Abstract
Ornithine decarboxylase (ODC), the key regulatory enzyme for polyamine biosynthesis, is known to have a short intracellular half-life, and antizyme, an ODC-binding protein induced by polyamines, has been suggested to be involved in the process of ODC degradation. In the present study we demonstrated that antizyme markedly accelerated ATP-dependent degradation of ODC in vitro in an extract from ODC-overproducing Chinese-hamster ovary cells.
Full text
PDF
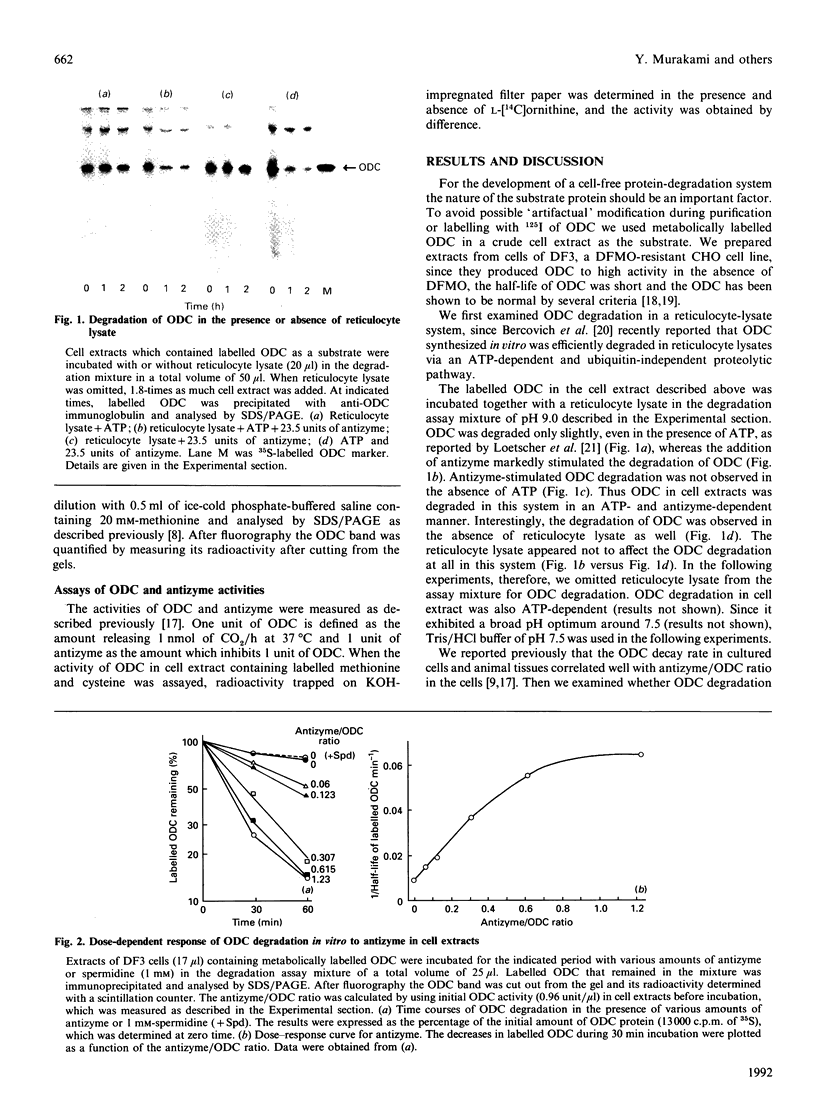


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bercovich Z., Rosenberg-Hasson Y., Ciechanover A., Kahana C. Degradation of ornithine decarboxylase in reticulocyte lysate is ATP-dependent but ubiquitin-independent. J Biol Chem. 1989 Sep 25;264(27):15949–15952. [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Choi J. H., Scheffler I. E. Chinese hamster ovary cells resistant to alpha-difluoromethylornithine are overproducers of ornithine decarboxylase. J Biol Chem. 1983 Oct 25;258(20):12601–12608. [PubMed] [Google Scholar]
- Dircks L., Grens A., Slezynger T. C., Scheffler I. E. Posttranscriptional regulation of ornithine decarboxylase activity. J Cell Physiol. 1986 Mar;126(3):371–378. doi: 10.1002/jcp.1041260307. [DOI] [PubMed] [Google Scholar]
- Flamigni F., Marmiroli S., Guarnieri C., Caldarera C. M. Effect of ATP depletion and phenanthroline on the spermidine-mediated decay of ornithine decarboxylase in erythroleukemia cells. Biochem Biophys Res Commun. 1990 Oct 30;172(2):939–944. doi: 10.1016/0006-291x(90)90766-g. [DOI] [PubMed] [Google Scholar]
- Flamigni F., Marmiroli S., Guarnieri C., Caldarera C. M. Stabilization of ornithine decarboxylase in erythroleukemia cells depleted of ATP. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1217–1222. doi: 10.1016/0006-291x(89)91107-8. [DOI] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Ghoda L., Phillips M. A., Bass K. E., Wang C. C., Coffino P. Trypanosome ornithine decarboxylase is stable because it lacks sequences found in the carboxyl terminus of the mouse enzyme which target the latter for intracellular degradation. J Biol Chem. 1990 Jul 15;265(20):11823–11826. [PubMed] [Google Scholar]
- Ghoda L., van Daalen Wetters T., Macrae M., Ascherman D., Coffino P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science. 1989 Mar 17;243(4897):1493–1495. doi: 10.1126/science.2928784. [DOI] [PubMed] [Google Scholar]
- Glass J. R., Gerner E. W. Spermidine mediates degradation of ornithine decarboxylase by a non-lysosomal, ubiquitin-independent mechanism. J Cell Physiol. 1987 Jan;130(1):133–141. doi: 10.1002/jcp.1041300119. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Control of ornithine decarboxylase in Chinese hamster ovary cells by polyamines. Translational inhibition of synthesis and acceleration of degradation of the enzyme by putrescine, spermidine, and spermine. J Biol Chem. 1986 Jul 15;261(20):9502–9508. [PubMed] [Google Scholar]
- Kanamoto R., Utsunomiya K., Kameji T., Hayashi S. Effects of putrescine on synthesis and degradation of ornithine decarboxylase in primary cultured hepatocytes. Eur J Biochem. 1986 Feb 3;154(3):539–544. doi: 10.1111/j.1432-1033.1986.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Loetscher P., Pratt G., Rechsteiner M. The C terminus of mouse ornithine decarboxylase confers rapid degradation on dihydrofolate reductase. Support for the pest hypothesis. J Biol Chem. 1991 Jun 15;266(17):11213–11220. [PubMed] [Google Scholar]
