Abstract
Background:
Prenatal ethylene oxide exposure may have adverse effects on fetal development. We examined the relationships between ethylene oxide hemoglobin (Hb) adduct levels and offspring’s size at birth in a prospective European mother–child study.
Methods:
This study included 1106 singletons from the NewGeneris project (2006–2010) with ethylene oxide Hb adducts measured in cord blood. We examined the relationships between adduct levels and offspring’s size at birth among all infants and separately among infants of nonsmokers, using linear regression models for birth weight and birth head circumference and logarithmic binomial regression models for small for gestational age. We examined potential interactions between CYP2E1 single nucleotide polymorphisms in cord blood and the effects of ethylene oxide Hb adduct levels on offspring birth size.
Results:
Higher quartiles of adduct levels as a measure of exposure were associated with decreasing birth weight and head circumference in the overall population. Compared to infants in the lowest quartile, those in the highest quartile exhibited lower birth weight (−70.73 g, 95% confidence interval = −141.16, −0.30) and reduced head circumference (−0.30 cm, 95% confidence interval = −0.58, −0.02). We observed similar, albeit less pronounced, patterns among infants of nonsmokers. There was no evidence of an association between ethylene oxide Hb adducts and risk of small for gestational age, nor consistent evidence of an interaction with CYP2E1 polymorphisms on the association between EO Hb adduct levels and offspring’s size at birth.
Conclusion:
Results suggest that higher ethylene oxide Hb adduct levels in cord blood are associated with a reduction in offspring birth size.
Keywords: Biomarker, Birth outcomes, Birth weight, Ethylene oxide, Head circumference, Hemoglobin adducts
Ethylene oxide (EO) is a volatile and reactive chemical substance known for its mutagenic and carcinogenic properties, as demonstrated by numerous in vitro and in vivo studies conducted in both animals and humans.1,2 The International Agency for Research on Cancer determined that EO is carcinogenic to humans (group 1).3 This determination was based on the available evidence among experimental animal studies, strong mechanistic evidence, and limited evidence in human epidemiologic studies.4–6
Prenatal exposure to EO may have adverse effects on health. Some reproductive outcomes have been associated with occupational EO exposure, including miscarriage and pregnancy loss in nurses with high exposure to EO at any stage of pregnancy compared to women with low occupational exposure during pregnancy.7,8 Animal studies also suggest adverse effects of EO on intrauterine growth as indicated by findings of decreased offspring body weight following intermittent exposure of maternal rats to EO vapor9; however, research in human populations is limited.
Exposure to EO can occur through either exogenous or endogenous means9 and also occurs via ethylene that is metabolized to EO. Exogenous exposure primarily stems from any of a range of sources. In the general population, exposure occurs via the inhalation of tobacco smoke containing both EO and ethylene10,11 or indoor air pollution from various household products, including cleaning agents, air fresheners, or personal care items. Exposure may also occur in a variety of occupational settings, including the healthcare industry where workers may encounter EO exposure during the sterilization of medical equipment or in the chemical industry, as the chemical is used in chemical industries for the production of ethylene glycol, nonionic surfactants, and other derivatives. Population environmental exposure can also occur in proximity to chemical plants that emit EO,12–14 as a result of emissions of ethylene from certain chemical plants, or from formation in combustion; for example, ethylene is found in automobile exhaust and is also emitted from natural sources.15 EO also occurs as a contaminant in food.16 Endogenously, EO originates from internally produced ethylene.17,18 Ethylene and EO are derived from lipid peroxidation, oxidation of free methionine, heme oxidation from hemoglobin (Hb), and the metabolism of intestinal bacteria.
The metabolism of ethylene and EO in humans involves both phase I and phase II reactions.19 In phase I, cytochrome P450 enzymes, primarily cytochrome P450 2E120 (CYP2E1), convert ethylene to EO, which is electrophilic and can react with Hb and DNA.21 In phase II, conjugation of EO with glutathione occurs facilitated by glutathione-S-transferase (mainly GSTT1), as well as hydrolysis facilitated with epoxide hydrolase, leading to the formation of metabolites that are excreted in urine.22 The reaction product (adduct) from EO to N-terminal valine in Hb, HOEtVal, can be used to monitor doses in blood from EO over a broad range of exposure levels, in the same way that Hb adducts have been used as a biomarker of exposure for other electrophilic chemicals or metabolites, to be used in risk assessment as an indicator of the internal dose or effective dose.2,23,24 In addition, the biomarker has been used to demonstrate that the general population is exposed to ethylene or EO, to a large part from endogenous sources.18
Considerable variation in EO Hb adduct levels exists in the general population, as has been shown in the US-based National Health and Nutrition Examination Survey (NHANES) study,25 which reflects heterogeneity in endogenous and exogenous pathways via exposure to ambient air pollution and/or tobacco smoke among others.26 Within the general population, higher adduct levels have been found among nonsmoking adults living closer to facilities emitting EO in the United States as compared with those living further away,27 and higher levels have also been found in cord blood samples from infants of smokers as compared to infants of nonsmokers.28
Concentrations of Hb adducts have demonstrated a linear correlation with EO exposure within the range of 0–200 ppm in inhaled air with EO DNA adducts and EO Hb adducts, which have been widely used as a biomarker of exposure in previous studies. EO Hb adducts in cord blood can be used as a biomarker in studies examining biologic mechanisms of EO exposure and birth outcomes.28,29 The average life span of red blood cells in adults is around 120 days30,31; therefore, EO Hb adducts in cord blood samples likely represent the average exposure during approximately the latter part of the second trimester and the third trimester of pregnancy.
Our study focuses on EO Hb adducts in cord blood measured in a European population32 and their potential associations with birth weight, birth head circumference, and small for gestational age (SGA), which are outcomes reflecting intrauterine growth. Intrauterine growth restriction is a strong predictor of perinatal survival of the child and has been linked to adverse effects later in life.33–37 We also incorporated data on several single nucleotide polymorphisms (SNPs) in the CYP2E1 locus to determine possible interactions of these SNPs with the associations between EO Hb levels and birth outcomes.
