Abstract
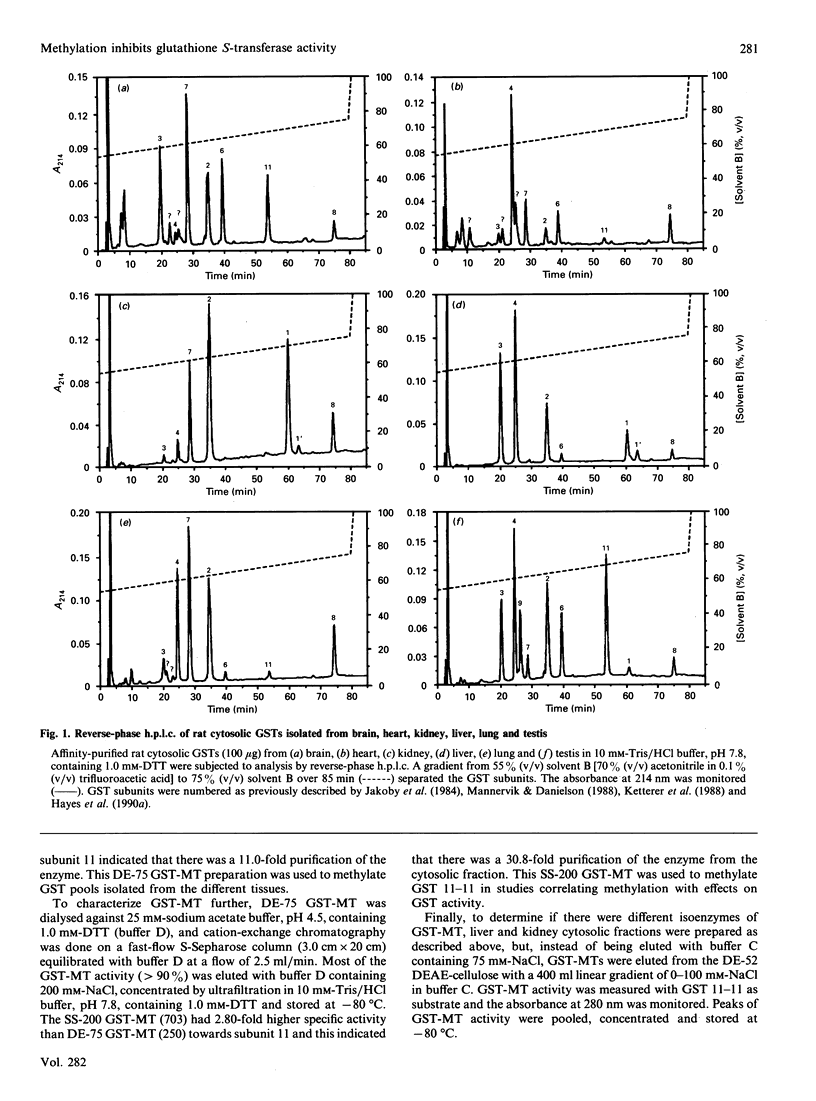
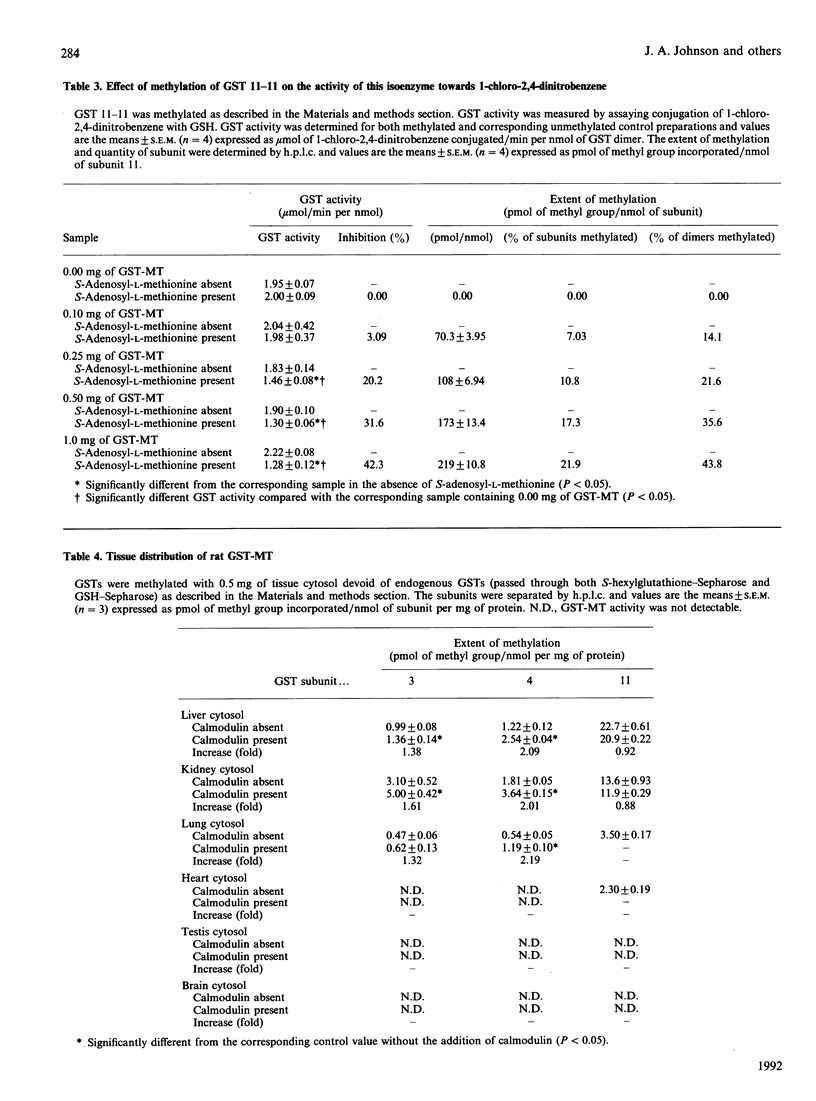
Glutathione S-transferases (GSTs) were isolated from rat liver, lung, heart, kidney, testis and brain by coupled affinity chromatography and subunits were resolved by reverse-phase h.p.l.c. The reverse-phase h.p.l.c. technique was improved from our previously published work [Johnson, Neal, Collins & Siegel (1990) Biochem. J. 270, 483-489] by changing from a C4 to a C18 wide-pore reverse-phase column; this resulted in baseline or near-baseline resolution of all GST subunits. There were significant tissue-dependent differences in the expression of GST subunits and the level of GST subunits present was quantitatively determined for each of the tissues. The extent of methylation of GSTs in vitro and distribution of GST methyltransferase (GST-MT) was determined in cytosolic fractions from each of these tissues. Purified GST isoenzymes were methylated with partially purified liver GST-MT. Methylation of Mu class subunits 3 and 4, the preferred substrates of methylation in liver, was substoichiometric in all tissues. The extent of methylation of subunit 3 ranged from 0.13% to 0.94% and subunit 4 from 0.03% to 0.60%. Methylation of Alpha class subunits was either not detectable or 5-10-fold less than that of Mu class subunits 3 and 4. Pi class subunit 7 was methylated to a greater extent than the Alpha class subunits but less than Mu class isoenzymes. A notable exception to this low level of methylation was GST 11-11, found mainly in testis and brain. Methylation of subunit 11 reached 21.9% (219 pmol of methyl group/nmol of subunit 11) when this isoenzyme was incubated with partially purified liver GST methyltransferase. Methylation of GST 11-11 was found to inhibit the conjugating activity of this isoenzyme towards 1-chloro-2,4-dinitrobenzene; the degree of inhibition of conjugating activity correlated with the extent of methylation of GST 11-11. GST-MT activity toward GST subunits 3, 4 and 11 was present in kidney and liver, detectable in lung and heart, but absent from brain and testis. Anion-exchange chromatography of GST-MTs from liver and kidney suggested the presence of four different forms of GST-MT (I-IV) and indicated that GST-MT isoenzymes III and IV were present at significantly lower concentrations in kidney than liver. The present paper shows that methylation is an enzyme-catalysed reaction that differs in substrate-specificity with respect to different GST isoenzymes, that expression of GST-MT is tissue-dependent and multiple forms of the enzyme are present in liver and kidney, and that methylation inhibits GST activity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Listowsky I. Developmental regulation of glutathione S-transferases. Xenobiotica. 1988 Nov;18(11):1249–1254. doi: 10.3109/00498258809042248. [DOI] [PubMed] [Google Scholar]
- Abramovitz M., Listowsky I. Selective expression of a unique glutathione S-transferase Yb3 gene in rat brain. J Biol Chem. 1987 Jun 5;262(16):7770–7773. [PubMed] [Google Scholar]
