaco after plan calculation.
The association between plan quality and MSW was qualitatively assessed with a 2 × 2 contingency table. Plans categorized as either unacceptable or clinically improvable were designated as “exposed cases.”
The optimized and calculated plans were irradiated to verify their reproducibility. The pretreatment verification criterion mandated that a minimum of 90%, and preferably at least 95%, of measurement points satisfy the volumetric and global gamma index criterion Г (3%, 2 mm), with a low-dose threshold set at 10% of the global maximum. For plans involving small volumes with high modulation requirements, the gamma index criterion was Г (2%, 2 mm). The plans were measured using the Octavius Detector 1500 and Octavius Detector 1000 SRS, which are embedded into the Octavius 4D phantom (PTW Freiburg, Germany).
RESULTS
Samples normality
As a remark in prostate SBRT plans, MU variation in samples where “Fluence Smoothing” changed from Off to Low or to Medium obtained a value of P = 0.005 in Shapiro–Wilk and the linear and clustered trend in the center of Q-Q plots confirmed normality. In gynecological cancer plans, MU variation in the IMRT sample replanned with VMAT, and regardless of the MSW value, the Shapiro–Wilk test confirmed normality (P = 0.101 > 0.005), but with a lower P value than the rest of the samples (P > 0.005). Normality was confirmed in the Q-Q plot.
Results from “Fluence smoothing” and minimum segment width variation in prostate and gynecological sites
The influence of “Fluence Smoothing,” MSW, and the introduction of the VMAT technique on MU and irradiation time for prostate and pelvic pathologies are in Table 2.
Table 2.
Average MU and delivery time differences for prostate and gynecological tumor sites
| Anatomical sites | Fixed | Change introduced | x | P | x̅ | CI95% Inf | CI95% Sup |
|---|---|---|---|---|---|---|---|
| Prostate | - | Fluence Smoothing Off-High | ΔMU | <0.001 | -102.72 | -136.78 | -68.64 |
| - | Fluence Smoothing Off-High | ΔMU(%) | <0.001 | -5.08 | -6.65 | -3.51 | |
| MSW=0.5cm | Fluence Smoothing Off-Low | ΔMU | 0.004 | 4.41 | 0.12 | 8.79 | |
| Gynecological | - | MSW 0.5cm-1.0cm | ΔMU(%) | <0.001 | -29.02 | -33.11 | -24.92 |
| IMRT | MSW 0.5cm-1.0cm | ΔMU(%) | <0.001 | -20.68 | -25.80 | -15.56 | |
| VMAT | MSW 0.5cm-1.0cm | ΔMU(%) | <0.001 | -37.56 | -40.81 | -33.90 | |
| - | MSW 0.5cm-1.0cm | ΔMU(%) | 0.513* | -4.27 | -13.27 | 7.68 | |
| - | IMRT- VMAT | ΔMU(%) | <0.001 | 21.46 | 13.55 | 29.37 | |
| MSW=1.0cm | IMRT-VMAT | ΔMU(%) | 0.019* | 6.87 | 1.28 | 12.47 | |
| MSW=0.5cm | IMRT-VMAT | ΔMU(%) | <0.001 | 36.06 | 24.42 | 47.69 | |
| - | MSW 0.5cm-1.0cm | Dt (s) | <0.001 | -42.15 | -50.39 | -33.91 | |
| IMRT | MSW 0.5cm-1.0cm | Dt (s) | <0.001 | -60.33 | -78.64 | -42.02 | |
| VMAT | MSW 0.5cm-1.0cm | Dt (s) | <0.001 | -34.78 | -45.40 | -24.17 | |
| - | IMRT-VMAT | Dt (s) | <.001 | -236.12 | -259.85 | -211.30 | |
| - | MSW 0.5cm-1.0cm | Dt(%) | <.001 | -28.32 | -37.11 | -14.50 | |
| IMRT | MSW 0.5cm-1.0cm | Dt(%) | <.001 | -14.08 | -46.16 | -9.27 | |
| VMAT | MSW 0.5cm-1.0cm | Dt(%) | <.001 | -10.89 | -13.24 | -8.25 | |
| - | IMRT- VMAT | Dt(%) | <.001 | -45.30 | -48.59 | -40.20 |
*Not significant. MSW: “Minimum Segment Width”. CI: Confidence Interval, VMAT: Volumetric-modulated arc therapy, IMRT: Intensity-modulated radiation therapy, MU=Monitor units
“Fluence Smoothing” in prostate cancer plans
The expected relative MU reduction was confirmed when changing from Off to High,  = −5.1% (P < 0.001), with a 95% confidence interval (6.6%–−3.5%). In absolute terms,
= −5.1% (P < 0.001), with a 95% confidence interval (6.6%–−3.5%). In absolute terms,  = −103MU, and the highest variation, −241MU.
= −103MU, and the highest variation, −241MU.
Results were not statistically significant when changing from Off to Medium or from Off to Low.
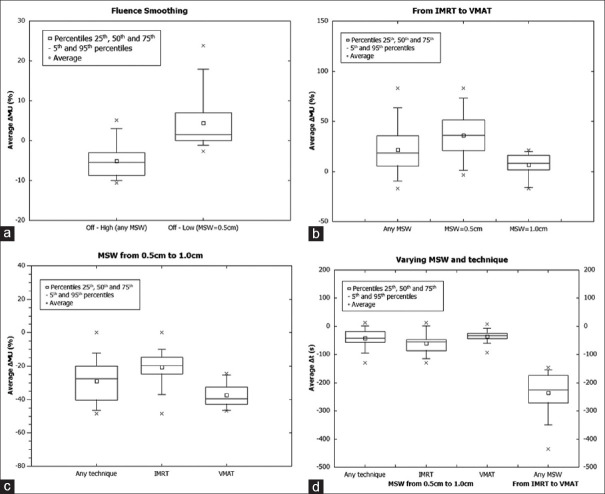
Changing from Off to Low showed an increase in MU in 73% of plans, instead of a reduction as expected. To elucidate this trend, the parameter MSW was examined in plans exhibiting a MU rising in this sample. In plans with MU increase, 63% featured a parameter MSW value of 0.5 cm; while the remaining plans had a value of 1.0 cm. Therefore, the sample was divided into subgroups with MSW = 0.5 cm and with MSW = 1.0 cm to examine the influence of MSW. The results were statistically significant in the Wilcoxon test in the MSW = 0.5 cm sample with  = +95MU or
= +95MU or  = +4.4% (P = 0.004 < 0.005). The P value is higher than desirable and close to the threshold, which may be due to a reduced sample size. Contrary to anticipated outcomes, the number of MU increased [Figure 1a].