METHODS
Study Population
Data for this study were from the NewGeneris project (www.newgeneris.org),32 which aimed to study selected genotoxic exposures from diet and the environment during pregnancy on child health through measurement of different biomarkers in cord blood. During 2006–2010, pregnant women were enrolled in 11 maternity units within Copenhagen, Denmark; Heraklion Greece; Oslo and Akershus, Norway (including participants enrolled in the Mother, father and child cohort study [MoBa]38); Barcelona, Spain; and Bradford, United Kingdom. Mothers were enrolled during pregnancy and/or around birth, and a total of 1108 singleton births had corresponding data on EO Hb adducts in cord blood. Of these, two participants withdrew consent before analyses were completed, resulting in a final population of 1,106 who were included in the present analyses. Detailed information on specific eligibility criteria applied in each of the 11 units is available elsewhere.39 Ethical approval was obtained from the local committees and all participating mothers gave their written informed consent.
We collected demographic, health, and lifestyle factors through detailed questionnaires. We obtained information on birth weight, birth head circumference, gestational age, sex, and mode of delivery from maternity records. Gestational age for participants in Denmark, Greece, and Spain was based on the interval between the last menstrual period and delivery date or ultrasound-based estimated date of conception in case the two results were discordant by 7 days or more. In Norway and the United Kingdom, gestational age was based on ultrasound-based estimation. We defined SGA cases as infants who weighed less than the 10th percentile of the cohort-specific reference (mean birth weight for all SGA children: 2838 g), stratified by completed week of gestation and sex.
Blood Collection and Ethylene Oxide Hemoglobin Adducts Analysis
Study staff collected 30 ml of cord blood after the delivery and 40 ml of maternal peripheral blood on the day of the delivery. We isolated red blood cells from cord blood and maternal blood and washed samples twice before shipping them to Stockholm, Sweden. We measured Hb adducts, determined by the “adduct FIRE procedure” (fluorescein isothiocyanate R (adducts) Edman) (using 250 µl samples). In brief, adducted N-terminal valines were detached using the Edman reagent fluoresceine-5-isothiocyanate. The detached analytes were purified by solid phase extraction and analyzed by liquid chromatography–tandem mass spectrometry.28,40 Deuterium-labeled internal standards were used for quantification and three control blood samples were included in each batch of analysis (24 samples/batch). The method, in combination with the used standards, allows for specific analysis of EO adducts to N-terminal valine in Hb, here described as “EO Hb adducts.”
The limit of quantification was evaluated for each sample and set to a peak height response of 10 times to noise. A limit of quantification of processed samples below 1 pmol/g Hb could be achieved for the analysis of EO Hb adducts but varied with the status of the HPLC column and mass spectrometer. In total, we analyzed 192 maternal blood and 1151 cord blood samples. A total of 35 maternal and 28 cord blood results did not fulfill the quality criteria for quantification and were not included in the final data set with results on levels of EO Hb adducts.
Candidate Gene Analysis: CYP2E1
Genomic DNA was isolated from 900 cord blood samples and was used for genome-wide genotyping with the Illumina HumanCytoSNP-12 v1 (Illumina Inc., Hayward, CA) array according to the manufacturer’s protocols. Genotype calling was done using Illumina GenomeStudio 2010. Quality control of the data was done following standard parameters with the PLINK tool (https://zzz.bwh.harvard.edu/plink/). Samples with genotype call rate <98.5% were filtered out. Sex consistency and relatedness was checked. SNPs with minor allele frequency <1% or not in Hardy Weinberg equilibrium (P value < 10−6) were filtered out. The final database consisted of 249,968 SNPs and 649 samples with exposure and outcome measures. From the genome-wide association studies dataset, 15 CYP2E1 SNPs were selected either because (1) they were in ±10 kb from the CYP2E1 gene coordinates or (2) they were CYP2E1 expression quantitative trait locus (eQTLs) according to GTEx project (https://gtexportal.org) (eTable 1; http://links.lww.com/EDE/C156).
Statistical Analysis
We computed descriptive statistics to provide an overview of the demographic data and birth outcomes. We also described EO Hb adduct levels based on the characteristics of the study population. In addition, we calculated the prevalence of SGA, taking into consideration weight, sex, and gestational age at birth.
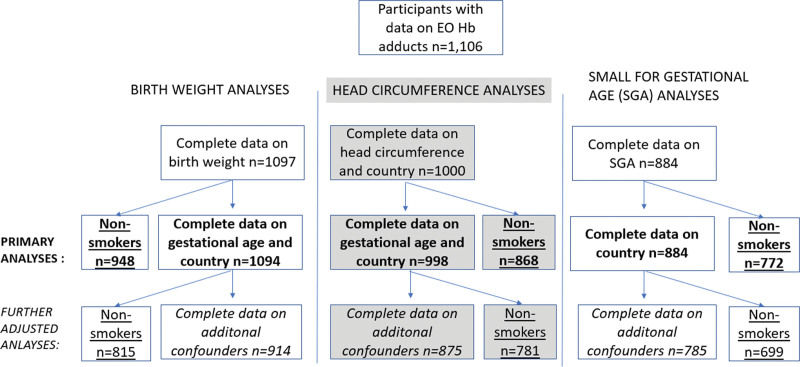
We modeled cord blood EO Hb adduct levels on a continuous scale, with effects estimated per 10 pmol/g Hb increments, and categorized them according to quartiles of the distribution. To assess the association between levels of EO Hb adducts with continuous birth weight and continuous birth head circumference, we constructed linear regression models. All models were adjusted for country and gestational age (continuous, in completed weeks). These models included n = 1,094 and n = 998, which had complete data on adjustment data and birth weight and adjustment data and head circumference, respectively. In further adjusted models, we also considered adjustment for additional potential confounders, selected a priori after reviewing prior literature and considering similar covariates as included in a previous analysis of acrylamide hemoglobin adducts and birth outcomes in the same study population,39 including child sex, maternal smoking habit toward the end of pregnancy (yes, no), parity (0, ≥1), maternal age (continuous, years), prepregnancy body mass index (BMI) (continuous BMI, kg/m²), maternal ethnicity (White European, non-White European), and maternal education (low, middle, high) as a marker of socioeconomic position. These further adjusted models included fewer participants than in the primary models (n = 914 and n = 875 for the birth weight and head circumference models, respectively) due to missingness of some of the additional adjustment variables (Figure 1). Models were run among the entire population and separately among nonsmoking mothers. In a sensitivity analysis, we additionally adjusted all models for passive smoking status. Given passive smoking status was missing for some participants (10%), these analyses were restricted to those with complete data on this variable, comparing associations adjusted with and without this variable. In an additional sensitivity analysis, we removed child sex from models.
FIGURE 1.
Flow chart for the number of participants included in each specified analysis.