- Alin P., Jensson H., Cederlund E., Jörnvall H., Mannervik B. Cytosolic glutathione transferases from rat liver. Primary structure of class alpha glutathione transferase 8-8 and characterization of low-abundance class Mu glutathione transferases. Biochem J. 1989 Jul 15;261(2):531–539. doi: 10.1042/bj2610531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad D. W. Stoichiometric methylation of porcine adrenocorticotropin by protein carboxyl methyltransferase requires deamidation of asparagine 25. Evidence for methylation at the alpha-carboxyl group of atypical L-isoaspartyl residues. J Biol Chem. 1984 Sep 10;259(17):10714–10721. [PubMed] [Google Scholar]
- Benson A. M., Hunkeler M. J., York J. L. Mouse hepatic glutathione transferase isoenzymes and their differential induction by anticarcinogens. Specificities of butylated hydroxyanisole and bisethylxanthogen as inducers of glutathione transferases in male and female CD-1 mice. Biochem J. 1989 Aug 1;261(3):1023–1029. doi: 10.1042/bj2611023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang M., Burgess J. R., Scholz R. W., Reddy C. C. The induction of specific rat liver glutathione S-transferase subunits under inadequate selenium nutrition causes an increase in prostaglandin F2 alpha formation. J Biol Chem. 1990 Apr 5;265(10):5418–5423. [PubMed] [Google Scholar]
- Chelsky D., Sobotka C., O'Neill C. L. Lamin B methylation and assembly into the nuclear envelope. J Biol Chem. 1989 May 5;264(13):7637–7643. [PubMed] [Google Scholar]
- Clarke S. Protein carboxyl methyltransferases: two distinct classes of enzymes. Annu Rev Biochem. 1985;54:479–506. doi: 10.1146/annurev.bi.54.070185.002403. [DOI] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Broach J. R. Fatty acylation is important but not essential for Saccharomyces cerevisiae RAS function. Mol Cell Biol. 1987 Jul;7(7):2344–2351. doi: 10.1128/mcb.7.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Stimmel J. B., Clarke S., Stock J., Broach J. R. RAS2 protein of Saccharomyces cerevisiae is methyl-esterified at its carboxyl terminus. J Biol Chem. 1989 Jul 15;264(20):11865–11873. [PubMed] [Google Scholar]
- Di Simplicio P., Jensson H., Mannervik B. Effects of inducers of drug metabolism on basic hepatic forms of mouse glutathione transferase. Biochem J. 1989 Nov 1;263(3):679–685. doi: 10.1042/bj2630679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G. J., Ding V. D., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. DNA sequence analysis of a Yb2 cDNA clone and regulation of the Yb1 and Yb2 mRNAs by phenobarbital. J Biol Chem. 1986 Jun 15;261(17):7952–7957. [PubMed] [Google Scholar]
