Abstract
The Escherichia coli cytochrome bd complex incorporates three haems as prosthetic groups. In the ferric form these are a predominantly high-spin chlorin (haem d), a high-spin haem b (b595) and a low-spin haem b (b558). The orientations of these three haems have been determined by e.p.r. studies on oriented multilayer preparations of cytoplasmic membrane fragments. The low-spin haem b (b558) and the high-spin haem d are oriented with their haem planes perpendicular to the membrane plane. The high-spin haem b595 is oriented with its haem plane at approx. 55 degrees to the membrane plane. A minor low-spin component, attributable to a low-spin subpopulation of the haem d, is also oriented with its haem plane perpendicular to the membrane plane.
Full text
PDF
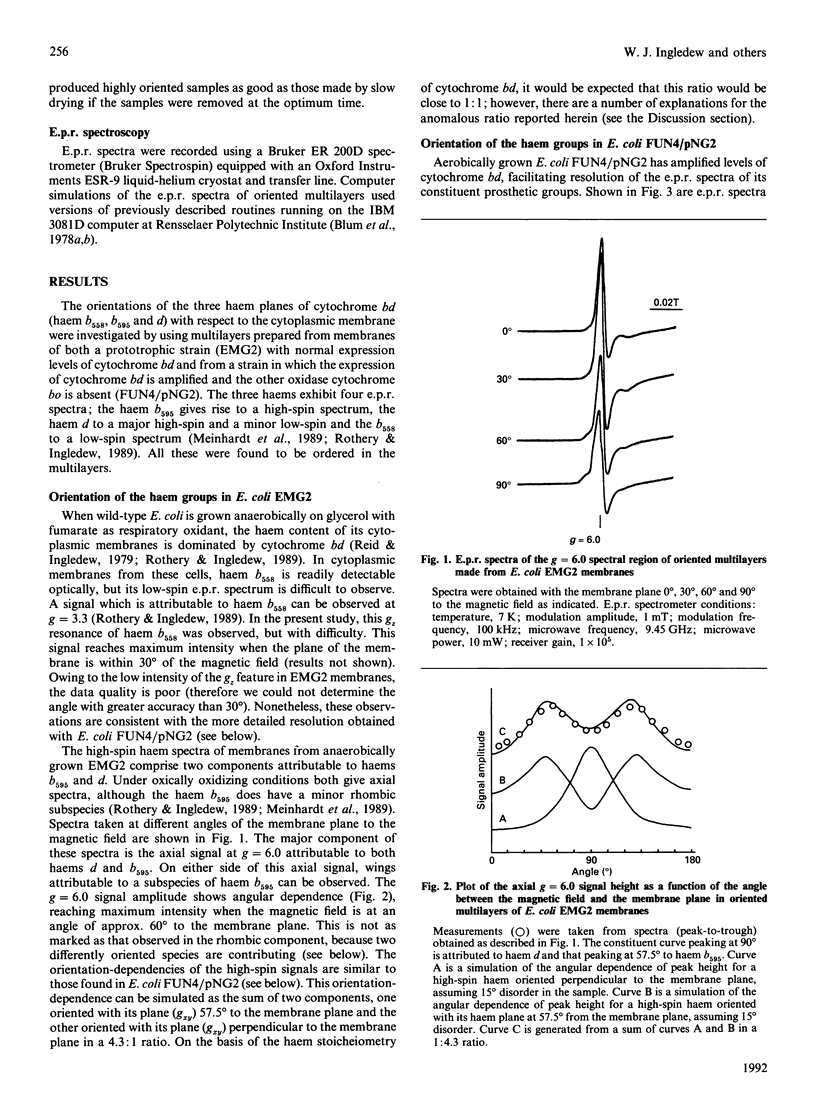


Selected References
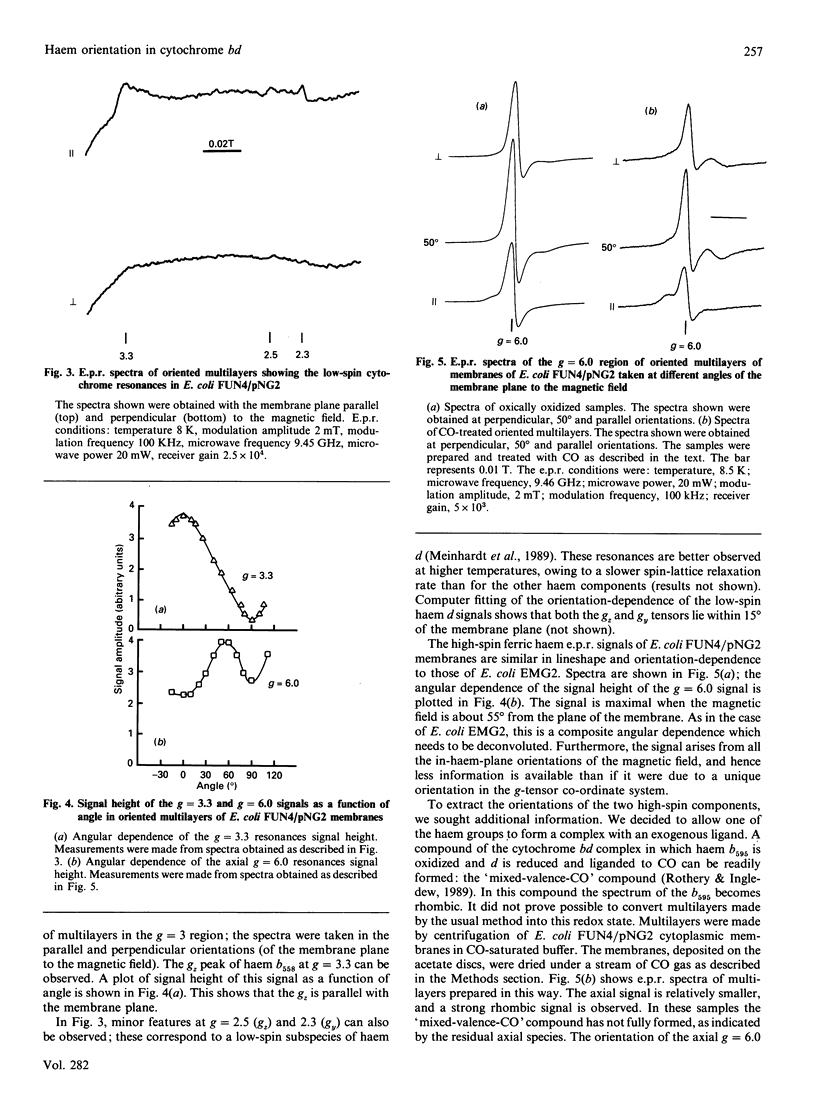
These references are in PubMed. This may not be the complete list of references from this article.