= +4.4% (P = 0.004 < 0.005). The P value is higher than desirable and close to the threshold, which may be due to a reduced sample size. Contrary to anticipated outcomes, the number of MU increased [Figure 1a].
Figure 1.
Results in prostate and gynecological plans. (a) Average monitor unit (ΔMU) (%) when switching “Fluence Smoothing” in prostate tumor sites, (b) Average ΔMU (%) when switching planning technique for different minimum segment width (MSW) values in gynecological cancer plans, (c) Average ΔMU (%) when switching MSW for different planning techniques in gynecological cancer plans, (d) Average Δt(s) when switching MSW for different techniques and when switching from intensity-modulated radiation therapy to volumetric-modulated arc therapy for any MSW in gynecological cancer plans. MSW: Minimum segment width. MU: Monitor unit, IMRT: Intensity-modulated radiation therapy, VMAT: Volumetric-modulated arc therapy
The average variations in exposure time are <2%, it can therefore be assumed to be constant.
Implications of minimum segment width and selected technique in monitor unit for gynecological cancer plans
The MU increase when changing from IMRT to VMAT is confirmed  = +21.5% [Figure 1b]. Separating the previous sample into one with MSW = 0.5 cm and another with MSW = 1.0 cm, statistically significant results were obtained only for the MSW = 0.5 cm sample, with
= +21.5% [Figure 1b]. Separating the previous sample into one with MSW = 0.5 cm and another with MSW = 1.0 cm, statistically significant results were obtained only for the MSW = 0.5 cm sample, with  = +36%. Therefore, with a lower MSW value, the increase in MU is even more noteworthy.
= +36%. Therefore, with a lower MSW value, the increase in MU is even more noteworthy.
Regardless of the technique used, changing from MSW = 0.5 cm to MSW = 1.0 cm results in  = −29% or −424.5MU, with the maximum variation being −46.7% or −983.3MU [Figure 1c]. Separating the previous sample in two regarding the technique used, statistically significant results were obtained in both samples. With IMRT technique fixed,
= −29% or −424.5MU, with the maximum variation being −46.7% or −983.3MU [Figure 1c]. Separating the previous sample in two regarding the technique used, statistically significant results were obtained in both samples. With IMRT technique fixed,  = −20.7% and With VMAT,
= −20.7% and With VMAT,  = −37.6%. Therefore, although MU increases with the VMAT technique, the MSW tool enables effective mitigation of this effect with an appropriate value.
= −37.6%. Therefore, although MU increases with the VMAT technique, the MSW tool enables effective mitigation of this effect with an appropriate value.
Minimum segment width tool and selected technique influence on irradiation time in gynecological cancer plans
Regardless of the MSW value, changing from IMRT to VMAT implied 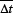 = −45% or − 236s. Extrapolated to an average of 55 patients treated per day and with a workload of 4 patients per hour, up to 14 more patients per day could be treated, which contrasts with
= −45% or − 236s. Extrapolated to an average of 55 patients treated per day and with a workload of 4 patients per hour, up to 14 more patients per day could be treated, which contrasts with  = +21.5% [see Figure 1d].
= +21.5% [see Figure 1d].
Regardless of the technique used, changing from MSW = 0.5–1.0 cm led to 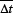 = −28.3% or −42s. With the same workload assumptions as in the previous statement, up to 2 more patients could be treated per day.
= −28.3% or −42s. With the same workload assumptions as in the previous statement, up to 2 more patients could be treated per day.
With the VMAT technique, 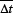 = −11% or − 35s; and up to 2 more patients could be treated per day.
= −11% or − 35s; and up to 2 more patients could be treated per day.
Influence of minimum segment width parameter in the remaining tumor locations
The MSW parameter dependence on MU and irradiation time in smaller numbers of patient cohort for the remaining tumor sites are illustrated in Table 3 and Figure 2.
Table 3.
Average MU and delivery time differences according to MSW in different tumor sites
| Anatomical sites | Change introduced | x | P | x̅ | CI95% Inf | CI95% Sup |
|---|---|---|---|---|---|---|
| Gynecological (with VMAT) | MSW from 0.5cm to 1.0cm | DMU(%) | <0.001 | -37.40 | -40.80 | -33.90 |
| Dt(%) | <0.001 | -10.90 | -13.20 | -8.74 | ||
| DMU | <0.001 | -677.90 | -776.40 | -579.52 | ||
| Dt (s) | <0.001 | -34.80 | -45.40 | -24.17 | ||
| Breast | MSW from 0.5cm to 1.0cm | DMU(%) | 0.007 | -16.80 | -21.30 | -10.80 |
| Dt(%) | 0.003 | -7.82 | -12.50 | -3.12 | ||
| DMU | <0.001 | -168.38 | -249.30 | -87.46 | ||
| Dt (s) | 0.004 | -11.74 | -19.10 | -4.39 | ||
| H&N | MSW from 0.5cm to 1.0cm | DMU(%) | <0.001 | -21.86 | -28.00 | -15.78 |
| Dt(%) | 0.002 | -7.83 | -11.90 | -3.79 | ||
| DMU | <0.001 | -256.56 | -340.70 | -172.43 | ||
| Dt (s) | 0.001 | -15.05 | -22.40 | -7.68 | ||
| SRS* | MSW from 0.5cm to 1.0cm | DMU(%) | 0.225* | -6.90 | -18.80 | 5.00 |
| Dt(%) | 0.824* | 0.65 | -5.69 | 7.00 | ||
| DMU | 0.411* | -110.72 | -398.35 | 176.92 | ||
| Dt (s) | 0.689* | 2.98 | -13.11 | 19.07 | ||
| MSW from 0.5cm to 1.0cm | DMU(%) | 0.194* | -9.02 | -23.89 | 5.84 | |
| SBRT* | Dt(%) | 0.363* | 4.21 | -6.03 | 14.46 | |
| DMU | 0.556* | -68.70 | -331.38 | 193.98 | ||
| Dt (s) | 0.278* | 9.92 | -10.04 | 29.89 | ||
| MSW from 0.5cm to 1.0cm | DMU(%) | <0.001 | -26.20 | -30.40 | -22.05 | |
| All** | Dt(%) | <0.001 | -9.22 | -10.90 | -7.42 | |
| DMU | <0.001 | -407.76 | -497.40 | -318.15 | ||
| Dt (s) | <0.001 | -22.16 | -28.20 | -16.20 |
*Not statistically significant, consequently excluded from “All”. **Gynecological with VMAT, breast and H&N plans. MSW: “Minimum Segment Width”. CI: Confidence interval, VMAT: Volumetric-modulated arc therapy, SBRT: Stereotactic body radiation therapy, SRS: Stereotactic radiosurgery, MU=Monitor units
Figure 2.
