Abstract
Objectives:
Different types of spinal cord stimulation (SCS) have been evaluated for the management of chronic nonsurgical refractory back pain (NSRBP). A direct comparison between the different types of SCS or between closed-loop SCS with conventional medical management (CMM) for patients with NSRBP has not been previously conducted, and therefore, their relative effectiveness and cost-effectiveness remain unknown. The aim of this study was to perform a systematic review, network meta-analysis (NMA) and economic evaluation of closed-loop SCS compared with fixed-output SCS and CMM for patients with NSRBP.
Methods:
Databases were searched to September 8, 2023. Randomized controlled trials of SCS for NSRBP were included. The results of the studies were combined using fixed-effect NMA models. A cost-utility analysis was performed from the perspective of the UK National Health Service with results reported as incremental cost per quality-adjusted life-year (QALY).
Results:
Closed-loop SCS resulted in statistically and clinically significant reductions in pain intensity (mean difference [MD] 32.72 [95% CrI 15.69-49.78]) and improvements in secondary outcomes (Oswestry Disability Index [ODI] and health-related quality of life [HRQoL]) compared with fixed-output SCS at 6-month follow-up. Compared with CMM, both closed-loop and fixed-output SCS resulted in statistically and clinically significant reductions in pain intensity (closed-loop SCS vs. CMM MD 101.58 [95% CrI 83.73-119.48]; fixed-output SCS versus CMM MD 68.86 [95% CrI 63.43-74.31]) and improvements in secondary outcomes (ODI and HRQoL). Cost-utility analysis showed that closed-loop SCS dominates fixed-output SCS and CMM, and fixed-output SCS also dominates CMM.
Discussion:
Current evidence showed that closed-loop and fixed-output SCS provide more benefits and cost-savings compared with CMM for patients with NSRBP.
Key Words: economic evaluation, network meta-analysis, neurostimulation, nonsurgical refractory back pain, spinal cord stimulation
Low back pain (LBP) affected 619 million people worldwide in 2020, with an estimated prevalence of 843 million by 2050.1 LBP is the single greatest cause of years lived with disability worldwide.1 After an initial acute episode of LBP, between 32% and 60% of people are estimated to have recurrences or experience chronic low back pain CLBP.2,3 The personal and socioeconomic burden is substantial. Approximately 15.4% of the United States workforce report, on average, 10.5 lost workdays per year due to CLBP, equivalent to approximately 264 million workdays lost every year.4 The cost of LBP has been estimated to account for approximately one-fifth of the total health care expenditure in the United Kingdom or 1.5% of the annual gross domestic product.5 The management of chronic back pain has been estimated to have annual costs of £11 billion in the United Kingdom.6 Back pain represented the highest amount of health care spending in the United States in 2016, with an estimated $134 billion per year.7
Treatment options for the management of CLBP start with the least costly and invasive options.8 Patients who do not obtain satisfactory pain relief or experience intolerable side effects are considered to experience chronic refractory back pain. For those patients, treatment options with greater costs and potential risks are then considered. Spinal surgery is an invasive, irreversible and expensive approach to manage LBP, although its benefit is often suboptimal.9 Estimates suggest that persistent spinal pain syndrome type 2 (PSPS-T2; ie, despite surgery) is observed in 5000 new patients annually with each new cohort costing the UK health care system in excess of £70 million over the first 10 years alone.10
Spinal cord stimulation (SCS) is a recommended intervention for the management of PSPS-T2.11 PSPS-T2 is characterized by pain in the low back area that radiates to one or both legs that did not resolve with back surgery.12 Earlier randomized controlled trials (RCTs) of fixed-output low-frequency stimulation aimed to evaluate improvements in leg pain due to challenges in obtaining paresthesia in the lower back region.13,14 Since then, novel modalities of SCS have been developed that do not rely on patient report of paresthesia to determine whether the electrical stimulation delivered activates the spinal cord cells and/or fibers that contribute to the inhibition of pain transmission in the dorsal horn of the spinal cord. The use of novel SCS modalities such as fixed-output high-frequency SCS, burst SCS, and closed-loop SCS has been evaluated in RCTs15–17 that included participants with nonsurgical refractory back pain (NSRBP), also referred to as persistent spinal pain syndrome type 1 (PSPS-T1).12 A direct comparison between the different types of SCS or between closed-loop SCS with conventional medical management (CMM) for patients with NSRBP has not been previously conducted, and therefore, their relative efficacy remains unknown. Network meta-analysis (NMA) can combine direct and indirect evidence, including all relevant data from studies with at least 2 treatment arms, and therefore allow assessment of interventions that may not have been evaluated in a head-to-head comparison. NMA results can also be used to inform clinical parameters of economic models.
The aim of this study was to perform an NMA and develop an economic model to assess the relative effectiveness and cost-effectiveness of closed-loop SCS compared with fixed-output SCS and CMM in patients with NSRBP.
MATERIALS AND METHODS
The systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses incorporating NMA (PRISMA-NMA).18 The protocol for the review is registered on PROSPERO as CRD42023449215. The economic evaluation is reported in line with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement19 and based on NICE reference methods.20
Search Strategy
The databases MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and WikiStim were searched from inception to September 8, 2023. The search strategies were designed using a combination of both indexing and free-text terms with no restriction on language or date. The search strategies are presented in Supplementary Material 1, Supplemental Digital Content 1, http://links.lww.com/CJP/B121. Database searches were supplemented by screening reference lists of topic-relevant systematic reviews and eligible studies.
Study Selection
The citations identified were assessed for inclusion in the review using a 2-stage process. First, 2 reviewers (RVD and NS) independently screened all titles and abstracts identified by the database searches to identify potentially relevant articles to be retrieved. Second, full-text copies of these studies were obtained and assessed independently by 2 reviewers (RVD and NS) for inclusion, using consensus for any disagreements. Studies were eligible for inclusion if they met the following criteria: (1) adult patients (18 y of age or older) with chronic NSRBP; (2) evaluation of SCS (any stimulation paradigm); (3) compared with CMM, an active intervention (including another stimulation paradigm) or placebo; (4) in a parallel group RCT study design; and (5) reported in a full-text publication.