- Gregori L., Marriott D., West C. M., Chau V. Specific recognition of calmodulin from Dictyostelium discoideum by the ATP, ubiquitin-dependent degradative pathway. J Biol Chem. 1985 May 10;260(9):5232–5235. [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Kerr L. A., Harrison D. J., Cronshaw A. D., Ross A. G., Neal G. E. Preferential over-expression of the class alpha rat Ya2 glutathione S-transferase subunit in livers bearing aflatoxin-induced pre-neoplastic nodules. Comparison of the primary structures of Ya1 and Ya2 with cloned class alpha glutathione S-transferase cDNA sequences. Biochem J. 1990 Jun 1;268(2):295–302. doi: 10.1042/bj2680295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Use of immuno-blot techniques to discriminate between the glutathione S-transferase Yf, Yk, Ya, Yn/Yb and Yc subunits and to study their distribution in extrahepatic tissues. Evidence for three immunochemically distinct groups of transferase in the rat. Biochem J. 1986 Feb 1;233(3):779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Selective elution of rodent glutathione S-transferases and glyoxalase I from the S-hexyglutathione-Sepharose affinity matrix. Biochem J. 1988 Nov 1;255(3):913–922. doi: 10.1042/bj2550913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka A., Sebata N., Kawashima K., Okuda H., Ogura K., Watabe T., Satoh K., Hatayama I., Tsuchida S., Ishikawa T. A new class of rat glutathione S-transferase Yrs-Yrs inactivating reactive sulfate esters as metabolites of carcinogenic arylmethanols. J Biol Chem. 1990 Jul 15;265(20):11973–11981. [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Neal T. L., Collins J. H., Siegel F. L. Characterization of methylation of rat liver cytosolic glutathione S-transferases by using reverse-phase h.p.l.c. and chromatofocusing. Biochem J. 1990 Sep 1;270(2):483–489. doi: 10.1042/bj2700483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A., Meyer D. J., Lalor E., Coles B., Ketterer B. Purification and characterization of a labile rat glutathione transferase of the Mu class. Biochem J. 1989 Jun 15;260(3):789–793. doi: 10.1042/bj2600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Okuno S., Kariya K. Propylthiouracil inducible glutathione transferases. Selective induction of ligandin (glutathione transferase 1-1). Biochem Pharmacol. 1986 Jun 1;35(11):1835–1839. doi: 10.1016/0006-2952(86)90300-x. [DOI] [PubMed] [Google Scholar]
- Listowsky I., Abramovitz M., Homma H., Niitsu Y. Intracellular binding and transport of hormones and xenobiotics by glutathione-S-transferases. Drug Metab Rev. 1988;19(3-4):305–318. doi: 10.3109/03602538808994138. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Lalor E., Coles B., Kispert A., Alin P., Mannervik B., Ketterer B. Single-step purification and h.p.l.c. analysis of glutathione transferase 8-8 in rat tissues. Biochem J. 1989 Jun 15;260(3):785–788. doi: 10.1042/bj2600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- Murata T., Hatayama I., Satoh K., Tsuchida S., Sato K. Activation of rat glutathione transferases in class mu by active oxygen species. Biochem Biophys Res Commun. 1990 Sep 14;171(2):845–851. doi: 10.1016/0006-291x(90)91223-f. [DOI] [PubMed] [Google Scholar]