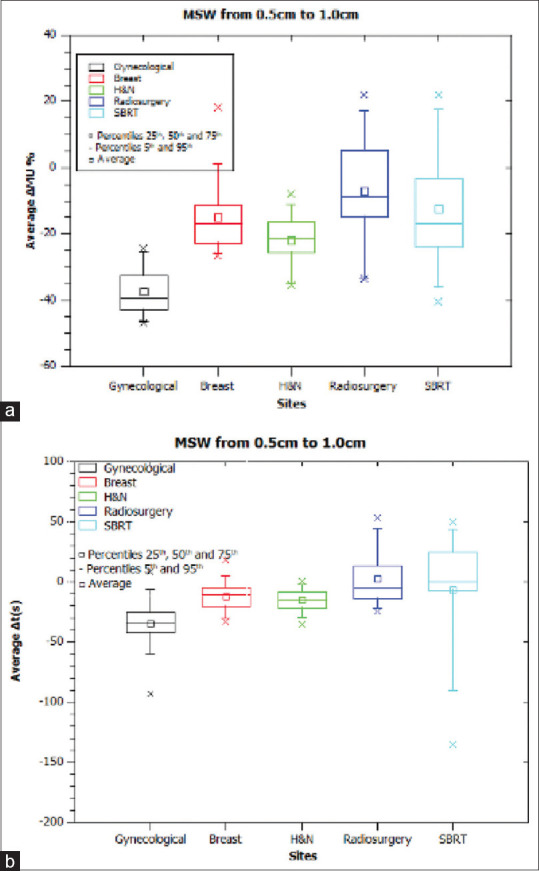
Results when modifying Minimum segment width (MSW) in all tumor sites. (a) Average Δmonitor unit (%) when changing MSW from 0.5 to 1.0 cm, (b) Average Δt(s) when changing MSW from 0.5 to 1.0 cm. MSW: Minimum segment width, MU: Monitor unit
Dosimetric indices goals
Regarding the dosimetric indices, plans have been qualitatively classified according to clinically improvable or unacceptable cases (“exposed”), and optimal cases (“nonexposed”). Cases are optimal as long as the plan meets the dosimetric constraints, PTV coverage is increased or preserved and/or OARs dose decreases. Contingency tables are shown in Table 4. The null hypothesis of the Chi-square test of association (α = 0.05) is that a higher value of MSW does not imply a worse plan quality. When P value is > 0.05, the null hypothesis is accepted.
Table 4.
Contingency 2×2 tables
| Fail plan quality | Pass plan quality | Total | P for Chi-square of association | |
|---|---|---|---|---|
| Breast | ||||
| MSW=1.0 | 0 | 15 | 15 | 0.55 |
| MSW=0.5 | 2 | 13 | 15 | |
| Total | 2 | 28 | 30 | |
| H and N | ||||
| MSW=1.0 | 1 | 9 | 10 | 0.41 |
| MSW=0.5 | 2 | 8 | 10 | |
| Total | 3 | 17 | 20 | |
| Gynecological | ||||
| MSW=1.0 | 1 | 29 | 30 | 0.47 |
| MSW=0.5 | 0 | 30 | 30 | |
| Total | 1 | 59 | 60 | |
| SRS | ||||
| MSW=1.0 | 4 | 7 | 11 | 0.16 |
| MSW=0.5 | 1 | 10 | 11 | |
| Total | 5 | 17 | 22 | |
| SBRT | ||||
| MSW=1.0 | 3 | 7 | 10 | 0.25 |
| MSW=0.5 | 1 | 9 | 10 | |
| Total | 4 | 16 | 20 | |
| All | ||||
| MSW=1.0 | 9 | 67 | 76 | 0.54 |
| MSW=0.5 | 6 | 70 | 76 | |
| Total | 15 | 137 | 152 |
SBRT: Stereotactic body radiation therapy, SRS: Stereotactic radiosurgery, MSW: Minimum segment width
All average differences in dosimetric indices met the dosimetric criteria and optimization constraints.
As remarks in SBRT prostate plans, mean differences were <1.2% in all dosimetric parameters (P < 0.001). The highest variations were observed in the bladder when changing “Fluence Smoothing” from Off to High in two isolated cases, with an increase of V32 Gy = 4% and of D¯ = 2.0 Gy; clinically acceptable variations.
In gynecological cancer plans, mean differences were <0.8% in all dosimetric parameters (P < 0.001). The highest variation was observed in isolation in bladder V37.5 Gy= +6% for an IMRT plan when changing MSW from 0.5 cm to 1.0 cm. Particularly, part of the bowel infiltrated PTV and the plan required the extra modulation provided by a lower MSW value. It is classified as “exposed.”
In breast tumors, when changing from MSW = 0.5 cm to MSW = 1.0 cm the changes are hardly clinically appreciable. The small variations are far from exceeding the dosimetric constraints. Several cases stand out for a considerable increase in PTV coverage when MSW = 1.0 cm and a decrease in volumes with maximum doses. Therefore, with an MSW = 0.5 cm, there might be an excess of modulation. Particularly, three plans are classified as optimal due to a higher V95% coverage in the breast PTV. Two of them with ΔV100%= +60% in the breast PTV, ΔV100%= +30% in the tumor bed; and both without increasing maximum dose. The third plan with ΔV100%= +18.2% in the breast, ΔV100%= +6.2% in the tumor bed.