We also used logarithmic binomial regression models to determine the risk ratios (RR) and 95% confidence intervals (CIs) for SGA. We examined effect estimates adjusted by country and also effect estimates adjusted for the additional aforementioned a priori potential confounders. Estimates were again given for the whole cohort and separately for infants of nonsmoking mothers.
In addition to estimating pooled associations adjusted for country, we estimated country-specific associations between EO Hb adducts and birth weight and head circumference, visually presenting results so that differences between countries for effect estimates could be seen.
Finally, we tested the interaction of the 15 CYP2E1 SNPs with the exposure–outcome relationships between EO Hb adducts and birth weight and head circumference, adjusting for gestational age, country, and the four genome-wide association studies principal components, which we found separated European versus non-European ancestries and subgroups within European ancestries. We provide estimates among the population of European ancestry mothers and additionally provide estimates among nonsmoking European ancestry mothers. SNPs were treated under an additive genetic model. In total, data from 649 participants were included in the CYP2E1 analysis for birth weight and 605 in the analysis for head circumference. Multiple-testing correction was applied considering all the SNPs tested using the Bonferroni method. All statistical analyses were conducted using Stata S.E. version 16.0 (StataCorp, College Station, TX).
RESULTS
General Sociodemographic and Adduct Results
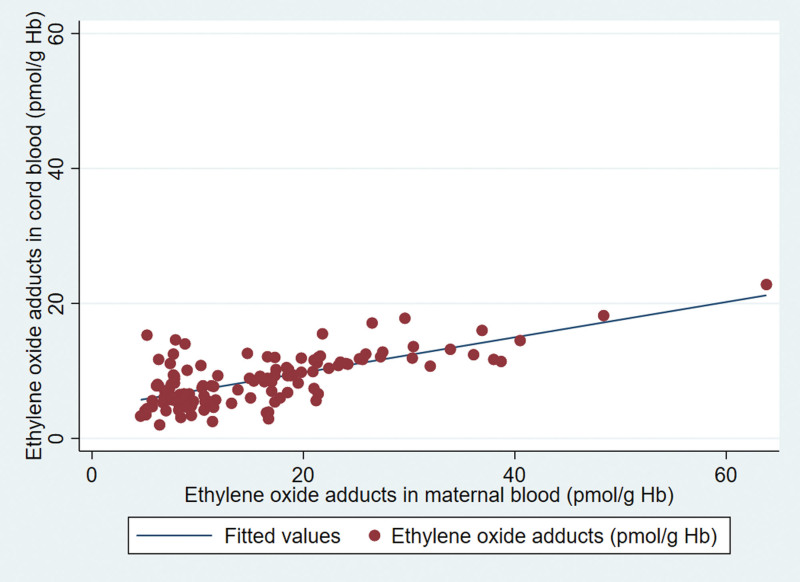
In this study, we analyzed EO Hb adducts in 1106 cord blood samples. The mothers’ average age was 31 (25th percentile–75th percentile [Q1–Q3]: 28–34) years. Among them, 382 (36%) were first-time mothers (nulliparous), 906 (83%) identified as White European, 129 (12%) smoked at the end of their pregnancies, 356 (36%) reported exposure to second-hand smoke, and 618 (56%) had vaginal deliveries. The median birth weight of offspring in the full population was 3394 (Q1–Q3: 3,090–3,714) g and for nonsmokers 3430 (Q1–Q3: 3,120–3,740) g. Meanwhile, the median head circumference of offspring regardless of maternal smoking status was 35 (Q1–Q3: 34–36) cm. The median gestational age was 39 weeks (Q1–Q3: 38–40), as shown in Table 1. The median EO adduct level was 9 (Q1–Q3: 7–15) pmol/g of Hb for all samples of cord blood and 16 (Q1–Q3: 8–25) pmol/g for maternal blood (n = 146 available pairs with data on maternal blood levels in addition to cord blood levels), with a positive correlation between the two values (correlation 0.94), as shown in Figure 2. Median cord Hb adduct levels were higher among smoking mothers (25, Q1–Q3: 14–41 pmol/g, n = 129; not shown) compared with nonsmoking mothers (9, Q1–Q3: 6–13 pmol/g, n = 951). In addition, among those reporting exposures to second-hand smoke (passive smoking), median EO cord blood Hb adducts were slightly higher in both nonsmokers (9, Q1–Q3: 6–13 pmol/g, n=271) and smokers (27, Q1–Q3: 15–46 pmol/g, n = 85).
TABLE 1.
Characteristics of the Study Population and Stratified by Smoking Status
| Variablesa | All (N = 1,106) | Nonsmokers (n = 951) | ||||
|---|---|---|---|---|---|---|
| n | %b | Median (Q1–Q3) | n | %b | Median (Q1–Q3) | |
| Country of birth | ||||||
| Greece | 246 | 22% | 184 | 20% | ||
| Spain | 225 | 20% | 185 | 19% | ||
| Norway | 231 | 21% | 225 | 24% | ||
| United Kingdom | 187 | 17% | 154 | 16% | ||
| Denmark | 217 | 20% | 203 | 21% | ||
| Smoking at end of pregnancy | 129 | 12% | - | - | - | |
| Maternal passive smoking exposure | 356 | 36% | 271 | 31% | ||
| Maternal ethnicity | ||||||
| White European | 906 | 83% | 771 | 81% | ||
| Non-White European | 187 | 17% | 179 | 19% | ||
| Maternal age (years) | 31 (28–34) | 31 (28–35) | ||||
| Prepregnancy BMI (kg/m2) | ||||||
| <18.5 | 43 | 4% | 35 | 4% | ||
| 18.5–25 | 616 | 63% | 556 | 64% | ||
| ≥25–30 | 204 | 21% | 180 | 21% | ||
| ≥30 | 110 | 11% | 91 | 11% | ||
| Maternal education | ||||||
| High | 348 | 36% | 333 | 39% | ||
| Middle | 373 | 39% | 328 | 38% | ||
| Low | 242 | 25% | 197 | 23% | ||
| Nulliparous | 382 | 36% | 347 | 37% | ||
| Birth weight (g) | 3,394 (3,090–3,714) | 3,430 (3,120–3,740) | ||||
| Low birth weight (<2500 g) | 18 | 2% | 11 | 1% | ||
| Small for gestational age | 70 | 8% | 57 | 7% | ||
| Delivery type | ||||||
| Vaginal | 618 | 56% | 550 | 58% | ||
| C-section | 479 | 44% | 399 | 42% | ||
| Gestational age (weeks) | 39 (38–40) | 39 (38–40) | ||||
| Preterm delivery (≤37 completed weeks) | 39 | 4% | 33 | 3% | ||
| Head circumference (cm) | 35 (34–36) | 35 (34–36) | ||||
| Offspring body length (cm) | 51 (50–52) | 51 (50–52) | ||||
| EO Hb adducts (pmol/g) | ||||||
| Cord blood | 9 (7–15) | 9 (6–13) | ||||
| Maternal blood (n=146) | 16 (8–25) | 13 (8–21) | ||||
The following variables had some amount of missing data: smoking at end of pregnancy (n = 26, 2%), maternal passive smoking (n = 116, 10%), maternal ethnicity (n = 11, 1%), maternal age (n = 12, 1%), maternal education (n = 143, 13%), mode of delivery (n = 9, <1%), sex of child (n = 8, <1%), maternal BMI (n = 133, 12%), birthweight (n = 9, <1%), small for gestational age (n = 222, 20%), parity (n = 42, 4%), gestational age (n = 9, <1%), birth head circumference (n = 106, 10%), birth length (n = 261, 24%), and maternal EO Hb adducts were only available for n = 146 (13%) of the population.