- Murtaugh T. J., Wright L. S., Siegel F. L. Posttranslational modification of calmodulin in rat brain and pituitary. J Neurochem. 1986 Jul;47(1):164–172. doi: 10.1111/j.1471-4159.1986.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Neal T. L., Wright L. S., Siegel F. L. Identification of glutathione S-transferase as a substrate and glutathione as an inhibitor of in vitro calmodulin-stimulated protein methylation in rat liver cytosol. Biochem Biophys Res Commun. 1988 Oct 14;156(1):368–374. doi: 10.1016/s0006-291x(88)80850-7. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Solubilization and partial purification of protein methylase 3 from calf thymus nuclei. J Biol Chem. 1970 Nov 25;245(22):6010–6015. [PubMed] [Google Scholar]
- Park K. S., Frost B., Tuck M., Ho L. L., Kim S., Paik W. K. Enzymatic methylation of in vitro synthesized apocytochrome c enhances its transport into mitochondria. J Biol Chem. 1987 Oct 25;262(30):14702–14708. [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Pyerin W., Taniguchi H., Horn F., Oesch F., Amelizad Z., Friedberg T., Wolf C. R. Isoenzyme-specific phosphorylation of cytochromes P-450 and other drug metabolizing enzymes. Biochem Biophys Res Commun. 1987 Feb 13;142(3):885–892. doi: 10.1016/0006-291x(87)91496-3. [DOI] [PubMed] [Google Scholar]
- Roberts D. M., Rowe P. M., Siegel F. L., Lukas T. J., Watterson D. M. Trimethyllysine and protein function. Effect of methylation and mutagenesis of lysine 115 of calmodulin on NAD kinase activation. J Biol Chem. 1986 Feb 5;261(4):1491–1494. [PubMed] [Google Scholar]
- Rowe P. M., Wright L. S., Siegel F. L. Calmodulin N-methyltransferase. Partial purification and characterization. J Biol Chem. 1986 May 25;261(15):7060–7069. [PubMed] [Google Scholar]
- Rushmore T. H., King R. G., Paulson K. E., Pickett C. B. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990 May;87(10):3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases by glutathione-affinity chromatography. Methods Enzymol. 1981;77:235–237. doi: 10.1016/s0076-6879(81)77031-9. [DOI] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Pyerin W. Glutathione S-transferase is an in vitro substrate of Ca++-phospholipid-dependent protein kinase (protein kinase C). Biochem Biophys Res Commun. 1989 Aug 15;162(3):903–907. doi: 10.1016/0006-291x(89)90757-2. [DOI] [PubMed] [Google Scholar]
- Tsuchida S., Sato K. Rat spleen glutathione transferases. A new acidic form belonging to the Alpha class. Biochem J. 1990 Mar 1;266(2):461–465. doi: 10.1042/bj2660461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Weiss M. J., Li N. Q., Reddy C. C. Tissue-specific expression of the rat glutathione S-transferases. J Biol Chem. 1983 Apr 25;258(8):4659–4662. [PubMed] [Google Scholar]
- Vincent P. L., Siegel F. L. Carboxylmethylation of calmodulin in cultured pituitary cells. J Neurochem. 1987 Nov;49(5):1613–1622. doi: 10.1111/j.1471-4159.1987.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]