In H and N locations, mean differences were <2% in all dosimetric parameters (P < 0.005), which is surprising due to the presence of high-dose gradients and OARs adjacent to PTVs. As remarks, one case was classified as optimal when increasing MSW to 1.0 cm, keeping unaltered the OARs indices and an optimum coverage in V100% and V95%, but decreasing by 77% the maximum dose in PTV,  = −273.38MU and
= −273.38MU and 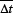 =−23s. It was noted that an improbable “exposed” case, characterized by minimum requested tumor coverage with V95%=95% in the three PTVs with MSW = 0.5 cm; becomes clinically acceptable when MSW = 1.0 cm, with ΔV100%= + (10%–15%) for the three PTVs and ΔV107%= +10% in the second highest prescription dose PTV. Despite the increase in the volume of maximum dose, it is clinically a more satisfactory plan. At last, one case where the highest prescription dose PTV variated ΔV100%= −12.82% is classified as “exposed” because of the coverage loss. The irregular tumor shape in this plan might indicate this is an extreme case.
=−23s. It was noted that an improbable “exposed” case, characterized by minimum requested tumor coverage with V95%=95% in the three PTVs with MSW = 0.5 cm; becomes clinically acceptable when MSW = 1.0 cm, with ΔV100%= + (10%–15%) for the three PTVs and ΔV107%= +10% in the second highest prescription dose PTV. Despite the increase in the volume of maximum dose, it is clinically a more satisfactory plan. At last, one case where the highest prescription dose PTV variated ΔV100%= −12.82% is classified as “exposed” because of the coverage loss. The irregular tumor shape in this plan might indicate this is an extreme case.
In SRS, 7 cases with a MU decrease led to a tumor coverage loss of ΔV100%= (−7.2%–−27.6%). The maximum delivery time decrease is 12s. OARs remain practically unaltered and all average differences are under 1% (P < 0.001). Original radiosurgery plans were optimized with higher priorities in OARs dosimetric constraints than in PTV dose targets, thus it was expected to obtain dosimetric variations in PTV coverage rather than in OARs when recalculating with a higher MSW. Neither MU nor delivery time variations are statistically significant; being V100% tumor coverage the main parameter that determines if the case is “exposed” or optimal. According to the energy 6 MV or 6 FFF, no differences are observed, although the small sample sizes should be noted.
In SBRT of several locations, when MU is decreased with MSW = 1.0 cm, a reduction in tumor coverage and an increase in maximum dose are observed. The treatment planning system (TPS) is not able to reproduce the required modulation with MSW = 1.0 cm. It seems reasonable to deduce that imposing an MSW of 1.0 cm may be counterproductive, as the TPS’s response is to modulate the maximum allowable modulation, increasing the highest dose without a corresponding increase in tumor coverage. No statistically significant results were obtained. Two extreme cases are noted. One favorable: ΔMU= −1688.2MU; Δt= −136s; OARs and PTVs variations <2%. One unfavorable: ΔV100%= −18.4%; ΔV95%= −4.29%; ΔV107%= +20%; ΔMU= −91.5MU, and Δt= +94.4s. Despite MU decrease, dosimetric indices get worse and the delivery time is longer.
In all plans except SRS and SBRT, the average decrease in treatment time is 22s. Extrapolated to daily workload, 1.3 patients per day are increased. Globally,  = −26%.
= −26%.
Pretreatment verifications
Pretreatment verification outcomes satisfy the gamma index criterion. In H and N plans, the percentage of points that pass the verification criterion is higher in plans with MSW = 1.0 cm. Consequently, plans with MSW = 1.0 cm exhibit greater reproducibility than those with MSW = 0.5 cm. OARs and PTVs dosimetric indices support the notion that the excessive modulation with MSW = 0.5 cm is not justified and introduces higher uncertainty in reproducibility. A positive variation of the percentage of points meeting the Г criterion gamma criterion (GP) ensures better treatment reproducibility. This variation is defined as the GP of MSW = 1.0 cm plan minus the GP of MSW = 0.5 cm plan.
According to anatomical locations, gynecological sites exhibit ΔGP (%) ranging between −1 and +4; with GP (Г [3%, 2 mm]) = (93.2%–98.3%) [see Supplementary Material]. In breast plans, ΔGP (%) range was between −1 and +5. The maximum variation corresponds to an extreme case favorable to change to MSW = 1.0 cm, with ΔMU= −273MU and Δt = −33s. The PTV is optimized with the presence of an expander-type implant, which might interfere heterogeneously with radiation. Both MSW = 0.5 cm and MSW = 1.0 cm plans meet the Г criterion with GP (Г [3%, 2 mm] = (91.9%–98.5%). In H and N location, ΔGP (%) ranged from −2 and + 19.4. The maximum variation is observed in a plan where the quality plan improves with MSW = 1.0 cm, with ΔV100%= +4%, Δ107%=1%, ΔMU= −373MU, and Δt= −23s. Considering all H and N plans, GP (Г [3%, 2 mm]) = [76.2%–98.7%]. Two cases with MSW = 0.5 cm do not meet the Г criterion, with GP values of 76.2% and 85.3%. Plan quality and the verification criterion are suitable when replanning with MSW = 1.0 cm. At last, in SRS and SBRT plans, obtained ΔGP (%) were between −1 and + 2; where the extreme variations corresponded to radiosurgeries. In the case with ΔGP (%) = −1%, coverage decreases by ΔV100%= −13%, ΔMU= −463MU, and Δt= −25s. Despite the reduction in MU and treatment time, PTV coverage with MSW = 0.5 cm plays a decisive role in plan selection. In the case with ΔGP(%)=+2%; the obtained results were ΔMU=-594MU and Δt=-17s. All plans passing the Г (2%, 2 mm) criterion exhibit GP values between (95.5% and 100%). The 1000 SRS detector, with lower uncertainty in spatial and dosimetric resolution, was expected to yield improved results.
DISCUSSION
The risk of inducing secondary malignancies due to secondary radiation dose in radiotherapy treatments is an issue of concern when introducing high-intensity modulation treatment techniques.[5,6,7,8,9] The flattening filter is one of the main sources of secondary radiation. Removal of the flattening filter allows for a dose rate up to four times higher and might mitigate the risk of secondary cancers by substantially reducing the irradiation time, particularly in hypo-fractioned treatments.[11,12,13,14] With the Monaco TPS tools of “Fluence Smoothing” and MSW, a practical method of MU and delivery time reduction that maintains the plan quality in high-modulated techniques treated with a flattening filter and 6 MV is investigated. As an assumption, a lower MU is obtained for higher values of both parameters.