Calculated from nonmissing values.
FIGURE 2.
Association between ethylene oxide hemoglobin adducts in cord and maternal blood (n = 146).
We also described EO Hb adduct levels based on study characteristics, with some suggested variability based on country of birth, smoking status, and maternal age among the entire population, while few differences remained among the nonsmoking participants (eTable 2; http://links.lww.com/EDE/C156).
Ethylene Oxide Cord Blood Hemoglobin Adduct Levels and Birth Weight
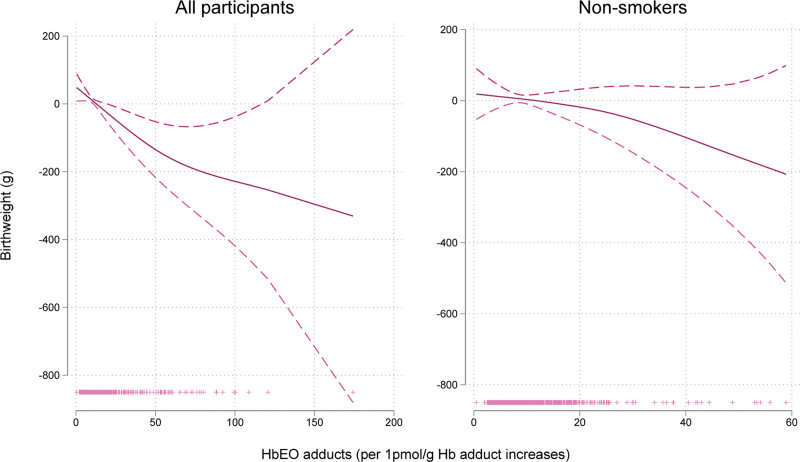
Higher levels of EO Hb adducts in cord blood were associated with a decrease in birth weight (Table 2). In the primary model, the mean birth weight was 3.30 g lower (95% CI = 5.11, −1.48) with each 10 pmol/g Hb increase in EO Hb adduct cord blood levels after adjusting for country of birth and gestational age in the total population, and 3.00 g lower (95% CI = −6.95, 0.94) among the nonsmokers, and the curve of relationship had a linear shape (Figure 3). The estimated difference in mean birth weight for infants in the highest versus lowest quartile of EO cord blood Hb adduct levels after adjusting for gestational age and country was −70.73 g (95% CI = −141.16, −0.30) in the full population and −22.72 g (95% CI = −102.39, 56.94) among the nonsmoker group (Table 2).
TABLE 2.
Prenatal Exposure to Ethylene Oxide (EO) Hemoglobin Adducts in Cord Blood, and Associations With Birth Weight for the Whole Population and Separately Among Nonsmokers
| Birth Weight | Median EO Hb adduct level (±SD) (pmol/g) | Coefficienta | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|
| Primary adjustedb | ||||
| All (N = 1,094) | ||||
| Change per 10 pmol/g Hb | −3.30 | −5.11 | −1.48 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 8 ± 1 | 38.45 | −31.59 | 108.50 |
| Quartile 3 | 12 ± 1 | −20.15 | −90.64 | 50.34 |
| Quartile 4 (highest) | 28 ± 21 | −70.73 | −141.16 | −0.30 |
| Nonsmokers (n = 948) | ||||
| Change per 10 pmol/g Hb | −3.00 | −6.95 | 0.94 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 8 ± 1 | 27.26 | −43.65 | 98.17 |
| Quartile 3 | 11 ± 1 | −3.06 | −74.79 | 68.67 |
| Quartile 4 (highest) | 19 ± 8 | −22.72 | −102.39 | 56.94 |
| Further adjustedc | ||||
| All (n = 914) | ||||
| Change per 10 pmol/g Hb | −2.61 | −5.00 | −0.21 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 8 ± 1 | 32.70 | −40.29 | 105.69 |
| Quartile 3 | 12 ± 1 | −46.50 | −120.78 | 27.77 |
| Quartile 4 (highest) | 26 ± 19 | −26.00 | −105.34 | 53.34 |
| Nonsmokers (n = 815) | ||||
| Change per 10 pmol/g Hb | −3.76 | −7.89 | 0.38 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 7 ± 1 | 22.53 | −51.03 | 96.09 |
| Quartile 3 | 11 ± 1 | −34.66 | −109.65 | 40.33 |
| Quartile 4 (highest) | 19 ± 8 | −25.88 | −108.45 | 56.68 |
Beta coefficient interpreted as change in grams per 10 pmol/g increase in EO cord blood Hb adduct levels.
Adjustment for country and gestational age.
Adjustment for country, gestational age, sex, maternal smoking, maternal age, prepregnancy BMI, maternal ethnicity, and maternal education.
FIGURE 3.
Generalized additive model plots for ethylene oxide hemoglobin adducts and birth weight adjusted for gestational age, child sex, and country. In the y-axis is the β coefficient for birth weight and in the x-axis is the level of ethylene oxide hemoglobin adducts (n = 1094 for all participants, n = 948 for nonsmokers).
