Abstract
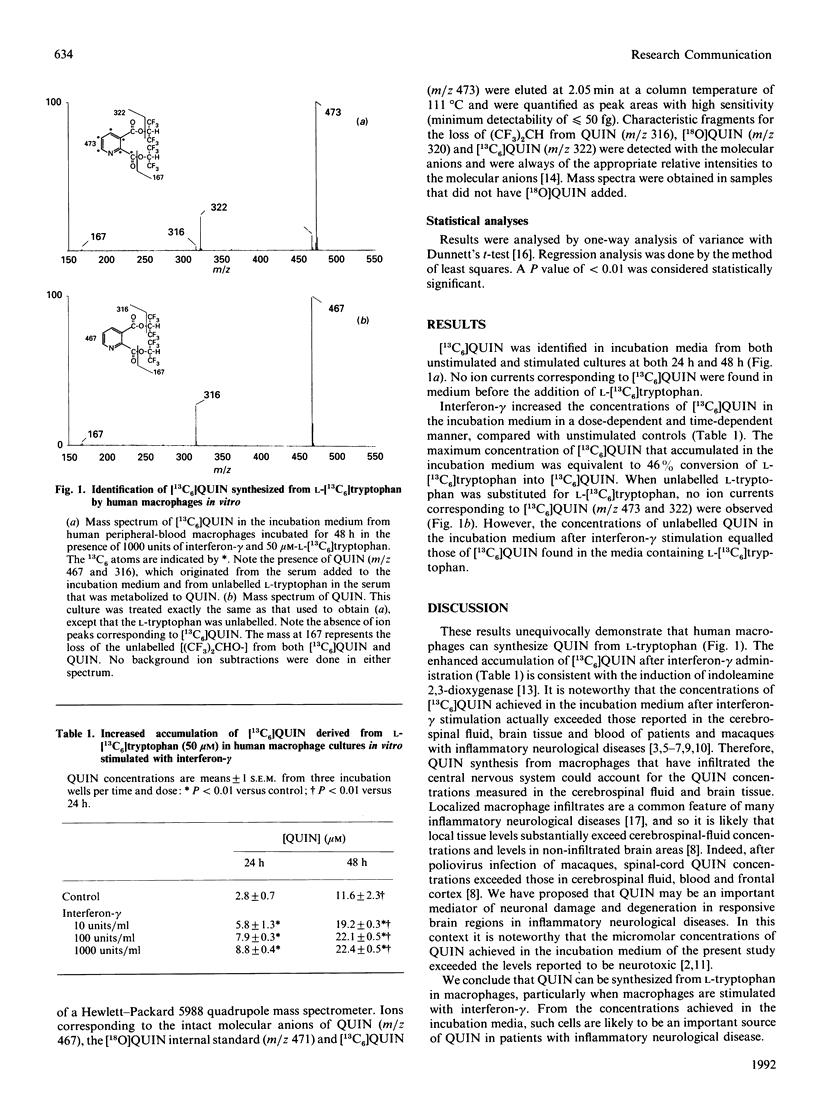
Substantial increases in the concentrations of the excitotoxin and N-methyl-D-aspartate-receptor agonist quinolinic acid (QUIN) occur in human patients and non-human primates with inflammatory diseases. Such increases were postulated to be secondary to induction of indoleamine 2,3-dioxygenase in inflammatory cells, particularly macrophages, by interferon-gamma. To test this hypothesis, human peripheral-blood macrophages were incubated with L-[13C6]tryptophan in the absence or presence of interferon-gamma. [13C6]QUIN was quantified by gas chromatography and electron-capture negative-chemical-ionization mass spectrometry. [13C6]QUIN was detected in the incubation medium of both unstimulated and stimulated cultures. Exposure to interferon-gamma substantially increased the accumulation of [13C6]QUIN in a dose- and time-dependent manner. The QUIN concentrations achieved exceeded those reported in both cerebrospinal fluid and blood of patients and of non-human primates with inflammatory diseases. Macrophages stimulated with interferon-gamma may be an important source of accelerated L-tryptophan conversion into QUIN in inflammatory diseases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Halperin J. J., Heyes M. P. Neuroactive kynurenines in Lyme borreliosis. Neurology. 1992 Jan;42(1):43–50. doi: 10.1212/wnl.42.1.43. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Brew B. J., Martin A., Price R. W., Salazar A. M., Sidtis J. J., Yergey J. A., Mouradian M. M., Sadler A. E., Keilp J. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991 Feb;29(2):202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Gravell M., London W. T., Eckhaus M., Vickers J. H., Yergey J. A., April M., Blackmore D., Markey S. P. Sustained increases in cerebrospinal fluid quinolinic acid concentrations in rhesus macaques (Macaca mulatta) naturally infected with simian retrovirus type-D. Brain Res. 1990 Oct 29;531(1-2):148–158. doi: 10.1016/0006-8993(90)90768-7. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Jordan E. K., Lee K., Saito K., Frank J. A., Snoy P. J., Markey S. P., Gravell M. Relationship of neurologic status in macaques infected with the simian immunodeficiency virus to cerebrospinal fluid quinolinic acid and kynurenic acid. Brain Res. 1992 Jan 20;570(1-2):237–250. doi: 10.1016/0006-8993(92)90587-y. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Lackner A. Increased cerebrospinal fluid quinolinic acid, kynurenic acid, and L-kynurenine in acute septicemia. J Neurochem. 1990 Jul;55(1):338–341. doi: 10.1111/j.1471-4159.1990.tb08857.x. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Markey S. P. (18O)quinolinic acid: its esterification without back exchange for use as internal standard in the quantification of brain and CSF quinolinic acid. Biomed Environ Mass Spectrom. 1988 Mar 1;15(5):291–293. doi: 10.1002/bms.1200150509. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Markey S. P. Quantification of quinolinic acid in rat brain, whole blood, and plasma by gas chromatography and negative chemical ionization mass spectrometry: effects of systemic L-tryptophan administration on brain and blood quinolinic acid concentrations. Anal Biochem. 1988 Oct;174(1):349–359. doi: 10.1016/0003-2697(88)90556-8. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Mefford I. N., Quearry B. J., Dedhia M., Lackner A. Increased ratio of quinolinic acid to kynurenic acid in cerebrospinal fluid of D retrovirus-infected rhesus macaques: relationship to clinical and viral status. Ann Neurol. 1990 Jun;27(6):666–675. doi: 10.1002/ana.410270614. [DOI] [PubMed] [Google Scholar]
- Saito K., Markey S. P., Heyes M. P. Chronic effects of gamma-interferon on quinolinic acid and indoleamine-2,3-dioxygenase in brain of C57BL6 mice. Brain Res. 1991 Apr 12;546(1):151–154. doi: 10.1016/0006-8993(91)91171-v. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Taylor M. W., Feng G. S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991 Aug;5(11):2516–2522. [PubMed] [Google Scholar]
- Whetsell W. O., Jr, Schwarcz R. Prolonged exposure to submicromolar concentrations of quinolinic acid causes excitotoxic damage in organotypic cultures of rat corticostriatal system. Neurosci Lett. 1989 Feb 27;97(3):271–275. doi: 10.1016/0304-3940(89)90609-5. [DOI] [PubMed] [Google Scholar]