Outcomes
The primary outcome was percentage reduction from baseline in back pain score according to the visual analog scale (VAS) or numeric rating scale (NRS). Secondary outcomes were:
proportion of patients achieving ≥50% reduction in back pain VAS or NRS score
proportion of patients achieving ≥80% reduction in back pain VAS or NRS score
proportion of patients with ≥10-point reduction in Oswestry Disability Index (ODI) score
change from baseline in ODI score
change from baseline in health-related quality of life (HRQoL [EQ-5D-5L index score])
proportion of patients improved or very much improved/better or a great deal better according to Patient Global Impression of Change (PGIC)
Primary and secondary outcomes were considered at 3 months and 6 months post-implantation. Crossover was permitted within both groups in the SENZA-NSRBP15 and DISTINCT RCT17 at 6 months post-implantation, therefore only data before crossover were considered for inclusion in analysis. Adverse events and device explants were considered at last follow-up reported.
Risk of Bias Assessment
The risk of bias (RoB) was assessed by using the revised Cochrane RoB tool (RoB 2.0).21 RoB assessment of the included studies was undertaken by one reviewer and verified for agreement by a second reviewer. Authors involved in the systematic review who were involved in the original studies did not assess RoB of included RCTs. Any disagreements were resolved by consensus.
Data Extraction and Statistical Analysis
Data extracted were study author and year of publication, study design characteristics, demographic data (ie, age and sex), details on the intervention and comparators, and outcome data, including the number of participants and the measurement time of the outcome. Data were extracted from the subgroup of patients with NSRBP only from the EVOKE RCT.16
Outcome data were extracted for inclusion in analysis in the first instance from intention-to-treat (ITT) populations with or without imputed missing data (eg, last value carried forward [LVCF]). Where data from ITT populations were not available, outcome data from those completing 3 months and 6 months in the study respectively were included in analysis.
To standardize outcome data to a single scale for pain intensity, it was assumed that the VAS scale (0 to 10 cm) and the NRS scale (0 to 10) were equivalent, and the VAS scale (0 to 100 mm) was converted by dividing pain scores by 10.22,23
In addition to the direct comparisons of fixed-output SCS versus CMM and closed-loop SCS versus fixed-output SCS made within the 3 RCTs, an indirect comparison of closed-loop SCS and CMM was made using NMA methodology. To conduct an NMA, high-frequency SCS used in SENZA-NSRBP,15 open-loop SCS used in EVOKE16 and burst SCS used in DISTINCT17 were assumed to form a single treatment node of fixed-output SCS in the network (Fig. 1).
FIGURE 1.
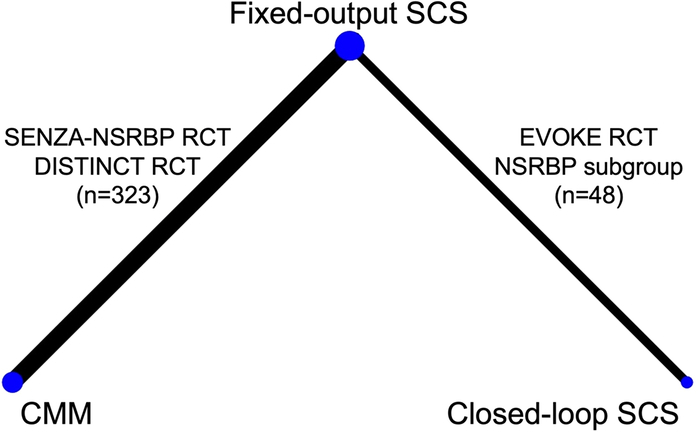
Network diagram of fixed-output SCS, closed-loop SCS and CMM at 6 months follow-up. CMM indicates conventional medical management; NSRBP, non-surgical refractory back pain; RCT, randomized controlled trial; SCS, spinal cord stimulation.
The measure of treatment effect for outcomes measured as a continuous change from baseline was mean difference (MD; ie, the absolute difference in the average change from baseline between intervention and control groups), and for outcomes measured as proportion of patients was risk difference (RD; ie, absolute difference in the proportion of patients experiencing an outcome between intervention and control groups, a measure that can be calculated and interpreted when the number of people experiencing an outcome is very low or zero in one or both of the treatment groups). Descriptive statistics were used to summarize adverse events and device explants.
Statistical analyses were conducted in a Bayesian framework using the multinma24 command in R version 4.2.0. All results were generated using 100,000 iterations on 3 chains after a burn-in of 100,000 and vague prior distributions were used for intercept, treatment, and heterogeneity parameters. NMAs were performed using fixed-effect and random-effects models; however, due to sparse data, convergence issues occurred for random-effects NMA models, resulting in unstable effect estimates. Therefore, treatment effect estimates and 95% credible intervals (CrIs) estimated using fixed-effect NMA models only are presented. The probability that each treatment is the best is also presented for each outcome based on the results of fixed-effect NMA.
Health Economic Analysis
A cost-utility analysis with a 15-year time horizon was performed from the perspective of the UK National Health Service to estimate the long-term costs and benefits of closed-loop SCS, fixed-output SCS, and CMM for a population of adult patients with NSRBP. The economic evaluation is based on previously published methodology and aligns with the same set of assumptions.25 Health care resource use was costed at 2022 prices. An annual discount rate of 3.5% was applied for both costs and outcomes in line with NICE recommendations.20 The results were reported as incremental cost-utility ratio (ICUR) by calculating the ratio of the difference in mean costs and mean change in quality-adjusted life years (QALYs). Full details of the health economic analysis methods are presented in Supplementary Material 2, Supplemental Digital Content 1, http://links.lww.com/CJP/B121.
RESULTS
The searches resulted in the identification of 2075 potentially eligible records after deduplication. Following the screening of titles and abstracts, 16 records were retrieved for assessment of the full-text publication. After review of the full-text publications, 3 unique studies (371 participants) were included in the review. Thirteen studies were excluded on review of the full-text publication; 2 studies were not RCT in design,26,27 1 study was only available as conference abstract,28 2 follow-up reports of the EVOKE RCT were excluded because data were not presented for the subpopulation of NSRBP,29,30 1 follow-up report of SENZA-NSRBP was excluded due to only presenting data after crossover,31 7 studies were not in a population with NSRBP, or this was a subpopulation with the results not presented specifically for patients with NSRBP.32–38 Adverse events and device explants were extracted from the last follow-up reports of the included studies.29–31 The PRISMA flow diagram detailing the study selection process is presented in Figure 2.
FIGURE 2.