- Blum H., Harmon H. J., Leigh J. S., Salerno J. C., Chance B. The orientation of a heme of cytochrome c oxidase in submitochondrial particles. Biochim Biophys Acta. 1978 Apr 11;502(1):1–10. doi: 10.1016/0005-2728(78)90125-1. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Fang H., Lin R. J., Gennis R. B. Location of heme axial ligands in the cytochrome d terminal oxidase complex of Escherichia coli determined by site-directed mutagenesis. J Biol Chem. 1989 May 15;264(14):8026–8032. [PubMed] [Google Scholar]
- Green G. N., Fang H., Lin R. J., Newton G., Mather M., Georgiou C. D., Gennis R. B. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J Biol Chem. 1988 Sep 15;263(26):13138–13143. [PubMed] [Google Scholar]
- Green G. N., Kranz R. G., Lorence R. M., Gennis R. B. Identification of subunit I as the cytochrome b558 component of the cytochrome d terminal oxidase complex of Escherichia coli. J Biol Chem. 1984 Jun 25;259(12):7994–7997. [PubMed] [Google Scholar]
- Ingledew W. J. The electron transport chain of Escherichia coli grown anaerobically with fumarate as terminal electron acceptor: an electron paramagnetic resonance study. J Gen Microbiol. 1983 Jun;129(6):1651–1659. doi: 10.1099/00221287-129-6-1651. [DOI] [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984 Mar 10;259(5):3375–3381. [PubMed] [Google Scholar]
- Lorence R. M., Carter K., Gennis R. B., Matsushita K., Kaback H. R. Trypsin proteolysis of the cytochrome d complex of Escherichia coli selectively inhibits ubiquinol oxidase activity while not affecting N,N,N',N'-tetramethyl-p-phenylenediamine oxidase activity. J Biol Chem. 1988 Apr 15;263(11):5271–5276. [PubMed] [Google Scholar]
- Lorence R. M., Koland J. G., Gennis R. B. Coulometric and spectroscopic analysis of the purified cytochrome d complex of Escherichia coli: evidence for the identification of "cytochrome a1" as cytochrome b595. Biochemistry. 1986 May 6;25(9):2314–2321. doi: 10.1021/bi00357a003. [DOI] [PubMed] [Google Scholar]
- Meinhardt S. W., Gennis R. B., Ohnishi T. EPR studies of the cytochrome-d complex of Escherichia coli. Biochim Biophys Acta. 1989 Jun 23;975(1):175–184. doi: 10.1016/s0005-2728(89)80216-6. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The cytochrome d complex is a coupling site in the aerobic respiratory chain of Escherichia coli. J Biol Chem. 1985 Nov 15;260(26):14003–14008. [PubMed] [Google Scholar]
- Poole R. K., Kumar C., Salmon I., Chance B. The 650 and chromophore in Escherichia coli is an 'oxy-' or oxygenated compound, not the oxidized form of cytochrome oxidase d: an hypothesis. J Gen Microbiol. 1983 May;129(5):1335–1344. doi: 10.1099/00221287-129-5-1335. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Scott R. I., Chance B. The light-reversible binding of carbon monoxide to cytochrome a1 in Escherichia coli K12. J Gen Microbiol. 1981 Aug;125(2):431–438. doi: 10.1099/00221287-125-2-431. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Finel M., Virkki M., Wikström M. Cytochrome o (bo) is a proton pump in Paracoccus denitrificans and Escherichia coli. FEBS Lett. 1989 Jun 5;249(2):163–167. doi: 10.1016/0014-5793(89)80616-7. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Ingledew W. J. Characterization and phenotypic control of the cytochrome content of Escherichia coli. Biochem J. 1979 Aug 15;182(2):465–472. doi: 10.1042/bj1820465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothery R. A., Houston A. M., Ingledew W. J. The respiratory chain of anaerobically grown Escherichia coli: reactions with nitrite and oxygen. J Gen Microbiol. 1987 Nov;133(11):3247–3255. doi: 10.1099/00221287-133-11-3247. [DOI] [PubMed] [Google Scholar]
- Rothery R. A., Ingledew W. J. The cytochromes of anaerobically grown Escherichia coli. An electron-paramagnetic-resonance study of the cytochrome bd complex in situ. Biochem J. 1989 Jul 15;261(2):437–443. doi: 10.1042/bj2610437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno J. C., Bolgiano B., Poole R. K., Gennis R. B., Ingledew W. J. Heme-copper and heme-heme interactions in the cytochrome bo-containing quinol oxidase of Escherichia coli. J Biol Chem. 1990 Mar 15;265(8):4364–4368. [PubMed] [Google Scholar]
- Salerno J. C., Ingledew W. J. Orientation of the haems of the ubiquinol oxidase:O2 reductase, cytochrome bo of Escherichia coli. Eur J Biochem. 1991 Jun 15;198(3):789–792. doi: 10.1111/j.1432-1033.1991.tb16082.x. [DOI] [PubMed] [Google Scholar]


