Abstract
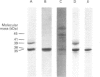
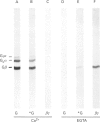
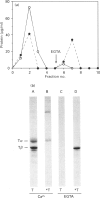
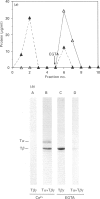
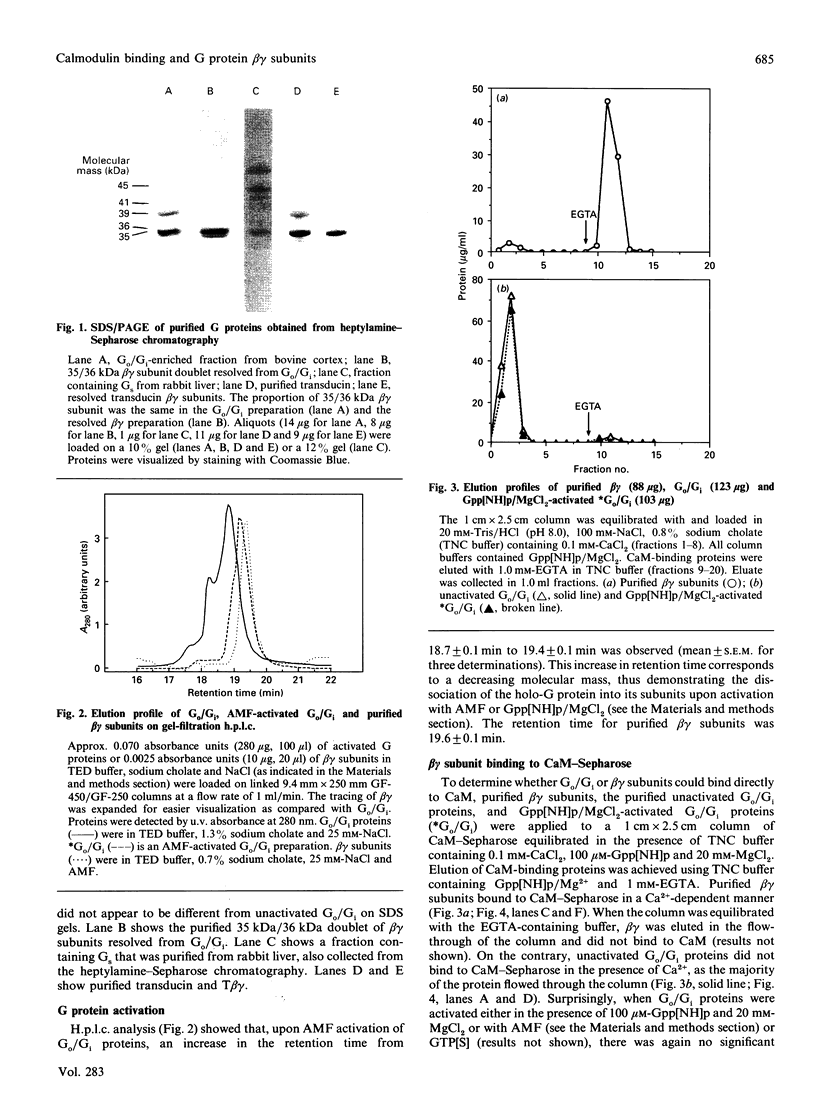
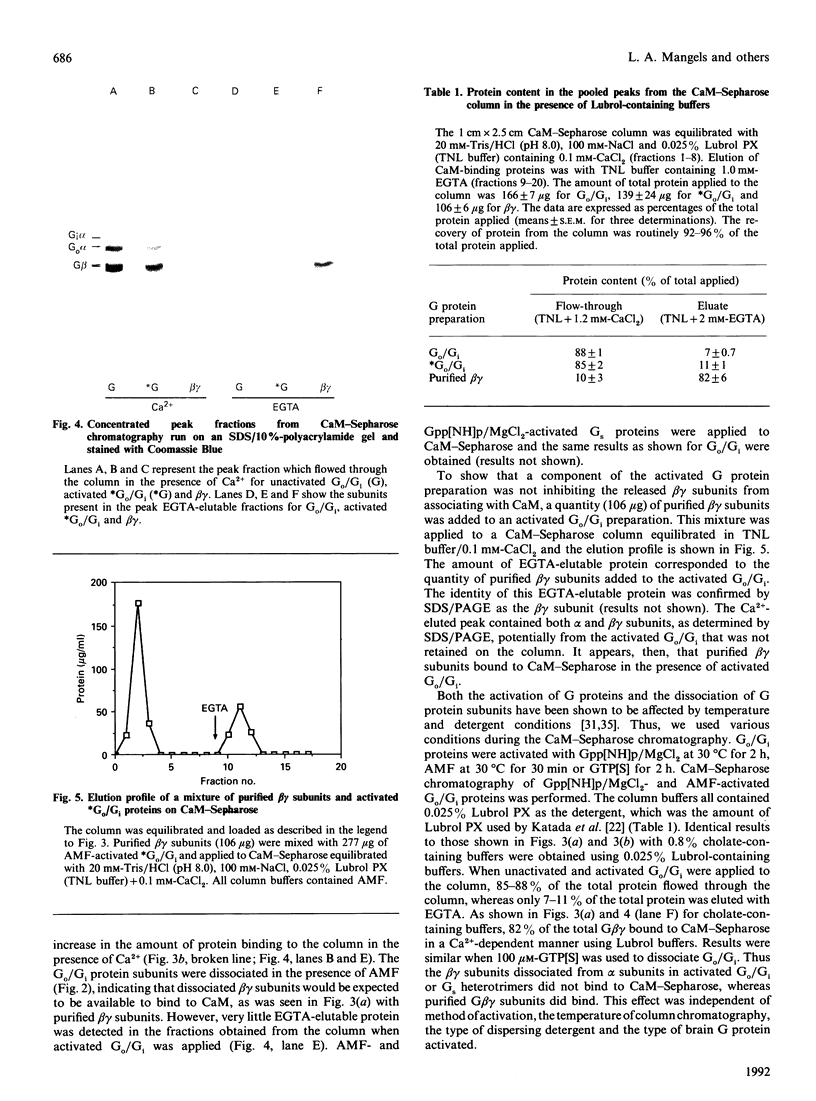
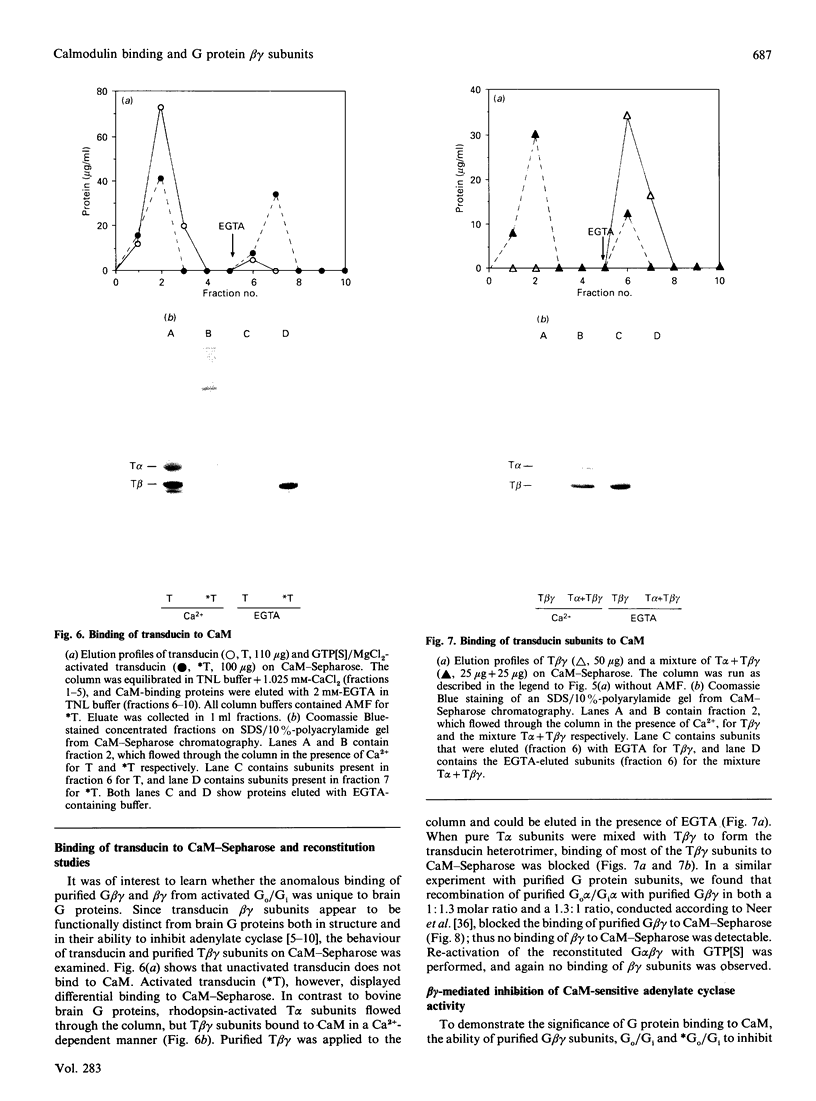
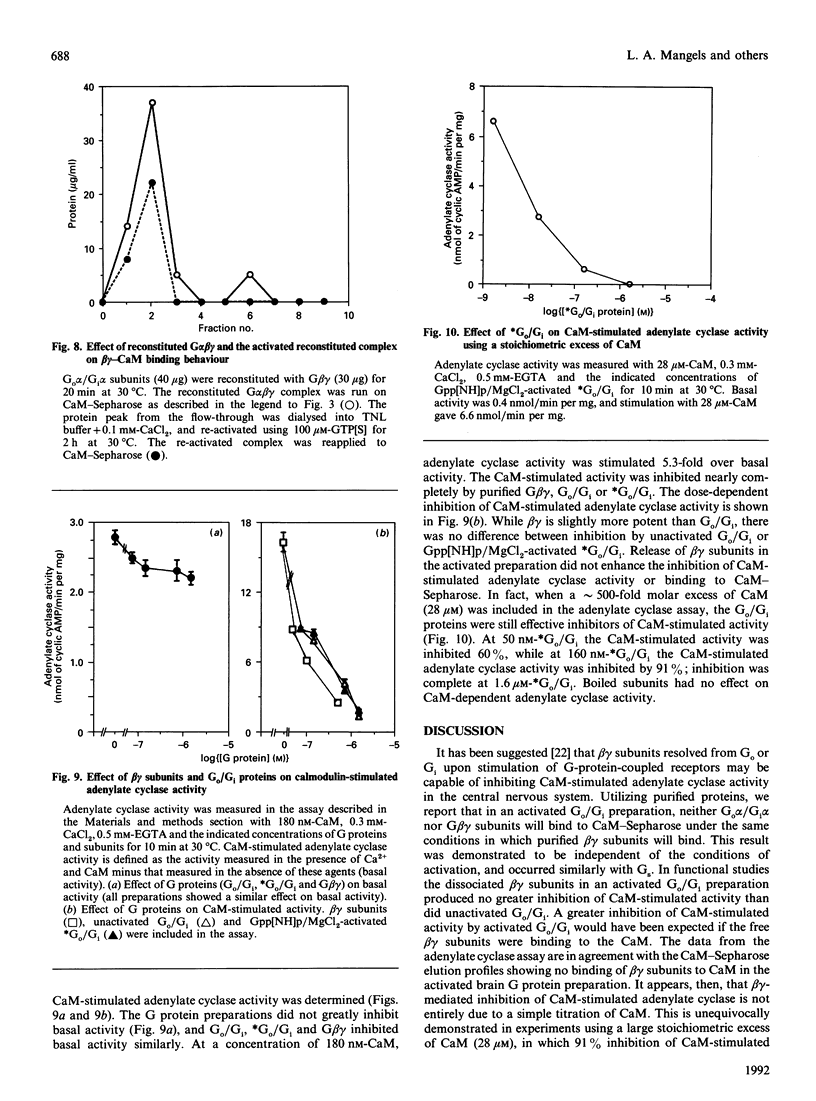
The interactions between guanine nucleotide regulatory proteins and the Ca(2+)-binding protein calmodulin were studied using calmodulin-Sepharose affinity chromatography. Purified bovine brain beta gamma subunits bound to calmodulin-Sepharose in a Ca(2+)-dependent manner. On the contrary, beta gamma subunits produced in an activated Go/Gi preparation did not bind to calmodulin-Sepharose. The effect was independent of the type of bovine brain G protein (Go/Gi, Gs), method of activation and the presence of magnesium. To distinguish whether the binding of purified beta gamma subunits to calmodulin was unique to brain beta gamma or to the method of purification, similar experiments were performed using transducin. In contrast to bovine brain G proteins, both purified transducin beta gamma subunits and beta gamma released from rhodopsin-activated transducin bound to calmodulin-Sepharose in a Ca(2+)-dependent manner. To assess the functional significance of the binding of bovine brain beta gamma subunits to calmodulin, the ability of purified beta gamma and of beta gamma in unactivated and activated Go/Gi to inhibit partially purified calmodulin-sensitive adenylate cyclase was determined. Purified beta gamma was highly effective in inhibiting calmodulin-stimulated adenylate cyclase activity. However, unactivated Go/Gi and preactivated Go/Gi inhibited calmodulin-stimulated adenylate cyclase activity to the same extent. This Go/Gi-mediated inhibition also occurred in the presence of