In analyses further adjusted for additional maternal characteristics and sex, results remained similar, although 95% CIs were wider. Only the estimate between continuous EO Hb cord blood adduct levels and birth weight among the whole population remained different with an estimated change of −2.61 g (95% CI = −5.00, −0.21) per 10 pmol/g Hb increase in EO cord blood Hb adduct levels. Estimates also remained stable when we adjusted for passive smoking (eTable 3; http://links.lww.com/EDE/C156).
Results from country-specific estimates showed some variability (eFigure 1; http://links.lww.com/EDE/C156). Among nonsmokers a reduction in mean birth weight among those with higher exposure to EO Hb adducts (per 10 pmol/g) was observed in four countries while an increase in mean birth weight was observed in the subset from Norway.
Ethylene Oxide Cord Blood Hemoglobin Adduct Levels and Small for Gestational Age
There was no evidence of an association between EO Hb adduct levels and risk of SGA in the whole population (RR = 1.00, 95% CI = 0.99, 1.02) based on 884 observations and 70 SGA births and for infants of nonsmokers (RR = 0.97, 95% CI = 0.92, 1.02), based on 772 observations and 57 SGA births (Table 3).
TABLE 3.
Prenatal Exposure to Ethylene Oxide Hemoglobin Adducts in Cord Blood, and Associations With Small for Gestational Age for the Whole Population and Separately Among Nonsmokers
| Small for Gestational Age | n | Risk Ratioa | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|
| Primary adjustedb | ||||
| All | 884 | 1.00 | 0.99 | 1.02 |
| Nonsmokers | 772 | 0.97 | 0.92 | 1.02 |
| Further adjustedc | ||||
| All | 785 | 0.99 | 0.98 | 1.01 |
| Nonsmokers | 699 | 0.98 | 0.93 | 1.02 |
Risk ratios are interpreted per 10 pmol/g increase in EO Hb adduct level.
Adjustment for country and gestational age.
Adjustment for country, gestational age, sex, maternal smoking, maternal age, prepregnancy BMI, maternal ethnicity, and maternal education.
Ethylene Oxide Cord Blood Hemoglobin Adduct Levels and Head Circumference at Birth
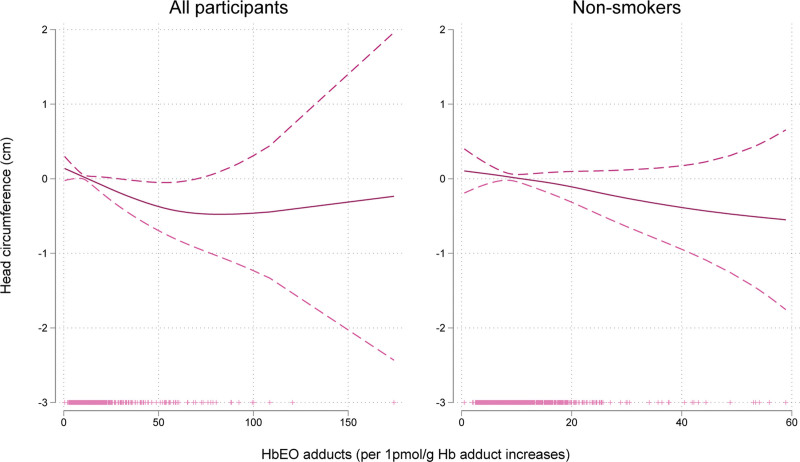
The median head circumference measurement at birth was 35 (Q1–Q3: 34–36). The estimated difference in head circumference for infants in the highest versus lowest quartile of EO Hb adduct levels after applying the primary adjustment for gestational age and country was −0.30 (95% CI = −0.58, −0.02) cm for all participants (Table 4). Increasing EO Hb adduct levels were also associated with decreasing head circumference among nonsmokers in a linear dose-response manner (Figure 4). Effect estimates remained stable when adjusting for passive smoking (eTable 4; http://links.lww.com/EDE/C156).
TABLE 4.
Prenatal Exposure to Ethylene Oxide Hemoglobin Adducts in Cord Blood, and Associations With Birth Head Circumference for the Whole Population and Separately Among Nonsmokers
| Birth Head Circumference | Median EO Hb Adduct Level (±SD) (pmol/g) |
Coefficient | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|
| Primary adjusteda | ||||
| All (n = 998) | ||||
| Change per 10 pmol/g Hbb | −0.01 | −0.02 | 0.00 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 8 ± 1 | −0.06 | −0.35 | 0.22 |
| Quartile 3 | 12 ± 1 | −0.20 | −0.48 | 0.08 |
| Quartile 4 (highest) | 29 ± 20 | −0.30 | −0.58 | −0.02 |
| Nonsmokers (n = 868) | ||||
| Change per 10 pmol/g Hbb | −0.01 | −0.03 | 0.00 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 8 ± 1 | −0.07 | −0.36 | 0.22 |
| Quartile 3 | 11 ± 1 | −0.16 | −0.45 | 0.13 |
| Quartile 4 (highest) | 19 ± 9 | −0.22 | −0.55 | 0.11 |
| Further adjustedc | ||||
| All (n = 875) | ||||
| Change per 10 pmol/g Hbb | 0.00 | −0.01 | 0.01 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 8 ± 1 | −0.02 | −0.32 | 0.27 |
| Quartile 3 | 12 ± 1 | −0.19 | −0.49 | 0.10 |
| Quartile 4 (highest) | 26 ± 18 | −0.12 | −0.44 | 0.20 |
| Nonsmokers (n = 781) | ||||
| Change per 10 pmol/g Hbb | −0.01 | −0.03 | 0.01 | |
| Quartile 1 (lowest) | 5 ± 1 | |||
| Quartile 2 | 8 ± 1 | −0.03 | −0.34 | 0.27 |
| Quartile 3 | 11 ± 1 | −0.17 | −0.48 | 0.14 |
| Quartile 4 (highest) | 19 ± 8 | −0.15 | −0.49 | 0.19 |
Adjustment for country and gestational age.
Beta coefficient interpreted as change in cm per 10 pmol/g increase in EO Hb adduct level.
Adjustment for country, gestational age, sex, maternal smoking, maternal age, prepregnancy BMI, maternal ethnicity, and maternal education.
FIGURE 4.
Generalized additive model plots for ethylene oxide hemoglobin adducts and head circumference adjusted for gestational age, child sex, and country. In the y-axis is the β coefficient for head circumference and in the x-axis is the level of ethylene oxide hemoglobin adducts (n = 998 for all participants, n = 868 for nonsmokers).