- Lu L., Stanley B. A., Pegg A. E. Identification of residues in ornithine decarboxylase essential for enzymic activity and for rapid protein turnover. Biochem J. 1991 Aug 1;277(Pt 3):671–675. doi: 10.1042/bj2770671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsufuji S., Miyazaki Y., Kanamoto R., Kameji T., Murakami Y., Baby T. G., Fujita K., Ohno T., Hayashi S. Analyses of ornithine decarboxylase antizyme mRNA with a cDNA cloned from rat liver. J Biochem. 1990 Sep;108(3):365–371. doi: 10.1093/oxfordjournals.jbchem.a123207. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Mahan D. W., McCann P. P., Qasba P. Dicyclohexylamine effects on HTC cell polyamine content and ornithine decarboxylase activity. Biochim Biophys Acta. 1985 Jul 5;840(3):309–316. doi: 10.1016/0304-4165(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Fujita K., Kameji T., Hayashi S. Accumulation of ornithine decarboxylase-antizyme complex in HMOA cells. Biochem J. 1985 Feb 1;225(3):689–697. doi: 10.1042/bj2250689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Hayashi S. Role of antizyme in degradation of ornithine decarboxylase in HTC cells. Biochem J. 1985 Mar 15;226(3):893–896. doi: 10.1042/bj2260893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Marumo M., Hayashi S. Ornithine decarboxylase antizyme in kidneys of male and female mice. Biochem J. 1988 Sep 1;254(2):367–372. doi: 10.1042/bj2540367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Nishiyama M., Hayashi S. Involvement of antizyme in stabilization of ornithine decarboxylase caused by inhibitors of polyamine synthesis. Eur J Biochem. 1989 Mar 1;180(1):181–184. doi: 10.1111/j.1432-1033.1989.tb14630.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Matsufuji S., Kanamoto R., Takano M., Murakami Y., Hayashi S. Two-step purification of mouse kidney ornithine decarboxylase. Prep Biochem. 1988;18(2):227–238. doi: 10.1080/00327488808062524. [DOI] [PubMed] [Google Scholar]
- Prouty W. F. Ornithine decarboxylase inactivation in HeLa cells. J Cell Physiol. 1976 Sep;89(1):65–76. doi: 10.1002/jcp.1040890107. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Hasson Y., Bercovich Z., Ciechanover A., Kahana C. Degradation of ornithine decarboxylase in mammalian cells is ATP dependent but ubiquitin independent. Eur J Biochem. 1989 Nov 6;185(2):469–474. doi: 10.1111/j.1432-1033.1989.tb15138.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Hasson Y., Bercovich Z., Kahana C. Characterization of sequences involved in mediating degradation of ornithine decarboxylase in cells and in reticulocyte lysate. Eur J Biochem. 1991 Mar 28;196(3):647–651. doi: 10.1111/j.1432-1033.1991.tb15861.x. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Effect of 1,3-diaminopropane on ornithine decarboxylase enzyme protein in thioacetamide-treated rat liver. Biochem J. 1983 Dec 15;216(3):701–707. doi: 10.1042/bj2160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Waxman L., Goldberg A. L. ATP serves two distinct roles in protein degradation in reticulocytes, one requiring and one independent of ubiquitin. J Cell Biol. 1983 Jun;96(6):1580–1585. doi: 10.1083/jcb.96.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]