a 500-fold molar excess of calmodulin over added G protein. These results demonstrate: (1) that beta gamma subunits may not be completely released upon G protein activation, and (2) that inhibition of calmodulin-stimulated adenylate cyclase by beta gamma subunits does not appear to be mediated by a direct beta gamma-calmodulin interaction. Differences in the binding properties of activated bovine brain G proteins versus those of transducin could be explained by differences in the gamma subunit between the proteins, or by differences in affinities of the alpha and beta gamma subunits for each other and for calmodulin. The different functional properties of purified beta gamma subunits and beta gamma subunits produced in situ by activation of G proteins indicates that extrapolation from the effects of purified subunits to events occurring in membranes should be done with caution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Morishita R., Kobayashi T., Kato K. Separation of two beta gamma subunit complexes of brain GTP-binding proteins composed of distinct gamma subunits. FEBS Lett. 1990 Jun 18;266(1-2):41–44. doi: 10.1016/0014-5793(90)81501-e. [DOI] [PubMed] [Google Scholar]
- Asano T., Ogasawara N., Kitajima S., Sano M. Interaction of GTP-binding proteins with calmodulin. FEBS Lett. 1986 Jul 28;203(2):135–138. doi: 10.1016/0014-5793(86)80729-3. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Graziano M. P., Gilman A. G. G protein beta gamma subunits from bovine brain and retina: equivalent catalytic support of ADP-ribosylation of alpha subunits by pertussis toxin but differential interactions with Gs alpha. Biochemistry. 1989 Jan 24;28(2):611–616. doi: 10.1021/bi00428a029. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Gierschik P., Staniszewski C., Benovic J. L., Codina J., Somers R., Birnbaumer L., Spiegel A. M., Lefkowitz R. J., Caron M. G. Functional differences in the beta gamma complexes of transducin and the inhibitory guanine nucleotide regulatory protein. Biochemistry. 1987 Mar 10;26(5):1485–1491. doi: 10.1021/bi00379a041. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Birnbaumer L., Sekura R. D. Effects of guanine nucleotides and Mg on human erythrocyte Ni and Ns, the regulatory components of adenylyl cyclase. J Biol Chem. 1984 Sep 25;259(18):11408–11418. [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Jackson R. L., Johnson J. D., Means A. R. Physicochemical properties of rat testis Ca2+-dependent regulator protein of cyclic nucleotide phosphodiesterase. Relationship of Ca2+-binding, conformational changes, and phosphodiesterase activity. J Biol Chem. 1977 Dec 10;252(23):8415–8422. [PubMed] [Google Scholar]
- Evans T., Fawzi A., Fraser E. D., Brown M. L., Northup J. K. Purification of a beta 35 form of the beta gamma complex common to G-proteins from human placental membranes. J Biol Chem. 1987 Jan 5;262(1):176–181. [PubMed] [Google Scholar]
- Gao B., Gilman A. G., Robishaw J. D. A second form of the beta subunit of signal-transducing G proteins. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6122–6125. doi: 10.1073/pnas.84.17.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N., Baetscher M., Aebersold R., Simon M. I. A G protein gamma subunit shares homology with ras proteins. Science. 1989 May 26;244(4907):971–974. doi: 10.1126/science.2499046. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Girardot J. M., Kempf J., Cooper D. M. Role of calmodulin in the effect of guanyl nucleotides on rat hippocampal adenylate cyclase: involvement of adenosine and opiates. J Neurochem. 1983 Sep;41(3):848–859. doi: 10.1111/j.1471-4159.1983.tb04818.x. [DOI] [PubMed] [Google Scholar]