The country-specific estimates showed slight variability (eFigure 2; http://links.lww.com/EDE/C156). Among nonsmokers, a reduction in head circumference among those with higher exposure to EO Hb adducts was again observed in four countries, while the estimate was null for those in Norway.
We conducted sensitivity analyses that removed child sex from the further adjusted models and found that results remained similar to those presented in the primary models (eTable 5; http://links.lww.com/EDE/C156).
Candidate Gene Analysis: CYP2E1
In the analyses examining interactions between the CYP2E1 SNPs and the exposure-outcome relationships between EO Hb adducts and birth weight and head circumference, we did not find evidence of any interaction among European ancestry mothers (eTable 6; http://links.lww.com/EDE/C156). In analyses among nonsmoking European ancestry mothers, the SNP rs6537616 modified the estimated effect of ethylene Hb adducts on birth weight; this sequence acts as an expression quantitative trait locus for CYP2E1 in some tissues (eTable 7; http://links.lww.com/EDE/C156). In particular, in the individuals bearing 0 copies of the A allele (GG), the estimated effect of EO Hb adducts on birth weight was 3.66 g (95% CI = −6.15, 13.47), for those with one copy (AG) the effect was −9.55 g (95% CI = −16.90, −2.19), and for those with two copies (AA) the estimate was −22.75 g (95% CI = −39.02, −6.49).
DISCUSSION
In our study, we examined birth outcomes related to EO Hb adducts in cord blood from a multicenter European population. We found that increasing levels of EO Hb adducts in cord blood were associated with decreasing birth weight and head circumference. We observed similar findings among nonsmoking mothers that remained after adjustment for passive smoking.
Prior studies examining EO exposure in relation to adverse birth outcomes have not examined EO Hb adducts in cord blood. In addition, prior studies focused on populations with high occupational exposures, such as dental assistants41 and nurses,8,42 finding that women exposed to higher occupational EO exposure during pregnancy were at greater risk of spontaneous abortions and preterm birth as compared with those with lower occupational exposure to EO sterilizing agents. Our results provide complementary evidence supporting the hypothesis that increased concentrations of Hb adducts in cord blood are linked with decreasing birth weight and head circumference in infants born to women who are likely to have no or very little occupational exposure to EO.
A strength of the present study is that we measured Hb adduct levels in cord blood that reflect exposure to EO. External exposure to EO is short term and difficult to assess. However, using Hb adducts as a biomarker results in a measure of the internal dose of or exposure to EO that reflects any endogenous formation and external exposure.43 The Hb adducts provide a quantitative estimation of internal dose that is highly sensitive and able to detect endogenous processes and low levels of exposure to EO that results in Hb adducts in the cord blood. In addition, this method integrates endogenous processes and exposures over the approximate 4-month lifespan of the erythrocytes. These aspects enhance the validity of our findings, allowing for more robust conclusions regarding the relationship between EO exposure and reproductive outcomes.
Mechanisms through which EO and EO Hb adduct-forming exposures or processes would impair intrauterine growth are not fully understood, but multiple known biologic mechanisms align with the findings of this study. First, EO has electrophilic reactivity and is recognized as a carcinogen.4,5,44 EO is an alkylating agent; alkylating agents have shown potent teratogenic effects in several animal species45 and are also mutagenic.46,47 EO can alter DNA bases, indicating the plausibility of subtle to clinically significant adverse effects on multiple body organs and functions during crucial stages of intrauterine development and growth. Second, tobacco smoking, both active and passive, the main source of exogenous exposure, has a negative impact on birth weight. While smoking is a major contributor to exogenous ethylene or EO exposure,48 we found consistent associations between EO exposure and birth outcomes among nonsmokers, associations that remained stable when adjusting for maternal exposure to passive smoking during pregnancy based on self-reported data. This suggests that babies with higher levels of EO Hb adduct exposure are more likely to have lower birth weight, irrespective of smoking status of the mothers.
Several limitations should be considered. While the use of EO Hb adducts is probably the most valid way to measure exposure that is reflective of both endogenous and exogenous pathways, it should be noted that endogenous levels are inherently influenced by metabolic processes, making them an imperfect measurement of exogenous exposure. We were not able to identify the numerous external sources of EO or ethylene exposure (e.g., occupational, food products), except for smoking, in the present study. It is noteworthy that the metabolism of EO exhibits substantial interindividual variability, underscoring the importance of endogenous exposure sources. These differences could be due to variation in other external exposures, modifying factors such as health status of the mother, the placenta and the child, maternal lifestyle (e.g., sleep, diet, and household products), maternal external exposure to environmental and occupational EO Hb adduct-forming exposures, and birth-related factors. In addition polymorphisms of genes that are central to conversion of ethylene into EO by CYP2E1, and detoxification of EO by phase II metabolism, for example, by glutathione-S-transferase theta 1 gene (GSTT1) mediated conjugation, could result in different individual susceptibility to the same EO exposure.49 Our joint evaluation of EO adduct levels and CYP2E1 SNPs did not show evidence of any interactions, but we could not test the GSTT1 deletion through the array used in the study. It was not within the scope of our study to evaluate the multiple potential maternal, pregnancy, placental, child, and birth-related factors that could influence the biologic pathways such as lipid peroxidation, which contributes to EO Hb adduct formation in cord blood. Furthermore, we only included pregnancies resulting in liveborn births, therefore we were unable to study the effects on early and late fetal loss. Finally, results were not fully homogeneous between countries. This could, in part, be explained by country-specific differences in the ambient air quality, diet and smoking habits, or other unmeasured factors.
Although there are multiple factors that can contribute to the formation of EO Hb adducts in cord blood, a portion of these cord blood adduct levels reflects external exposures. Exposure to ethylene or EO in the general population is of growing concern. Risk assessments have so far focused on cancer risk of occupationally exposed populations.50 Our study is based on a population of pregnant women and newborn infants recruited from the general population, rather than a specific population with known high occupational exposure levels. The findings of our study provide new evidence of an association between EO Hb adduct-forming exposures or processes in cord blood and offspring weight and head circumference at birth that may have long-lasting effects. Reduced head circumference has been linked to delayed neurodevelopment,36 and reduced birth weight increases the risk of cardiovascular disease, type 2 diabetes, and osteoporosis.37 The precise measurement of biomarkers can inform risk assessment processes, aiding regulatory bodies in setting exposure limits or guidelines for EO and safeguarding public health. Further studies are needed in order to identify and reduce external exposure levels, both occupational and nonoccupational, which contribute to the formation of EO Hb adducts.