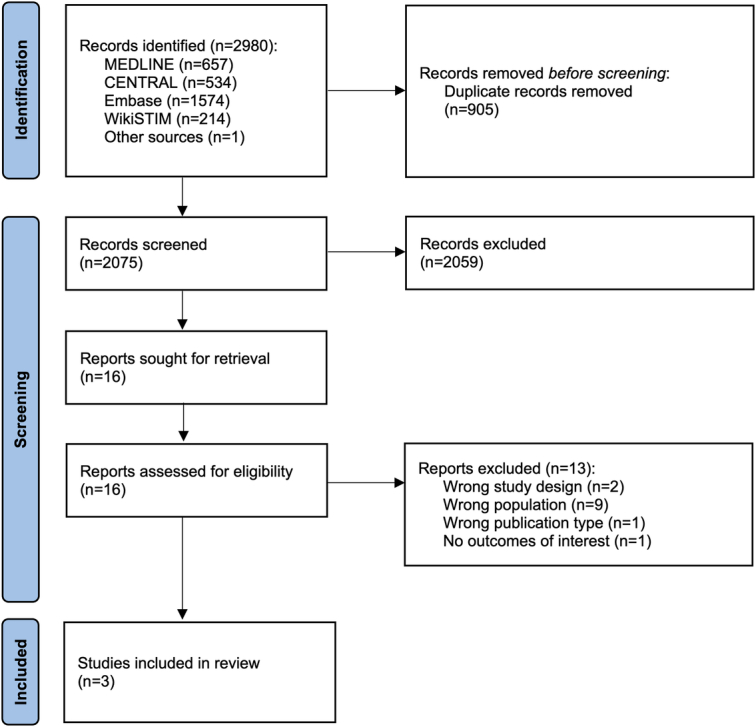
PRISMA 2020 flow diagram.
The characteristics and outcomes of the included RCTs are summarized in Table 1. The RCTs were funded by industry, multicenter and conducted in the United States. SENZA-NSRBP compared high-frequency SCS to CMM,15 DISTINCT compared Burst SCS to CMM,17 while EVOKE compared closed-loop SCS to open-loop SCS.16 All RCTs included a temporary screening trial before implantation of the permanent SCS device. The populations included in the RCTs were of similar age and had similar diagnosis. Patients in SENZA-NSRBP had shorter duration of pain than those reported in the other studies.
TABLE 1.
Characteristics of Randomized Controlled Trials Included in the Systematic Review
| References | Intervention | Control | Follow-up duration | Number randomized, sex, and mean age±SD | Most common diagnosis and duration of pain |
|---|---|---|---|---|---|
| Deer et al17 DISTINCT | B-SCS (fixed-output SCS) | CMM | 6 mo 6 mo until crossover, outcomes at longer follow-ups not yet reported |
B-SCS N=162, F=96 (59%), 58.1±13.0 y CMM N=107, F=52 (51%), 59.1±12.4 y |
Nonspecific low back pain, lumbar spondylosis, radiculopathy, degenerative disk disease, spinal stenosis B-SCS 11.9±10.6 y CMM 13.1±12.4 y |
| Kapural et al15 SENZA-NSRBP | HF-SCS (fixed-output SCS) | CMM | 12 mo 6 mo until crossover, only outcomes until 6 mo included in the analysis |
HF-SCS N=83, F=50 (60%), Mdn=53 y (29–87) CMM N=76, F=40 (53%), Mdn=58.5 y (26–77) |
Degenerative disk disease, spondylosis, radiculopathy, mild/moderate spinal stenosis, spondylolisthesis, sacroiliac dysfunction HF-SCS Mdn=8.5 y (0.5-52) CMM Mdn=8 y (1-59) |
| Mekhail et al16 EVOKE NSRBP subgroup | CL-SCS | OL-SCS (fixed-output SCS) | 36 mo 24 mo until crossover, only outcomes until 6 mo included in the analysis |
CL-SCS N=25, F=12 (48%), 53.0±8.3 y OL-SCS N=25, F=12 (48%), 55.6±11.3 y |
Degenerative disk disease, mild-moderate spinal stenosis, radiculopathy, spondylosis without myelopathy CL-SCS 17.2±12.1 y OL-SCS 12.4±10.6 y |
B-SCS indicates burst SCS; CL-SCS, closed-loop SCS; CMM, conventional medical management; HF-SCS, high-frequency spinal cord stimulation; Mdn, median; NSRBP, non-surgical refractory back pain; y, years.
Risk of Bias Assessment
The summary of the RoB assessment is presented in Table 2. The RCTs were judged to have a low RoB for the domains of the process of randomization and deviations from intended interventions. Patients randomized to the CMM group in SENZA-NSRBP received best standard of care, which was required to be consistent with clinical and interventional pain management guidelines.15 In DISTINCT, patients randomized to CMM could receive noninterventional therapies, medications, and interventional therapies as decided by the investigator.17 Although the descriptions of CMM are limited, RoB due to deviations from intended interventions was not considered to be present in the trials as the populations in SCS studies would already have tried several, if not all types of CMM. The level of missing data for the EVOKE NSRBP subgroup was judged as presenting some concerns due to imbalance in the number of completers between treatment groups, which may have biased outcome data in favor of fixed-output SCS. This bias does not apply to the trial as a whole but only to the EVOKE NSRBP subgroup. DISTINCT and SENZA-NSRBP were judged to have high RoB for the outcome measurement domain as the trial was open-label, with participants and outcome assessors aware of the interventions received.15,17 In EVOKE, patients, investigators, and staff, including outcome assessors were blinded and the study was therefore judged to have low RoB for the outcome measurement domain.16 DISTINCT was judged as presenting some concerns for the selection of the reported results domain because study protocol was not available and not explicitly mention that a prespecified statistical analysis plan was followed.17 The overall bias for DISTINCT and SENZA-NSRBP was judged to be high because at least 1 domain was judged to have a high RoB. The overall bias for the EVOKE NSRBP subgroup was judged to present some concerns.
TABLE 2.
Risk of Bias Assessment
| References | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|
| Deer et al17 DISTINCT | Low | Low | Low | High | Some concerns | High |
| Kapural et al15 SENZA-NSRBP | Low | Low | Low | High | Low | High |
| Mekhail et al16 EVOKE NSRBP subgroup | Low | Low | Some concerns | Low | Low | Some concerns |
Outcomes
Outcomes and adverse events reported in the included studies are presented in Table 3. Results were available for all outcomes from the EVOKE NSRBP subgroup with missing values imputed (LVCF; n=50 at 3 months and n=48 at 6 months) and for pain outcomes from the EVOKE NSRBP subgroup who completed the study (completers; n=41 at 3 months and n=39 at 6 months). Results were only available for completers from SENZA-NSRBP (n=143 at 3 months and n=140 at 6 months) and from completers from DISTINCT at 6 months (n=183).
TABLE 3.