- Harrison J. K., Mickevicius C. K., Gnegy M. E. Differential regulation by calmodulin of basal, GTP-, and dopamine-stimulated adenylate cyclase activities in bovine striatum. J Neurochem. 1988 Aug;51(2):345–352. doi: 10.1111/j.1471-4159.1988.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Heideman W., Wierman B. M., Storm D. R. GTP is not required for calmodulin stimulation of bovine brain adenylate cyclase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1462–1465. doi: 10.1073/pnas.79.5.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekman M., Holzhöfer A., Gierschik P., Im M. J., Jakobs K. H., Pfeuffer T., Helmreich E. J. Regulation of signal transfer from beta 1-adrenoceptor to adenylate cyclase by beta gamma subunits in a reconstituted system. Eur J Biochem. 1987 Dec 1;169(2):431–439. doi: 10.1111/j.1432-1033.1987.tb13630.x. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Codina J., Rosenthal W., Birnbaumer L., Neer E. J., Yamazaki A., Bitensky M. W. Characterization by two-dimensional peptide mapping of the gamma subunits of Ns and Ni, the regulatory proteins of adenylyl cyclase, and of transducin, the guanine nucleotide-binding protein of rod outer segments of the eye. J Biol Chem. 1985 Nov 25;260(27):14867–14872. [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- Katada T., Kusakabe K., Oinuma M., Ui M. A novel mechanism for the inhibition of adenylate cyclase via inhibitory GTP-binding proteins. Calmodulin-dependent inhibition of the cyclase catalyst by the beta gamma-subunits of GTP-binding proteins. J Biol Chem. 1987 Sep 5;262(25):11897–11900. [PubMed] [Google Scholar]