ACKNOWLEDGMENTS
We thank the participants in the study and the doctors, nurses, midwives, and laboratory technicians who assisted with its conduct; We would also like to acknowledge the members of the NewGeneris consortium and particularly Jos C. Kleinjans (The Netherlands), Soterios A. Kyrtopoulos (Greece), and Dan Segerbäck (Sweden) for their contributions in developing this work.
Supplementary Material
Footnotes
The NewGeneris (Newborns and Genotoxic Exposure Risks) study was funded by the European Union (EU Contract FOOD-CT-2005-016320). The study was also supported by grants obtained locally, including the Swedish Cancer and Allergy Foundation and the Swedish Research Council Formas, the National Institute for Health Research, UK (programme grant RP-PG-0407-10044), the Norwegian Ministry of Health, the Norwegian Ministry of Education and Research, the Norwegian Research Council/FUGE (grant 151918/S10), the EU funded HiWATE (contract Food-CT-2006-036224), the US National Institutes of Health (NIH)/National Institute of Environmental Health Sciences (contract NO-ES75558), and the US NIH/National Institute of Neurological Disorders and Stroke (grant 1 UO1 NS 047537-01). M.P. holds a Juan de la Cierva postdoctoral fellowship awarded from the Spanish Ministry of Science and Innovation (JCI-2011-09479) while preparing the pooled data and conducting the initial statistical analyses for the current study and has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 758151) for CHIPS. B.N.H. has received funding from the Instituto de Salud Carlos III (ISCIII) and the European Union NextGenerationEU/PRTR (Grant IHMC22/00017).
Disclosure: The authors report no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
The data are not available for access outside of the consortium. The code used for analyses will be made available via github.com.
REFERENCES
- 1.Tates AD, Grummt T, Tornqvist M, et al. Biological and chemical monitoring of occupational exposure to ethylene oxide. Mutat Res. 1991;250:483–497. [DOI] [PubMed] [Google Scholar]
- 2.van Sittert NJ, Boogaard PJ, Natarajan AT, Tates AD, Ehrenberg LG, Tornqvist MA. Formation of DNA adducts and induction of mutagenic effects in rats following 4 weeks inhalation exposure to ethylene oxide as a basis for cancer risk assessment. Mutat Res. 2000;447:27–48. [DOI] [PubMed] [Google Scholar]
- 3.IARC. Chemical agents and related occupations. In: IARC Monographs. 2012:100F. [PMC free article] [PubMed] [Google Scholar]
- 4.Steenland K, Stayner L, Deddens J. Mortality analyses in a cohort of 18 235 ethylene oxide exposed workers: follow up extended from 1987 to 1998. Occup Environ Med. 2004;61:2–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Steenland K, Whelan E, Deddens J, Stayner L, Ward E. Ethylene oxide and breast cancer incidence in a cohort study of 7576 women (United States). Cancer Causes Control. 2003;14:531–539. [DOI] [PubMed] [Google Scholar]
- 6.National Toxicology Program. Ethylene oxide: CAS No. 75-21-8. In: 15th Report on Carcinogens. 2021. Available at: https://www.ncbi.nlm.nih.gov/books/NBK590871/. Accessed 23 October 2023. [Google Scholar]
- 7.Hemminki K, Mutanen P, Saloniemi I, Niemi ML, Vainio H. Spontaneous abortions in hospital staff engaged in sterilising instruments with chemical agents. Br Med J (Clin Res Ed). 1982;285:1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gresie-Brusin DF, Kielkowski D, Baker A, Channa K, Rees D. Occupational exposure to ethylene oxide during pregnancy and association with adverse reproductive outcomes. Int Arch Occup Environ Health. 2007;80:559–565. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Toxic Substances and Disease Registry (US). Chapter 2, Health effects. In: Toxicological Profile for Ethylene Oxide. 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK589511/. Accessed 23 October 2023. [PubMed] [Google Scholar]
- 10.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–790. [DOI] [PubMed] [Google Scholar]
- 11.Persson KA, Berg S, Tornqvist M, Scalia-Tomba GP, Ehrenberg L. Note on ethene and other low-molecular weight hydrocarbons in environmental tobacco smoke. Acta Chem Scand B. 1988;42:690–696. [DOI] [PubMed] [Google Scholar]
- 12.Jones RR, Fisher JA, Medgyesi DN, et al. Ethylene oxide emissions and incident breast cancer and non-Hodgkin lymphoma in a US cohort. J Natl Cancer Inst. 2023;115:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galarneau E, Yacovitch TI, Lerner B, et al. From hotspots to background: high-resolution mapping of ethylene oxide in urban air. Atmos Environ. 2023;307:119828. [Google Scholar]
- 14.Morgott DA. Anthropogenic and biogenic sources of ethylene and the potential for human exposure: a literature review. Chem Biol Interact. 2015;241:10–22. [DOI] [PubMed] [Google Scholar]
- 15.Chang C. Q&A: How do plants respond to ethylene and what is its importance? BMC Biol. 2016;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudkiewicz A, Dutta P, Kołożyn-Krajewska D. Ethylene oxide in foods: current approach to the risk assessment and practical considerations based on the European food business operator perspective. Eur Food Res Technol. 2022;248:1951–1958. [Google Scholar]
- 17.Swenberg JA, Lu K, Moeller BC, et al. Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and risk assessment. Toxicol Sci. 2011;120:S130–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filser JG, Denk B, Tornqvist M, Kessler W, Ehrenberg L. Pharmacokinetics of ethylene in man; body burden with ethylene oxide and hydroxyethylation of hemoglobin due to endogenous and environmental ethylene. Arch Toxicol. 1992;66:157–163. [DOI] [PubMed] [Google Scholar]
- 19.Filser JG, Klein D. A physiologically based toxicokinetic model for inhaled ethylene and ethylene oxide in mouse, rat, and human. Toxicol Lett. 2018;286:54–79. [DOI] [PubMed] [Google Scholar]
- 20.Fennell TR, Snyder RW, Parkinson C, Murphy J, James RA. The effect of ethylene exposure on ethylene oxide in blood and on hepatic cytochrome p450 in Fischer rats. Toxicol Sci. 2004;81:7–13. [DOI] [PubMed] [Google Scholar]