Outcomes of Randomized Controlled Trials Included in the Systematic Review
| References | Intervention | Control | Outcomes included in the analysis at 6 mo | Key findings | Adverse events at last follow-up | Explants at last follow-up |
|---|---|---|---|---|---|---|
| Deer et al17 DISTINCT | B-SCS (fixed-output SCS) | CMM | Percent pain reduction from baseline Proportion of patients with ≥50% pain reduction Proportion of patients with ≥80% pain reduction Change in ODI Proportion of patients with MCID ≥10 in ODI Proportion of patients reporting “better or a great deal better” in PGIC |
↑ P<0.001 ↑ P<0.001 B-SCS 42.2%/CMM 1.2% ↑ P<0.001 B-SCS 91.2%/CMM 16.0% ↑ P<0.001 |
Fourteen nonserious device- or procedure-related events were reported in the B-SCS arm (14/162, 8.6%), of which 6 lead migrations (3.7%), 2 infections (1.2%), 2 skin reactions, 2 IPG pocket pain, 1 CSF leakage, and 1 IPG migration. Three SAEs were reported, of which 2 were infections that required explant, 1 was postprocedural abdominal pain resolved with pain management without sequelae | At 6 mo there were 2 explants due to infections; no explants reported due to LoE |
| Kapural et al15 SENZA-NSRBP | HF-SCS (fixed-output SCS) | CMM | Percent pain reduction from baseline Proportion of patients with ≥50% pain reduction Proportion of patients with ≥80% pain reduction Proportion of patients with MCID ≥10 in ODI Change in HRQoL (EQ-5D utility) Proportion of patients reporting “better or a great deal better” in PGIC |
↑ P<0.001 ↑ P<0.001 HF-SCS 58.5%/CMM 0% ↑ P<0.001 ↑ P<0.001 ↑ P<0.001 |
At 12 mo there were 5 SAEs (2 implant site infections, 1 poor wound healing, 1 lethargy, 1 osteomyelitis). Over 24 mo, there were 51 study-related AEs.32 The most common AE was implant site pain (13 events in 12/145 patients [8.3%]; 8 [5.5%] required IPG repositioning. Implant site infection was reported by 6 patients (4.1%), and 3 patients (2%) had transient CSF leakage. Six patients (4.1%) underwent lead revision due to lead dislodgment | Over 24 mo there were 6 (4.8%) explants out of 125 SCS devices.(32) Three (2.4%) were due to patient dissatisfaction with SCS therapy (inefficacy, LoE), and 3 (2.4%) due to infection (2 of these patients received a replacement device) |
| Mekhail et al16 EVOKE NSRBP subgroup | CL-SCS | OL-SCS (fixed-output SCS) | Percent pain reduction from baseline Proportion of patients with ≥50% pain reduction Proportion of patients with ≥80% pain reduction Change in ODI Proportion of patients with MCID ≥10 in ODI Change in HRQoL (EQ-5D utility) Proportion of patients reporting “improved or very much improved” in PGIC |
↑ P<0.001 ↑ P<0.01 ↑ P<0.05 ↑ P<0.01 ↑ P<0.01 ↑ P<0.001 ↑ P<0.001 |
At 36 mo there were 7 AEs in 6 (24%) patients in the OL-SCS group and 13 AEs in 8 (32%) patients in the CL-SCS group (rate difference 8.0 [95% CI: −16.8 to 32.8]). Most frequently reported AEs were IPG pocket pain (2 [8%] in OL-SCS group and 3 [12%] in CL-SCS group) and lead migration (2 [8%] in OL-SCS group and 2 [8%] in CL-SCS group) | Over 36 mo there were 5 (10%) explants. 1 (4%) explant due to LoE in OL-SCS, 2 explants due to need for MRI (1 [4%] in OL-SCS and 1 [4%] in CL-SCS), and 2 explants due to subsequent unrelated comorbid conditions (1 [4%] in OL-SCS and 1 [4%] in CL-SCS); no explants reported due to LoE for CL-SCS |
(-) no statistically significant differences between groups.
↑ statistically significant between groups in favor of intervention group.
AE indicates adverse event; B-SCS, burst SCS; CL-SCS, closed-loop SCS; CMM, conventional medical management; CSF, cerebrospinal fluid; HF-SCS, high-frequency spinal cord stimulation; HRQoL, health-related quality of life; IPG, implantable pulse generator; LoE, loss of efficacy; MCID, minimal clinical important difference; NSRBP, non-surgical refractory back pain; ODI, Oswestry Disability Index; OL-SCS, open-loop SCS; PGIC, Patient Global Impression of Change; SAE, serious adverse event; SCS, spinal cord stimulation.
In this section, results are presented from NMAs, including the EVOKE NSRBP subgroup with LVCF and completers from SENZA-NSRBP and DISTINCT. Results of NMAs, including completers from the EVOKE NSRBP subgroup (where available) and completers from SENZA-NSRBP and DISTINCT are presented in Supplementary Material 3, Supplemental Digital Content 1, http://links.lww.com/CJP/B121. Results from NMAs at 3 months include SENZA-NSRBP and EVOKE only, as results at this timepoint were not reported for DISTINCT.
Primary Outcome: Change From Baseline in Pain Score
Meta-analysis results for primary and secondary outcomes are reported in Table 4. All RCTs reported the mean percentage reduction from baseline back pain VAS or NRS score and the proportions of patients with 50% reduction and 80% reduction from baseline back pain VAS score at 6 months.
TABLE 4.