An SBRT prostate protocol is selected to appreciate a greater change in the MU, given the low fractionation of the absorbed dose leads to a higher MU per fraction. In prostate plans, the expected impact of “Fluence Smoothing” on MU is confirmed. The greatest variation of MU occurs when changing from Off to High, with  = −5.1%= −103MU. The MU reduction is not particularly representative, but it might be noteworthy in specific cases that start from an excessive base modulation, with a potential reduction of up to 241MU. Since changing from Off to Medium or Low does not outcome substantial variations in MU, it is reasonable to deduce that the influence of this parameter exists but is not particularly essential. A closer examination of the Off-Low sample reveals that, in 73% of the cases, total MU increases, suggesting the presence of another parameter with a greater impact on MU calculation. In 63% of plans with increased MU, MSW is 0.5 cm, indicating that the influence of MSW on MU warrants further investigation. Moreover, it may be counterproductive to impose a higher value “Fluence Smoothing” in patients planned with a lower MSW.
= −5.1%= −103MU. The MU reduction is not particularly representative, but it might be noteworthy in specific cases that start from an excessive base modulation, with a potential reduction of up to 241MU. Since changing from Off to Medium or Low does not outcome substantial variations in MU, it is reasonable to deduce that the influence of this parameter exists but is not particularly essential. A closer examination of the Off-Low sample reveals that, in 73% of the cases, total MU increases, suggesting the presence of another parameter with a greater impact on MU calculation. In 63% of plans with increased MU, MSW is 0.5 cm, indicating that the influence of MSW on MU warrants further investigation. Moreover, it may be counterproductive to impose a higher value “Fluence Smoothing” in patients planned with a lower MSW.
In gynecological sites, a higher level of modulation is required in comparison to prostate plans, primarily due to the more asymmetric geometry and the restrictive dose constraints of nearby OARs. IMRT and VMAT plans are replanned with “Fluence Smoothing” fixed, while only varying the MSW parameter. Regardless of the MSW value, there is a substantial increase in MU ( = +21.5%) when reoptimizing IMRT plans with VMAT technique; which is accompanied by a notable reduction in delivery time (
= +21.5%) when reoptimizing IMRT plans with VMAT technique; which is accompanied by a notable reduction in delivery time (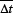 = −236s). The decreased treatment duration might result from prolonged firing time at the maximum allowable MU rate and extended uninterrupted irradiation at the highest achievable gantry speed. The mean reduction of 3.9 min obtained agrees with the mean reduction of (1.5–3) min reported in the VMAT techniques review by Otto.[15] Discrepancies in time differences may be attributed to a higher number of IMRT entry fields in our hospital, compared to other centers. The nearly 4 min reduction per patient translates to an increase of approximately 14 patients treated per day. Therefore, the introduction of VMAT signifies a valuable technological advancement in complex locations that require lengthier delivery times and pose a higher risk of compromised dose distributions due to with involuntary patient movements. For both techniques, a variation of
= −236s). The decreased treatment duration might result from prolonged firing time at the maximum allowable MU rate and extended uninterrupted irradiation at the highest achievable gantry speed. The mean reduction of 3.9 min obtained agrees with the mean reduction of (1.5–3) min reported in the VMAT techniques review by Otto.[15] Discrepancies in time differences may be attributed to a higher number of IMRT entry fields in our hospital, compared to other centers. The nearly 4 min reduction per patient translates to an increase of approximately 14 patients treated per day. Therefore, the introduction of VMAT signifies a valuable technological advancement in complex locations that require lengthier delivery times and pose a higher risk of compromised dose distributions due to with involuntary patient movements. For both techniques, a variation of  = −29% is obtained when replanning with MSW = 1.0 cm instead of MSW = 0.5 cm, resulting in a reduction of up to 983MU. According to the technique employed, the variation in MU is more pronounced in VMAT
= −29% is obtained when replanning with MSW = 1.0 cm instead of MSW = 0.5 cm, resulting in a reduction of up to 983MU. According to the technique employed, the variation in MU is more pronounced in VMAT  = −37.6% than in IMRT plans
= −37.6% than in IMRT plans  = −20.7%. Opting for the VMAT technique implies that, on average, an additional two patients could be treated per day due to shorter treatment durations. Despite the associated increase in MU with VMAT techniques, Monaco provides tools for mitigating intensity modulation, with the MSW parameter proving to be the most effective.
= −20.7%. Opting for the VMAT technique implies that, on average, an additional two patients could be treated per day due to shorter treatment durations. Despite the associated increase in MU with VMAT techniques, Monaco provides tools for mitigating intensity modulation, with the MSW parameter proving to be the most effective.
When looking at the literature,[16,17,18,19] several articles investigate the influence of MSW on both the total MU and the quality plan. In general, the consensus drawn from these studies is that MSW values between 0.5 cm and 1.0 cm offer optimal optimization. Consequently, these specific values were selected as the focal point of this study.
In Wang et al. article,[16] 19 VMAT plans of cervical pathologies are replanned with MSW = 0.5 cm, 1.0 cm, and 1.5 cm. Cervical tumor plans are complex and entail high modulation, similar to the gynecological and H and N plans studied in this work. When changing MSW from 0.5cm to 1.0 cm, the variation was  = (−14.5 ± 6.1%); and from 0.5 cm to 1.5 cm,
= (−14.5 ± 6.1%); and from 0.5 cm to 1.5 cm,  = (−20.9 ± 7.9%). They also obtained shorter delivery times in both cases. In Yoosuf et al. publication,[17] they reoptimize 5 cases of 5 different pathologies for 5 MSW values. Their findings align with a decrease in MU when changing from MSW = 0.5 cm to 1.0 cm, with
= (−20.9 ± 7.9%). They also obtained shorter delivery times in both cases. In Yoosuf et al. publication,[17] they reoptimize 5 cases of 5 different pathologies for 5 MSW values. Their findings align with a decrease in MU when changing from MSW = 0.5 cm to 1.0 cm, with  = −390.9MU and
= −390.9MU and 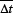 = −30s. A MU variation associated with the same MSW change of
= −30s. A MU variation associated with the same MSW change of  = −14.2% is found in the Hong et al. paper,[18] based on the reoptimization of 20 esophagus plans with 4 MSW values. This analysis was accompanied by enhanced statistical accuracy attributable to the substantial sample size. Similarly, in the publication of Nithiyanantham et al.,[19] the mean reduction in MU when comparing from 0.5 cm to 1.0 cm or 1.5 cm is
= −14.2% is found in the Hong et al. paper,[18] based on the reoptimization of 20 esophagus plans with 4 MSW values. This analysis was accompanied by enhanced statistical accuracy attributable to the substantial sample size. Similarly, in the publication of Nithiyanantham et al.,[19] the mean reduction in MU when comparing from 0.5 cm to 1.0 cm or 1.5 cm is  = (−12.7 ± 6%), with 9 VMAT cases involving various pathologies.