- 21.Csanady GA, Denk B, Putz C, et al. A physiological toxicokinetic model for exogenous and endogenous ethylene and ethylene oxide in rat, mouse, and human: formation of 2-hydroxyethyl adducts with hemoglobin and DNA. Toxicol Appl Pharmacol. 2000;165:1–26. [DOI] [PubMed] [Google Scholar]
- 22.Haufroid V, Merz B, Hofmann A, Tschopp A, Lison D, Hotz P. Exposure to ethylene oxide in hospitals: biological monitoring and influence of glutathione S-transferase and epoxide hydrolase polymorphisms. Cancer Epidemiol Biomarkers Prev. 2007;16:796–802. [DOI] [PubMed] [Google Scholar]
- 23.Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:279–308. [DOI] [PubMed] [Google Scholar]
- 24.von Stedingk H., Osterman-Golkar S., Törnqvist M. Biomarkers of exposure: hemoglobin adducts. In: Knudsen LE, Merlo DF, eds. Biomarkers and Human Biomonitoring, Vol 2: Selected Biomarkers of Current Interest. The Royal Society of Chemistry; 2012:1–22. [Google Scholar]
- 25.Center for Disease Control and Prevention. National Center for Environmental Health. National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2022. Updated March 2024. Available at: https://www.cdc.gov/environmental-exposure-report/about/suggested-citation.html. Accessed 10 November 2023. [Google Scholar]
- 26.Tornqvist M. Is ambient ethene a cancer risk factor? Environ Health Perspect. 1994;102:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szwiec E, Friedman L, Buchanan S. Levels of ethylene oxide biomarker in an exposed residential community. Int J Environ Res Public Health. 2020;17:8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Stedingk H, Vikstrom AC, Rydberg P, et al. Analysis of hemoglobin adducts from acrylamide, glycidamide, and ethylene oxide in paired mother/cord blood samples from Denmark. Chem Res Toxicol. 2011;24:1957–1965. [DOI] [PubMed] [Google Scholar]
- 29.Wild CP, Kleinjans J. Children and increased susceptibility to environmental carcinogens: evidence or empathy? Cancer Epidemiol Biomarkers Prev. 2003;12:1389–1394. [PubMed] [Google Scholar]
- 30.Zhang HD, Ma YJ, Liu QF, et al. Human erythrocyte lifespan measured by Levitt’s CO breath test with newly developed automatic instrument. J Breath Res. 2018;12:036003. [DOI] [PubMed] [Google Scholar]
- 31.Dziegiel MH, Koldkjaer O, Berkowicz A. Massive antenatal fetomaternal hemorrhage: evidence for long-term survival of fetal red blood cells. Transfusion. 2005;45:539–544. [DOI] [PubMed] [Google Scholar]
- 32.Merlo DF, Wild CP, Kogevinas M, Kyrtopoulos S, Kleinjans J; NewGeneris Consortium. NewGeneris: a European study on maternal diet during pregnancy and child health. Cancer Epidemiol Biomarkers Prev. 2009;18:5–10. [DOI] [PubMed] [Google Scholar]
- 33.Oudgenoeg-Paz O, Mulder H, Jongmans MJ, van der Ham IJM, Van der Stigchel S. The link between motor and cognitive development in children born preterm and/or with low birth weight: a review of current evidence. Neurosci Biobehav Rev. 2017;80:382–393. [DOI] [PubMed] [Google Scholar]
- 34.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Srinivasan SR, Yao L, et al. Low birth weight is associated with higher blood pressure variability from childhood to young adulthood: the Bogalusa Heart Study. Am J Epidemiol. 2012;176:S99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale CR, O’Callaghan FJ, Bredow M, Martyn CN; Avon Longitudinal Study of Parents and Children Study Team. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. 2006;118:1486–1492. [DOI] [PubMed] [Google Scholar]
- 37.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol. 2016;45:382–388. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen M, von Stedingk H, Botsivali M, et al. ; NewGeneris Consortium. Birth weight, head circumference, and prenatal exposure to acrylamide from maternal diet: the European prospective mother–child study (NewGeneris). Environ Health Perspect. 2012;120:1739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Stedingk H, Rydberg P, Tornqvist M. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2483–2490. [DOI] [PubMed] [Google Scholar]
- 41.Rowland AS, Baird DD, Shore DL, Darden B, Wilcox AJ. Ethylene oxide exposure may increase the risk of spontaneous abortion, preterm birth, and postterm birth. Epidemiology. 1996;7:363–368. [DOI] [PubMed] [Google Scholar]
- 42.Lawson CC, Rocheleau CM, Whelan EA, et al. Occupational exposures among nurses and risk of spontaneous abortion. Am J Obstet Gynecol. 2012;206:327.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preston RJ. Cytogenetic effects of ethylene oxide, with an emphasis on population monitoring. Crit Rev Toxicol. 1999;29:263–282. [DOI] [PubMed] [Google Scholar]
- 44.Steenland K, Stayner L, Greife A, et al. Mortality among workers exposed to ethylene oxide. N Engl J Med. 1991;324:1402–1407. [DOI] [PubMed] [Google Scholar]
- 45.Mattison DR. Environmental exposures and development. Curr Opin Pediatr. 2010;22:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ethylene Oxide (EtO): Evidence of Carcinogenicity. DHHS (NIOSH) PUBLICATION NUMBER 81-130. National Institute for Occupational Safety and Health. May 1981. Available at: https://www.cdc.gov/niosh/docs/81-130/default.html. Accessed 23 October 2023. [Google Scholar]
- 47.Embree JW, Lyon JP, Hine CH. The mutagenic potential of ethylene oxide using the dominant--lethal assay in rats. Toxicol Appl Pharmacol. 1977;40:261–267. [DOI] [PubMed] [Google Scholar]
- 48.Kenwood BM, McLoughlin C, Zhang L, et al. Characterization of the association between cigarette smoking intensity and urinary concentrations of 2-hydroxyethyl mercapturic acid among exclusive cigarette smokers in the National Health and Nutrition Examination Survey (NHANES) 2011-2016. Biomarkers. 2021;26:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiebel FA, Dommermuth A, Thier R. The hereditary transmission of the glutathione transferase hGSTT1-1 conjugator phenotype in a large family. Pharmacogenetics. 1999;9:251–256. [PubMed] [Google Scholar]
- 50.U.S. EPA. Evaluation of the Inhalation Carcinogenicity of Ethylene Oxide (Final Report). EPA/635/R-16/350F; 2016. [Google Scholar]