Primary and Secondary Outcomes: Fixed-effect NMA Results
| Mean percentage reduction from baseline back pain VAS/NRS score | ||
|---|---|---|
| Mean difference (95% CrI): % | ||
| Comparison* | 3 mo† | 6 mo‡ |
| Closed-loop SCS vs. Fixed-output SCS | 26.38 (8.18-44.59) | 32.72 (15.69-49.78) |
| Fixed-output SCS vs. CMM | 74.34 (66.62-82.06) | 68.86 (63.43-74.31) |
| Closed-loop SCS vs. CMM | 100.72 (80.92-120.51) | 101.58 (83.73-119.48) |
| Proportion of patients with 50% and 80% reduction from baseline back pain VAS/NRS score at 6 mo | ||
| Risk Difference (95% CrI) | ||
| Comparison§ | 50% reduction‡ | 80% reduction‡,∥ |
| Closed-loop SCS vs. fixed-output SCS | 0.46 (0.21-0.71) | 0.29 (0.05-0.53) |
| Fixed-output SCS vs. CMM | 0.78 (0.72-0.85) | 0.48 (0.40-0.56) |
| Closed-loop SCS vs. CMM | 1.24 (0.98-1.50) | 0.77 (0.52-1.02) |
| Proportion of patients with ≥10-point reduction in ODI score (ODI responders) from baseline | ||
| Risk Difference (95% CrI) | ||
| Comparison§ | 3 mo† | 6 mo‡ |
| Closed-loop SCS vs Fixed-output SCS | 0.36 (0.13-0.58) | 0.37 (0.14-0.61) |
| Fixed-output SCS vs CMM | 0.69 (0.57-0.81) | 0.75 (0.68-0.82) |
| Closed-loop SCS vs CMM | 1.05 (0.79-1.30) | 1.12 (0.87-1.37) |
| Mean change from baseline in ODI | ||
| Mean difference (95% CrI) | ||
| Comparison* | ODI (6 mo)¶ | |
| Closed-loop SCS vs. fixed-output SCS | 14.48 (5.97-23.03) | |
| Fixed-output SCS vs. CMM | 28.68 (24.06-33.29) | |
| Closed-loop SCS vs. CMM | 43.15 (33.47-52.84) | |
| Mean change from baseline in EQ-5D-5L Index score | ||
| Mean difference (95% CrI) | ||
| Comparison* | 3 mo† | 6 mo# |
| Closed-loop SCS vs. fixed-output SCS | 0.07 (−0.03 to 0.18) | 0.12 (0.02-0.22) |
| Fixed-output SCS vs. CMM | 0.20 (0.16-0.25) | 0.24 (0.20-0.29) |
| Closed-loop SCS vs. CMM | 0.28 (0.17-0.39) | 0.36 (0.25-0.47) |
Mean difference >0 favors the first intervention in the comparison over the second intervention.
EVOKE NSRBP subgroup with LVCF (n=50) and completers from SENZA-NSRBP (n=143).
EVOKE NSRBP subgroup with LVCF (n=48) and completers from SENZA-NSRBP (n=140) and DISTINCT (n=183).
Risk difference > 0 favors the first intervention in the comparison over the second intervention.
Convergence problems occurred due to very low numbers of patients (0 to 1) achieving ≥80% pain reduction in CMM group of SENZA-NSRBP and DISTINCT.
Evoke NSRBP subgroup with LVCF (n=48) and completers from DISTINCT (n=183).
Evoke NSRBP subgroup with LVCF (n=48) and completers from SENZA-NSRBP (n=140).
CMM indicates conventional medical management; CrI, Credible interval; NMA, network meta-analysis; NRS, numeric rating scale; ODI, Oswestry Disability Index; SCS, spinal cord stimulation; VAS, visual analog scale.
A much larger percentage reduction in back pain VAS or NRS score was achieved on both fixed-output SCS and closed-loop SCS compared with CMM at both 3 months and 6 months follow-up. Furthermore at 6 months, more patients achieved 50% reduction and 80% reduction in back pain VAS score on fixed-output SCS or closed-loop SCS compared with CMM.
Closed-loop SCS was superior to fixed-output SCS in reducing back pain VAS or NRS score, achieving 50% reduction and 80% reduction of baseline back pain VAS or NRS score.
Results of NMAs, including completers from the Evoke NSRBP subgroup and completers from SENZA-NSRBP and DISTINCT (Table S3, Supplemental Digital Content 1, http://links.lww.com/CJP/B121, Table S4, Supplemental Digital Content 1, http://links.lww.com/CJP/B121) and the proportions of patients with 50% reduction from baseline back pain VAS or NRS score at 3 months (Table S5, Supplemental Digital Content 1, http://links.lww.com/CJP/B121) were similar for fixed-output SCS and closed-loop SCS compared with CMM. When including completers from the EVOKE NSRBP subgroup and completers from SENZA-NSRBP, no difference was found between closed-loop SCS and fixed-output SCS for most pain outcomes (Table S3–S5, Supplemental Digital Content 1, http://links.lww.com/CJP/B121). However, it should be noted that all noncompleters from the EVOKE NSRBP subgroup were in the fixed-output SCS group and the imbalance between the number of completers in the treatment groups was likely to have biased the change in pain score outcome data in favor of fixed-output SCS.
Secondary Outcomes
All 3 trials reported the proportion of patients with ≥10-point reduction in ODI score from baseline. The mean change in ODI score was reported in DISTINCT and EVOKE NSRBP subgroup at 6 months. The mean change from baseline in EQ-5D-5L index score was reported in SENZA-NSRBP and EVOKE NSRBP subgroup at 3 months and at 6 months.
There were more ODI responders and a greater improvement from baseline in EQ-5D-5L index score on fixed-output SCS or closed-loop SCS compared with CMM at both 3 months and 6 months. There were also more ODI responders on closed-loop SCS compared with fixed-output SCS at both 3 months and 6 months and a greater improvement from baseline in EQ-5D-5L index score on closed-loop SCS compared with fixed-output SCS by 6 months, but not at 3 months. There was also a greater improvement from baseline in ODI score on fixed-output SCS or closed-loop SCS compared with CMM and on closed-loop SCS compared with fixed-output SCS at 6 months.
More patients were improved or very much improved/better or a great deal better according to PGIC on fixed-output SCS or closed-loop SCS compared with CMM, and also on closed-loop SCS compared with fixed-output SCS at both 3 months and 6 months (Table S6, Supplemental Digital Content 1, http://links.lww.com/CJP/B121).
Anticipated absolute effect for primary and secondary outcomes is presented in Table S7, Supplemental Digital Content 1, http://links.lww.com/CJP/B121.
Closed-loop SCS has the highest probability of being the best treatment option for all outcomes at all timepoints (Table 5).
TABLE 5.
Probability Each Treatment Is the Best Option
| Treatment (%) | ||||
|---|---|---|---|---|
| Outcome* | Timepoint (mo) | Fixed-output SCS | Closed-loop SCS | Control: CMM |
| Percentage reduction from baseline back pain VAS/NRS score | 3 | 0 | 100 | 0 |
| 6 | 0 | 100 | 0 | |
| Proportion of patients with 50 reduction from baseline back pain VAS / NRS score | 6 | 0 | 100 | 0 |
| Proportion of patients with 80 reduction from baseline back pain VAS/NRS score | 6 | 1 | 99 | 0 |
| Proportion of ODI responders | 3 | 0 | 100 | 0 |
| 6 | 0 | 100 | 0 | |
| Change from baseline in ODI | 6 | 0 | 100 | 0 |
| Change from baseline in EQ-5D-5L index score | 3 | 7 | 93 | 0 |
| 6 | 1 | 99 | 0 | |
Probabilities each treatment is the best are calculated from fixed-effect network meta-analysis using outcome data from EVOKE NSRBP subgroup with LVCF and completers from SENZA-NSRBP and DISTINCT (where reported).