= (−12.7 ± 6%), with 9 VMAT cases involving various pathologies.
In Yoosuf et al.,[17] Hong et al.[18] and Nithiyanantham et al.[19] as well, the optimal starting parameter discussed is MSW = 1.0 cm. The authors argue that increasing this value would compromise the plan quality by violating the dosimetric requirements for PTVs and OARs, while reducing it would result in an unjustified increase in the MU for minimal dosimetric gains. Our findings align with the existing literature, confirming that a practical method of reducing MU and delivery time is to start from MSW = 1.0 cm in the first planning. Furthermore, the VMAT technique provides shorter delivery times compared to the IMRT technique. Using VMAT technique and MSW = 1.0 cm, an average of 1.3 patients treated per day could potentially be increased. In conjunction with other Monaco tools and beam arrangements, this method may contribute to treating a higher number of patients. In the articles discussed[16,17,18,19] like in our work, dosimetric variations in OARs and PTVs are negligible. Nevertheless, it is noteworthy that two plans of H and N are not reproducible with a satisfactory gamma criterion with MSW = 0.5 cm. Consequently, an unjustified and excessive modulation may increase the physicist’s workload by demanding a second optimization that meets the preverification criterion.
The results offer a challenging interpretation and lack statistical significance when this method is applied to more intricate scenarios, such as radiosurgery plans and SBRT treatments for lung or vertebral tumors. In the literature,[16,17,18,19] similar complex modulation plans are not examined.
In SRS plans, which involve delivering a high dose in a single fraction, there were 2 cases with an increase in MU, 1 case with no change in MU, and 7 cases with decreased MU. A total of 4 plans increased irradiation time, while 6 decreased it. The mean MU and treatment duration variations were not statistically significant. However, the samples exhibit normal distributions representative of the universal population. Therefore, it appears reasonable to conclude that MSW could not be a decisive factor, and the focus of the conclusions should be on dosimetric indices variations. Since OARs, in the original plans, are prioritized over PTVs to achieve stringent dosimetric constraints and requested dose gradients, all OARs satisfy dosimetric indices. The clinically significant variations lie in tumor coverage, specifically V100% and V95%. Even with the highest treatment time reduction of 12 s, it barely diminishes the risk of an incorrect plan reproducibility; and to ensure a satisfactory PTV coverage leads to choose an MSW value of 0.5 cm. In addition, the cranial stereotaxic frame immobilizer used in radiosurgery treatments reduces patient positioning uncertainty to <1 mm.
In SBRT plans, as in SRS plans, mean MU and treatment duration variations were also found to be not statistically significant. While in 2 plans MU increases, in 4 plans delivery time increases, indicating that their relationship is not always linear. Plans with high modulation entail the linear accelerator technology operating under limiting conditions, and it is crucial to closely examine potential associations with variables. Noteworthy, with MSW = 1.0 cm, it is worth emphasizing that one plan kept OARs and PTV indices unaltered, but  = −1688.2MU and
= −1688.2MU and 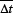 = −136s.
= −136s.
No exceptional features are observed in this plan, and both MSW plans meet the preverification criterion. Nevertheless, in a paraspinal vertebral SBRT plan with a dose ring sparing the spinal cord, there are no remarkable dosimetric variations, with  = +467 and
= +467 and 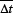 = +33s when MSW = 1.0 cm. A plausible explanation for this observation is that when an extreme basal modulation is not reproducible with MSW = 1.0 cm, the TPS might respond by considerably increasing the allowable modulation while maintaining the same dosimetric outcomes.
= +33s when MSW = 1.0 cm. A plausible explanation for this observation is that when an extreme basal modulation is not reproducible with MSW = 1.0 cm, the TPS might respond by considerably increasing the allowable modulation while maintaining the same dosimetric outcomes.
Rather than definitively establishing a suitable MSW value for highly modulated plans, it seems prudent to recommend individualized investigations. In addition, a lower MSW value may result in insufficient plan reproducibility due to the required modulation, and an intermediate value might be more appropriate. A higher value may not adequately cover the PTV.
No correlation was identified between the energy used (6 MV or 6 FFF) and the MSW value. This leads to the conclusion that a higher MSW value reduces MU regardless of the radiation beam energy, without necessarily correlating it with the magnitude of expected differences in MU and delivery time reductions.
Expanding the sample size and replicating the study in anatomical sites characterized by high baseline modulation plans is advisable, emphasizing SRS and SBRT treatments, which have been scarcely addressed in the literature. In addition, exploring lung or conventional abdominal sites where low-density volumes may potentially impact the calculation algorithm is also of interest.
Exploring MSW values between 0.5 cm and 1.0 cm in the pursuit of an optimal value for highly modulated plans, and values >1.0 cm even though the potential compromise in plan quality, would be relevant. However, it is crucial to emphasize that any potential deterioration in plan quality should not be deemed acceptable in exchange for mitigating the risk of secondary cancers, and preverification measurements must adhere to satisfactory standards.
Given the limited availability of publications with 6 MV, 6 FFF, and Monaco tools, there exists significant scope for research and for unveiling potential associations among the variables relevant to the initial stages of a radiotherapy plan.
CONCLUSIONS
Planning anatomical sites characterized by Low and Medium baseline modulation (prostate, gynecological, head and neck, and breast tumors), using an MSW value of 1.0 cm in the optimization process significantly reduces both MU and delivery time without compromising plan quality. Our work suggests to start with a “Fluence Smoothing” value set to “High” and, if additional modulation is necessary, to adjust this parameter while maintaining MSW at 1.0 cm. Despite the increase in MU associated with new intensity-modulated techniques like VMAT, the delivery time is considerably reduced. When MSW = 1.0 cm is also implemented, a substantial MU reduction can be achieved. Adopting this practical method for MU reduction could increase the average number of patients treated per day to 16, compared to an MSW value of 0.5 cm and IMRT technique settings. However, for treatments involving maximum complexity and high demands for radiation modulation, such as SRS and SBRT plans, it is advisable to analyze individually the optimal MSW value, paying particular attention to the V100% coverage of the PTV.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY MATERIAL
Supplement Table 1.