- Kim M. H., Neubig R. R. Membrane reconstitution of high-affinity alpha 2 adrenergic agonist binding with guanine nucleotide regulatory proteins. Biochemistry. 1987 Jun 16;26(12):3664–3672. doi: 10.1021/bi00386a061. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Smallwood P. M., Moen P. T., Jr, Helman L. J., Ahn T. G. Molecular cloning of beta 3 subunit, a third form of the G protein beta-subunit polypeptide. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2329–2333. doi: 10.1073/pnas.87.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil S., Lakey T., Tomlinson S. Calmodulin regulation of adenylate cyclase activity. Cell Calcium. 1985 Jun;6(3):213–216. doi: 10.1016/0143-4160(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Malnoë A., Cox J. A. Relationship among calmodulin-, forskolin-, and guanine nucleotide-dependent adenylate cyclase activities in cerebellar membranes: studies by limited proteolysis. J Neurochem. 1985 Oct;45(4):1163–1171. doi: 10.1111/j.1471-4159.1985.tb05537.x. [DOI] [PubMed] [Google Scholar]
- Marbach I., Bar-Sinai A., Minich M., Levitzki A. Beta subunit copurifies with GppNHp-activated adenylyl cyclase. J Biol Chem. 1990 Jun 15;265(17):9999–10004. [PubMed] [Google Scholar]
- Mazzoni M. R., Hamm H. E. Effect of monoclonal antibody binding on alpha-beta gamma subunit interactions in the rod outer segment G protein, Gt. Biochemistry. 1989 Dec 12;28(25):9873–9880. doi: 10.1021/bi00451a047. [DOI] [PubMed] [Google Scholar]
- Minocherhomjee M., Selfe S., Flowers N. J., Storm D. R. Direct interaction between the catalytic subunit of the calmodulin-sensitive adenylate cyclase from bovine brain with 125I-labeled wheat germ agglutinin and 125I-labeled calmodulin. Biochemistry. 1987 Jul 14;26(14):4444–4448. doi: 10.1021/bi00388a038. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Pulsifer L., Wolf L. G. The amino terminus of G protein alpha subunits is required for interaction with beta gamma. J Biol Chem. 1988 Jun 25;263(18):8996–8970. [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983 Sep 25;258(18):11369–11376. [PubMed] [Google Scholar]