CMM indicates conventional medical management; NRS, numeral rating scale; ODI, Oswestry Disability Index; SCS, spinal cord stimulation; VAS, visual analog scale.
Health Economic Analysis
Closed-loop SCS was estimated to result in lower costs per patient (£84,466, $106,847) than fixed-output SCS (£85,478, $108,127) and CMM (£106,949, $135,287). Closed-loop SCS generated more QALYs (6.973), than fixed-output SCS (5.627) and CMM (4.855). Cost-utility analysis shows that closed-loop SCS dominates fixed-output SCS and CMM (ie, cost-saving and generates more QALYs than the alternatives). Fixed-output SCS was also found to dominate CMM. Closed-loop SCS dominates fixed-output SCS as soon as year 1 and CMM at year 5. Both closed-loop SCS and fixed-output SCS incur higher initial costs due to device cost. Cost-effectiveness acceptability curve for the base-case analysis shows that closed-loop SCS has a 97% likelihood of being cost-effective at a willingness-to-pay threshold of £20,000/QALY when compared with fixed-output SCS and CMM. Full results of the cost-utility analysis, deterministic sensitivity analysis, probabilistic sensitivity analysis and scenario analysis, are presented in Supplementary Material 4, Supplemental Digital Content 1, http://links.lww.com/CJP/B121.
DISCUSSION
The results of the NMA of 3 RCTs and a total of 371 patients show that closed-loop SCS resulted in statistically and clinically significant reductions in pain intensity, a greater proportion of patients with ≥50% and with ≥80% pain reduction, a greater proportion of patients with ≥10-point reduction in ODI score, statistically and clinically significant improvements in ODI and HRQoL, and a greater proportion of patients improved or very much improved based on PGIC response when compared with fixed-output SCS. Closed-loop SCS and fixed-output SCS result in statistically and clinically significant reductions in pain intensity, a greater proportion of patients with ≥50% and with ≥80% pain reduction, a greater proportion of patients with ≥10-point reduction in ODI score, statistically and clinically significant improvements in ODI and HRQoL, and a greater proportion of patients improved or very much improved based on PGIC response when compared with CMM. The cost-utility analysis indicates that both closed-loop and fixed-output SCS are cost-saving and provide incremental benefits when compared with CMM, and closed-loop SCS dominates fixed-output SCS (lower costs and additional benefits).
The statistically and clinically significant improvements observed with SCS (both closed-loop and fixed-output) when compared with CMM were expected and also align with RCT evidence of SCS compared with CMM in other patient populations.14,35,39–42 Substantial evidence suggests that SCS (generally) is superior to CMM for PSPS-T2,14 painful diabetic neuropathy (PDN),39–41 and NSRBP.15,17 We suggest that SCS should be considered as the standard of care for these patient populations and therefore the comparator of choice for future treatment options. Currently, SCS is considered for patients with chronic pain refractory to CMM options. The trivial benefit observed for patients randomized to CMM in most trials can be explained by the fact that this population will have failed to obtain long-term benefit with most, if not all CMM options available before trial entry, thereby potentially generating negative expectations in the CMM participants.43 Recognition of SCS as standard of care is essential for comparisons of different forms of SCS to be considered for decision-making. NMAs can be a useful tool in decision-making to enable assessment of the relative treatment effects of interventions for which head-to-head comparisons may not have been conducted. The evidence reported herein suggests that closed-loop SCS is superior to fixed-output SCS.
The magnitude of the treatment effect of SCS when compared with CMM is expected to be greater than that observed for comparisons of different types of SCS. Future research should consider that the absence of significant treatment effects when comparing different types of SCS is plausible, given that this is an active intervention. In these instances, it may be more relevant to interpret clinically meaningful changes from baseline for the different types of SCS. Absence of significant differences between 2 different types of SCS should not translate or be interpreted as absence of clinically meaningful improvements when compared with baseline.
Burst SCS and high-frequency SCS provide different forms of stimulation; however, both use a fixed pattern of electrical output and do not provide measurements of neural activation to allow objective assessment of therapy delivery, outcomes or guide device programming. The current analysis uses all the evidence available in a single network. Analyses that consider burst SCS and high-frequency SCS as different types of stimulation would be limited given the small number of RCTs of SCS for an NSRBP population, and it would not be possible to do an NMA as the single node of “fixed-output” is required for the indirect comparison between closed-loop and CMM. As new studies are reported (eg, SOLIS [NCT04676022], NOVA [NCT04571242]), the NMA could be updated to include the new evidence. Despite the updates and emergence of new evidence, the conclusion that SCS is superior to CMM is unlikely to change. Consistent with the current results, a previous NMA of SCS in patients with PDN found low-frequency and high-frequency SCS to provide more patient benefits than CMM.23 A full NMA that includes all SCS RCT evidence is currently ongoing and may provide more granular comparative effects of different types of stimulation.
There were no important differences in baseline characteristics across the included studies, which are likely to impact the generalizability of the findings. In addition, the 3 RCTs were homogenous in that they were all funded by industry and conducted in the United States. A previous systematic review observed that outcomes reported in industry-funded RCTs that compared SCS with CMM were not significantly different from those observed in RCTs independent from industry.44 The same review observed greater improvements with SCS in studies based in the United States but noted that the results should be interpreted with caution since only one of the studies included in that specific meta-analysis had been conducted in that setting.44 Whether similar findings in an NSRBP population are reproduced in other health care and funding settings and in studies independent from industry should be further investigated.
Some bias may have been introduced because both SENZA-NSRBP and DISTINCT were open-label RCTs. While EVOKE was designed as a double-blind RCT and judged to have low risk of bias for the outcome measurement domain, this is an exception rather than the rule in parallel RCTs of SCS. Double-blind crossover RCTs of SCS have compared different types of SCS and their efficacy compared with placebo/sham stimulation.23,45,46 However, most of the SCS waveforms evaluated in these studies were experimental and not used in clinical practice.47,48 Therefore, current evidence from placebo-controlled crossover RCTs is of very limited value to inform decision-making.