Analysis of dose distributions measured varying the gamma index
| Tumor sites | Plan | MSW | GP Γ (3%, 3 mm) | GP Γ (3%, 2 mm) | GP Γ (2%, 3 mm) | GP Γ (2%, 2 mm) |
|---|---|---|---|---|---|---|
| Gynecological | 1 | 0.5 | 96.5 | 93.4 | 92.5 | 88.1 |
| 1.0 | 98.5 | 96.1 | 94.8 | 88.3 | ||
| 2 | 0.5 | 99.4 | 97.8 | 97.5 | 93 | |
| 1.0 | 98.4 | 96.9 | 96 | 92.1 | ||
| 3 | 0.5 | 95.5 | 93.5 | 93.1 | 82 | |
| 1.0 | 97.4 | 94.8 | 94 | 86.5 | ||
| 4 | 0.5 | 97.8 | 94.9 | 97.1 | 87.5 | |
| 1.0 | 99.1 | 96.1 | 97.5 | 90.9 | ||
| 5 | 0.5 | 96.5 | 93.2 | 89.9 | 85.5 | |
| 1.0 | 99.5 | 95.8 | 93.3 | 89.9 | ||
| 6 | 0.5 | 97.8 | 94.1 | 91.8 | 84.6 | |
| 1.0 | 98.9 | 96.5 | 93.5 | 89.1 | ||
| 7 | 0.5 | 97.8 | 95.0 | 95.3 | 91.5 | |
| 1.0 | 96.9 | 94.1 | 94.8 | 87.4 | ||
| 8 | 0.5 | 96.5 | 95.9 | 95.7 | 90.2 | |
| 1.0 | 95.9 | 94.9 | 93.8 | 88.9 | ||
| 9 | 0.5 | 98.2 | 94.5 | 90.5 | 81.3 | |
| 1.0 | 99.6 | 98.3 | 96.9 | 89.2 | ||
| 10 | 0.5 | 96.9 | 94.3 | 94.6 | 81.5 | |
| 1.0 | 97.3 | 94.6 | 95.5 | 89.8 | ||
| H and N | 1 | 0.5 | 95.0 | 91.5 | 88.9 | 72.5 |
| 1.0 | 98.7 | 96.5 | 93.2 | 78.8 | ||
| 2 | 0.5 | 78.8 | 76.2 | 71.6 | 63.5 | |
| 1.0 | 97.4 | 95.6 | 86.9 | 71.9 | ||
| 3 | 0.5 | 96.5 | 91.6 | 89.5 | 80 | |
| 1.0 | 98.8 | 97.8 | 91.9 | 80.2 | ||
| 4 | 0.5 | 92.3 | 91.7 | 89.2 | 71.8 | |
| 1.0 | 99.1 | 98.7 | 94.5 | 83.9 | ||
| 5 | 0.5 | 96.5 | 93.4 | 86.6 | 73.5 | |
| 1.0 | 98.9 | 96.5 | 92.9 | 85.4 | ||
| 6 | 0.5 | 89.9 | 85.3 | 64.5 | 58 | |
| 1.0 | 98.3 | 96.4 | 95.9 | 91.7 | ||
| 7 | 0.5 | 91.5 | 92.6 | 88.7 | 80.3 | |
| 1.0 | 98.9 | 98.7 | 96.5 | 90.7 | ||
| 8 | 0.5 | 96.5 | 92.9 | 91.8 | 84 | |
| 1.0 | 95.8 | 90.8 | 90.7 | 81.2 | ||
| 9 | 0.5 | 94.2 | 94.5 | 91.2 | 79.5 | |
| 1.0 | 96.4 | 94.3 | 92.3 | 82.7 | ||
| 10 | 0.5 | 95.1 | 94.6 | 76.2 | 60.9 | |
| 1.0 | 99.7 | 98.7 | 93.5 | 84.5 | ||
| Breast | 1 | 0.5 | 97.5 | 94.9 | 95.5 | 88 |
| 1.0 | 96.8 | 93.8 | 94.9 | 86.8 | ||
| 2 | 0.5 | 95.2 | 91.9 | 84.7 | 73.8 | |
| 1.0 | 99.4 | 97.0 | 97.5 | 91.3 | ||
| 3 | 0.5 | 95.8 | 93.5 | 93.4 | 84.5 | |
| 1.0 | 97.1 | 93.7 | 93.5 | 87.3 | ||
| 4 | 0.5 | 95.9 | 92.4 | 84.3 | 71.5 | |
| 1.0 | 99.4 | 97.4 | 98.1 | 90.4 | ||
| 5 | 0.5 | 94.5 | 92.8 | 92.5 | 89.8 | |
| 1.0 | 99.5 | 97.1 | 98.2 | 93.7 | ||
| 6 | 0.5 | 96.9 | 92.1 | 87.8 | 85 | |
| 1.0 | 98.6 | 94.9 | 95.6 | 87.9 | ||
| 7 | 0.5 | 98.2 | 95.4 | 96.3 | 90.7 | |
| 1.0 | 97.9 | 95.2 | 95.8 | 90.6 | ||
| 8 | 0.5 | 97.3 | 93.6 | 94.1 | 86.5 | |
| 1.0 | 96.9 | 92.8 | 93.1 | 84.7 | ||
| 9 | 0.5 | 95.5 | 93.1 | 93.3 | 89.5 | |
| 1.0 | 99.5 | 98.5 | 97.6 | 90.7 | ||
| 10 | 0.5 | 94.5 | 91.9 | 87.5 | 81.5 | |
| 1.0 | 98.8 | 94.7 | 93.8 | 88.2 | ||
|
| ||||||
| Tumor sites | Plan | MSW | GP Γ (2%, 2 mm) | GP Γ (2%, 1 mm) | GP Γ (1%, 2 mm) | GP Γ (1%, 1 mm) |
|
| ||||||
| SBRT and SRS | 1 | 0.5 | 100 | 97.5 | 98.8 | 85.5 |
| 1.0 | 99.3 | 96.5 | 98.4 | 85.9 | ||
| 2 | 0.5 | 98.5 | 95.7 | 97 | 82.9 | |
| 1.0 | 97.9 | 94.9 | 96.6 | 78.4 | ||
| 3 | 0.5 | 96.4 | 81.3 | 93.4 | 71.7 | |
| 1.0 | 95.5 | 82.5 | 92.8 | 66.7 | ||
| 4 | 0.5 | 99.4 | 96.7 | 99.1 | 92.1 | |
| 1.0 | 98.5 | 95.6 | 96.5 | 87.4 | ||
| 5 | 0.5 | 97.9 | 88.1 | 95.1 | 80 | |
| 1.0 | 96.9 | 87.5 | 93.2 | 71.5 | ||
| 6 | 0.5 | 100 | 99.4 | 99.9 | 97.4 | |
| 1.0 | 100 | 99.8 | 100 | 97.9 | ||
| 7 | 0.5 | 97.8 | 91.5 | 96.9 | 83.5 | |
| 1.0 | 96.7 | 90.8 | 95.1 | 81.6 | ||
| 8 | 0.5 | 100 | 96.5 | 98.9 | 91.5 | |
| 1.0 | 96.4 | 94.1 | 95.2 | 89.6 | ||
| 9 | 0.5 | 97.5 | 97 | 96.3 | 91.7 | |
| 1.0 | 98.6 | 97.9 | 98.1 | 93.1 | ||
| 10 | 0.5 | 96.5 | 92.6 | 95.3 | 89.8 | |
| 1.0 | 98.8 | 94.5 | 97.9 | 90.5 | ||
Passing rates of measured dose distributions with gamma index variation. GP: Passing rates or percentage of points meeting the Г criterion. SBRT: Stereotactic body radiation therapy, SRS: Stereotactic radiosurgery, MSW: Minimum segment width
REFERENCES
- 1.Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br J Radiol. 2011;84:967–96. doi: 10.1259/bjr/22373346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foroudi F, Wilson L, Bressel M, Haworth A, Hornby C, Pham D, et al. Adosimetric comparison of 3D conformal versus intensity modulated versus volumetric arc radiation therapy for muscle invasive bladder cancer. Radiat Oncol. 2012;7:111. doi: 10.1186/1748-717X-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff D, Stieler F, Welzel G, Lorenz F, Abo-Madyan Y, Mai S, et al. Volumetric modulated arc therapy (VMAT) versus serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93:226–33. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Vergalasova I, Cai J. A modern review of the uncertainties in volumetric imaging of respiratory-induced target motion in lung radiotherapy. Med Phys. 2020;47:e988–1008. doi: 10.1002/mp.14312. [DOI] [PubMed] [Google Scholar]