- Perez-Reyes E., Cooper D. M. Calmodulin stimulation of the rat cerebral cortical adenylate cyclase is required for the detection of guanine nucleotide- or hormone-mediated inhibition. Mol Pharmacol. 1987 Aug;32(1):212–216. [PubMed] [Google Scholar]
- Robishaw J. D., Kalman V. K., Moomaw C. R., Slaughter C. A. Existence of two gamma subunits of the G proteins in brain. J Biol Chem. 1989 Sep 25;264(27):15758–15761. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Calmodulin stimulation of adenylate cyclase in rat brain membranes does not require GTP. Life Sci. 1982 Apr 26;30(17):1457–1464. doi: 10.1016/0024-3205(82)90559-8. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Sugimoto K., Nukada T., Tanabe T., Takahashi H., Noda M., Minamino N., Kangawa K., Matsuo H., Hirose T., Inayama S. Primary structure of the beta-subunit of bovine transducin deduced from the cDNA sequence. FEBS Lett. 1985 Oct 28;191(2):235–240. doi: 10.1016/0014-5793(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Westcott K. R., La Porte D. C., Storm D. R. Resolution of adenylate cyclase sensitive and insensitive to Ca2+ and calcium-dependent regulatory protein (CDR) by CDR-sepharose affinity chromatography. Proc Natl Acad Sci U S A. 1979 Jan;76(1):204–208. doi: 10.1073/pnas.76.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager R. E., Heideman W., Rosenberg G. B., Storm D. R. Purification of the calmodulin-sensitive adenylate cyclase from bovine cerebral cortex. Biochemistry. 1985 Jul 2;24(14):3776–3783. doi: 10.1021/bi00335a054. [DOI] [PubMed] [Google Scholar]
- Yi F., Denker B. M., Neer E. J. Structural and functional studies of cross-linked Go protein subunits. J Biol Chem. 1991 Feb 25;266(6):3900–3906. [PubMed] [Google Scholar]