The cost-utility analysis presented further confirms the advantages of SCS over CMM and closed-loop SCS over fixed-output SCS. The health economic evidence in support of SCS is robust. Several studies and systematic reviews of economic evaluations have found SCS to be cost-effective or dominant when compared with CMM.49–54 Further, novel types of SCS seem to provide incremental benefits when compared with traditional types of SCS.25,55
Limitations
The results of the NMA are limited to evidence from 3 RCTs. Due to sparse data within the network of 3 RCTs, results for the primary and secondary outcomes were associated with uncertainty reflected in large mean differences or risk differences and wide credible intervals, particularly for the indirect comparison of closed-loop SCS versus CMM. Since the network is small and has no closed loops, inconsistency between “direct” and “indirect” evidence cannot be assessed, so it is unknown if any inconsistency is present in the results. The results of the NMA should be interpreted with caution also due to 2 of the RCTs being open-label and assessing subjective outcomes and different types of fixed-output stimulation, and due to imbalance in the number of completers between treatment groups of the EVOKE NSRBP subgroup, which may have biased outcome data in favor of fixed-output SCS. Data for closed-loop SCS were derived from an NSRBP subgroup from the EVOKE RCT and were not a randomized subgroup. Currently, no long-term data are available to inform comparison in the NMA beyond a 6-month follow-up. A report of the findings from the EVOKE NSRBP subgroup up to 24-month follow-up is in preparation. Strengths and weakness of the cost-utility analysis are similar to those reported in previous studies.25
CONCLUSIONS
Current evidence showed that both closed-loop and fixed-output SCS provide more benefits than CMM for an NSRBP population. Closed-loop SCS was found to result in improved outcomes compared with fixed-output SCS. The certainty in the magnitude of effect may change with more evidence. However, despite the absence of head-to-head RCT evidence and limitations of current head-to-head RCT evidence, it is highly unlikely that the conclusions for the comparison of both types of SCS versus CMM or closed-loop versus fixed-output SCS would change considerably given that all the patients who received an SCS device had tried and failed to obtain satisfactory benefits with CMM.
Supplementary Material
Footnotes
This work was supported by Saluda Medical. S.E. reports consultancy fees from Medtronic, and Mainstay Medical outside the submitted work. He has received department research funding from the National Institute of Health Research, Saluda Medical and Medtronic. S.N. reports consultancy fees from Saluda Medical. A.B. MTech Access was commissioned by Saluda Medical to participate in this project. N.A.M. reports receiving grants from Neuros, Mesoblast, and Vivex Biologics, as well as consulting as a medical monitor for Saluda Medical, Nevro, Vivex Biologics, Mainstay, Sollis Therapeutics, and Vertos outside the submitted work. C.G. reports consulting fees and stock options received from Mainstay, personal fees from Mainstay, Saluda Medical, Persica, and Iliad outside the submitted work, research funded by Sollis, expert witness testimony fees, and serves as Editor-in-Chief of Pain Practice. B.B. reports an educational grant from Saluda Medical and consultancy fees from Salvia Bioelectronics, Medtronic and Abbott outside the submitted work. P.S.S. has received consultancy fees from Medtronic, Saluda Medical, Nalu, and Biotronic outside the submitted work, and has stock options from Saluda Medical and Nalu. M.M. reports no conflicts of interest. N.S., A.L., and R.V.D. are employees of Saluda Medical. R.V.D. has previously received consultancy fees from Mainstay Medical, Medtronic, and Saluda Medical outside the submitted work.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.clinicalpain.com.
Contributor Information
Sam Eldabe, Email: seldabe@mac.com.
Sarah Nevitt, Email: sarah.nevitt@york.ac.uk.
Anthony Bentley, Email: anthony.bentley@mtechaccess.co.uk.
Nagy A. Mekhail, Email: MEKHAIN@ccf.org.
Christopher Gilligan, Email: Christopher.Gilligan@rwjbh.org.
Bart Billet, Email: bart.billet@azdelta.be.
Peter S. Staats, Email: peterstaats@hotmail.com.
Michelle Maden, Email: Michelle.Maden@liverpool.ac.uk.
Nicole Soliday, Email: Nicole.Soliday@saludamedical.com.
Angela Leitner, Email: Angela.Leitner@saludamedical.com.
Rui V. Duarte, Email: rui.duarte@liverpool.ac.uk.
REFERENCES
- 1.GBD 2021 Low Back Pain Collaborators . Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e316–e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Network Open. 2021;4:e2037371, e2037371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itz CJ, Geurts JW, van Kleef M, et al. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain. 2013;17:5–15. [DOI] [PubMed] [Google Scholar]
- 4.United States Bone and Joint Initiative . The burden of musculoskeletal diseases in the United States (BMUS). 2014. Accessed July 5, 2023. http://www.boneandjointburden.org
- 5.Fayaz A, Croft P, Langford RM, et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6:e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95–103. [DOI] [PubMed] [Google Scholar]
- 7.Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323:863–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37:29–42. [DOI] [PubMed] [Google Scholar]
- 9.Halicka M, Duarte R, Catherall S, et al. Systematic review and meta-analysis of predictors of return to work after spinal surgery for chronic low back and leg pain. J Pain. 2022;23:1318–1342. [DOI] [PubMed] [Google Scholar]
- 10.Weir S, Samnaliev M, Kuo TC, et al. The incidence and healthcare costs of persistent postoperative pain following lumbar spine surgery in the UK: a cohort study using the Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES). BMJ Open. 2017;7:e017585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence (NICE) . Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin. Technology appraisal guidance [TA159]. 2008. Accessed October 31, 2022. https://www.nice.org.uk/guidance/ta159 [Google Scholar]
- 12.Christelis N, Simpson B, Russo M, et al. Persistent spinal pain syndrome: a proposal for failed back surgery syndrome and ICD-11. Pain Med. 2021;22:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98–106; discussion 106-7. [DOI] [PubMed] [Google Scholar]
- 14.Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–188. [DOI] [PubMed] [Google Scholar]
- 15.Kapural L, Jameson J, Johnson C, et al. Treatment of nonsurgical refractory back pain with high-frequency spinal cord stimulation at 10 kHz: 12-month results of a pragmatic, multicenter, randomized controlled trial. J Neurosurg Spine. 2022;37:188–199. [DOI] [PubMed] [Google Scholar]
- 16.Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19:123–134. [DOI] [PubMed] [Google Scholar]
- 17.Deer T, Gilligan C, Falowski S, et al. Treatment of refractory low back pain using passive recharge burst in patients without options for corrective surgery: findings and results from the DISTINCT study, a prospective randomized multicenter controlled trial. Neuromodulation. 2023;26:1387–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. [DOI] [PubMed] [Google Scholar]