- 5.Rehman JU, Zahra S, Ahmad N, Khalid M, Noor ul Huda Khan Asghar HM, Gilani ZA, et al. Intensity modulated radiation therapy: A review of current practice and future outlooks. J Radiat Res Appl Sci. 2018;11:361–7. [Google Scholar]
- 6.Braunstein S, Nakamura JL. Radiotherapy-induced malignancies: Review of clinical features, pathobiology, and evolving approaches for mitigating risk. Front Oncol. 2013;3:73. doi: 10.3389/fonc.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facoetti A, Barcellini A, Valvo F, Pullia M. The role of particle therapy in the risk of radio-induced second tumors: A review of the literature. Anticancer Res. 2019;39:4613–7. doi: 10.21873/anticanres.13641. [DOI] [PubMed] [Google Scholar]
- 8.Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: A review article. Radiat Oncol J. 2018;36:85–94. doi: 10.3857/roj.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosset JM, Hetnal M, Chargari C. Second cancers after radiotherapy: Update and recommandations. Radioprotection. 2018;53:101–5. [Google Scholar]
- 10.Hernandez V, Hansen CR, Widesott L, Bäck A, Canters R, Fusella M, et al. What is plan quality in radiotherapy?The importance of evaluating dose metrics, complexity, and robustness of treatment plans. Radiother Oncol. 2020;153:26–33. doi: 10.1016/j.radonc.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Arslan A, Sengul B. Comparison of radiotherapy techniques with flattening filter and flattening filter-free in lung radiotherapy according to the treatment volume size. Sci Rep. 2020;10:8983. doi: 10.1038/s41598-020-66079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Y, Kry SF, Popple R, Yorke E, Papanikolaou N, Stathakis S, et al. Flattening filter-free accelerators: A report from the AAPM therapy emerging technology assessment work group. J Appl Clin Med Phys. 2015;16:5219. doi: 10.1120/jacmp.v16i3.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prendergast BM, Fiveash JB, Popple RA, Clark GM, Thomas EM, Minnich DJ, et al. Flattening filter-free linac improves treatment delivery efficiency in stereotactic body radiation therapy. J Appl Clin Med Phys. 2013;14:4126. doi: 10.1120/jacmp.v14i3.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang TM, Peters MJ, Hickey B, Semciw A. Efficacy of flattening-filter-free beam in stereotactic body radiation therapy planning and treatment: A systematic review with meta-analysis. J Med Imaging Radiat Oncol. 2017;61:379–87. doi: 10.1111/1754-9485.12583. [DOI] [PubMed] [Google Scholar]
- 15.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–7. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Chen L, Zhu F, Guo W, Zhang D, Sun W. A study of minimum segment width parameter on VMAT plan quality, delivery accuracy, and efficiency for cervical cancer using Monaco TPS. J Appl Clin Med Phys. 2018;19:609–15. doi: 10.1002/acm2.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoosuf AM, Ahmad MB, AlShehri S, Alhadab A, Alqathami M. Investigation of optimum minimum segment width on VMAT plan quality and deliverability: A comprehensive dosimetric and clinical evaluation using DVH analysis. J Appl Clin Med Phys. 2021;22:29–40. doi: 10.1002/acm2.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong J, Han JH, Luo HL, Song YQ. Optimization of minimum segment width parameter in the intensity-modulated radiotherapy plan for esophageal cancer. Int J Gen Med. 2021;14:9913–21. doi: 10.2147/IJGM.S336269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nithiyanantham K, Kadirampatti Mani G, Subramani V, Karukkupalayam Palaniappan K, Uthiran M, Vellengiri S, et al. Influence of segment width on plan quality for volumetric modulated arc based stereotactic body radiotherapy. Rep Pract Oncol Radiother. 2014;19:287–95. doi: 10.1016/j.rpor.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]