- 19.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence (NICE) . NICE health technology evaluations: the manual [PMG36]. 2022. Accessed June 2023. https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 22.Duarte RV, Nevitt S, McNicol E, et al. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain. 2020;161:24–35. [DOI] [PubMed] [Google Scholar]
- 23.Duarte RV, Nevitt S, Copley S, et al. Systematic review and network meta-analysis of neurostimulation for painful diabetic neuropathy. Diabetes Care. 2022;45:2466–2475. [DOI] [PubMed] [Google Scholar]
- 24.Phillippo DM. multinma: Bayesian Network Meta-Analysis of Individual and Aggregate Data (v0.5.0). 2023. Accessed March 29, 2023. 10.5281/zenodo.7966190 [DOI] [Google Scholar]
- 25.Duarte RV, Bentley A, Soliday N, et al. Cost-utility analysis of evoke closed-loop spinal cord stimulation for chronic back and leg pain. Clin J Pain. 2023;39:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Kaisy A, Van Buyten JP, Kapural L, et al. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: subanalysis of pooled data from two prospective studies. Anaesthesia. 2020;75:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baranidharan G, Feltbower R, Bretherton B, et al. One-year results of prospective research study using 10 kHz spinal cord stimulation in persistent nonoperated low back pain of neuropathic origin: Maiden back study. Neuromodulation. 2021;24:479–487. [DOI] [PubMed] [Google Scholar]
- 28.Al-Kaisy A, Baranidharan G, Gempt J, et al. Randomized, controlled trial comparing high-frequency SCS (10 kHz) to conventional medical management for treatment of non-surgical-refractory-back-pain. Neuromodulation. 2021;24:e143–e144. [Google Scholar]
- 29.Mekhail N, Levy RM, Deer TR, et al. Durability of clinical and quality-of-life outcomes of closed-loop spinal cord stimulation for chronic back and leg pain: a secondary analysis of the evoke randomized clinical trial. JAMA Neurology. 2022;3:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mekhail NA, Levy RM, Deer TR, et al. ECAP-controlled closed-loop versus open-loop SCS for the treatment of chronic pain: 36-month results of the EVOKE blinded randomized clinical trial. Reg Anesth Pain Med. 2024;49:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel NP, Jameson J, Johnson C, et al. Durable responses at 24 months with high-frequency spinal cord stimulation for nonsurgical refractory back pain. J Neurosurg Spine. 2023;40:229–239. [DOI] [PubMed] [Google Scholar]
- 32.Fishman M, Cordner H, Justiz R, et al. Twelve-month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021;21:912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT Randomized Controlled Trial. Anesthesiology. 2015;123:851–860. [DOI] [PubMed] [Google Scholar]
- 34.Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigoard P, Basu S, Desai M, et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain. 2019;160:1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigoard P, Billot M, Ingrand P, et al. How should we use multicolumn spinal cord stimulation to optimize back pain spatial neural targeting? A prospective, multicenter, randomized, double-blind, controlled trial (ESTIMET Study). Neuromodulation. 2021;24:86–101. [DOI] [PubMed] [Google Scholar]
- 37.van Heteren EPZ, van Roosendaal B-KWP, van Gorp E-JJAA, et al. Spinal cord stimulation with additional peripheral nerve/field stimulation versus spinal cord stimulation alone on back pain and quality of life in patients with persistent spinal pain syndrome. Neuromodulation. 2023;26:658–665. [DOI] [PubMed] [Google Scholar]
- 38.Wallace MS, North JM, Phillips GM, et al. Combination therapy with simultaneous delivery of spinal cord stimulation modalities: COMBO randomized controlled trial. Pain management. 2023;13:171–184. [DOI] [PubMed] [Google Scholar]
- 39.de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155:2426–2431. [DOI] [PubMed] [Google Scholar]
- 40.Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes care. 2014;37:3016–3024. [DOI] [PubMed] [Google Scholar]
- 41.Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of High-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA neurology. 2021;78:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canos-Verdecho A, Abejon D, Robledo R, et al. Randomized prospective study in patients with complex regional pain syndrome of the upper limb with high-frequency spinal cord stimulation (10-kHz) and low-frequency spinal cord stimulation. Neuromodulation. 2021;24:448–458. [DOI] [PubMed] [Google Scholar]
- 43.Colloca L, Barsky AJ. Placebo and nocebo effects. N Engl J Med. 2020;382:554–561. [DOI] [PubMed] [Google Scholar]
- 44.Eldabe S, Nevitt S, Copley S, et al. Does industry funding and study location impact findings from randomized controlled trials of spinal cord stimulation? A systematic review and meta-analysis. Reg Anesth Pain Med. 2024;49:272–284. [DOI] [PubMed] [Google Scholar]
- 45.O’Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev. 2021;12:CD013756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traeger AC, Gilbert SE, Harris IA, et al. Spinal cord stimulation for low back pain. The Cochrane Database Syst Rev. 2023;3:CD014789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eldabe S, Gilligan C, Taylor RS, et al. Issues in design, conduct, and conclusions of JAMA’s Hara et al.‘s randomized clinical trial of spinal cord burst stimulation versus placebo stimulation on disability in patients with chronic radicular pain after lumbar spine surgery. Pain Practice. 2023;23:232–233. [DOI] [PubMed] [Google Scholar]
- 48.Staats PS, Taylor RS, Gilligan C, et al. Limitations of the Cochrane review of spinal cord stimulation for low back pain. Pain Pract. 2023;23:868–872. [DOI] [PubMed] [Google Scholar]
- 49.Taylor RS, Ryan J, O’Donnell R, et al. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010;26:463–469. [DOI] [PubMed] [Google Scholar]
- 50.Annemans L, Van Buyten JP, Smith T, et al. Cost effectiveness of a novel 10 kHz high-frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). J Long Term Eff Med Implants. 2014;24:173–183. [DOI] [PubMed] [Google Scholar]
- 51.Kemler MA, Raphael JH, Bentley A, et al. The cost-effectiveness of spinal cord stimulation for complex regional pain syndrome. Value Health. 2010;13:735–742. [DOI] [PubMed] [Google Scholar]
- 52.Simpson EL, Duenas A, Holmes MW, et al. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess. 2009;13:iii–154. [DOI] [PubMed] [Google Scholar]
- 53.Niyomsri S, Duarte RV, Eldabe S, et al. A systematic review of economic evaluations reporting the cost-effectiveness of spinal cord stimulation. Value Health. 2020;23:656–665. [DOI] [PubMed] [Google Scholar]
- 54.Odonkor CA, Orman S, Orhurhu V, et al. Spinal cord stimulation vs conventional therapies for the treatment of chronic low back and leg pain: a systematic review of health care resource utilization and outcomes in the last decade. Pain Med. 2019;20:2479–2494. [DOI] [PubMed] [Google Scholar]
- 55.Taylor RS, Bentley A, Campbell B, et al. High-frequency 10 kHz spinal cord stimulation for chronic back and leg pain: cost-consequence and cost-effectiveness analyses. Clin J Pain. 2020;36:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]


