Abstract
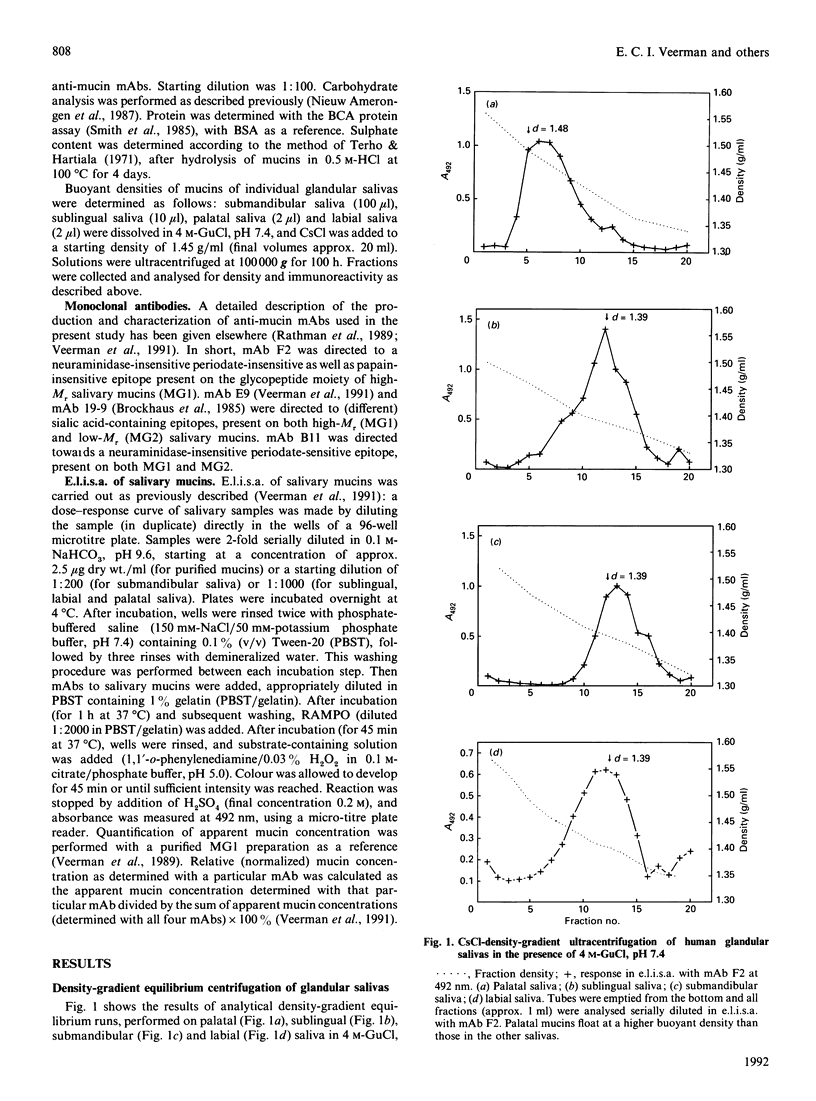
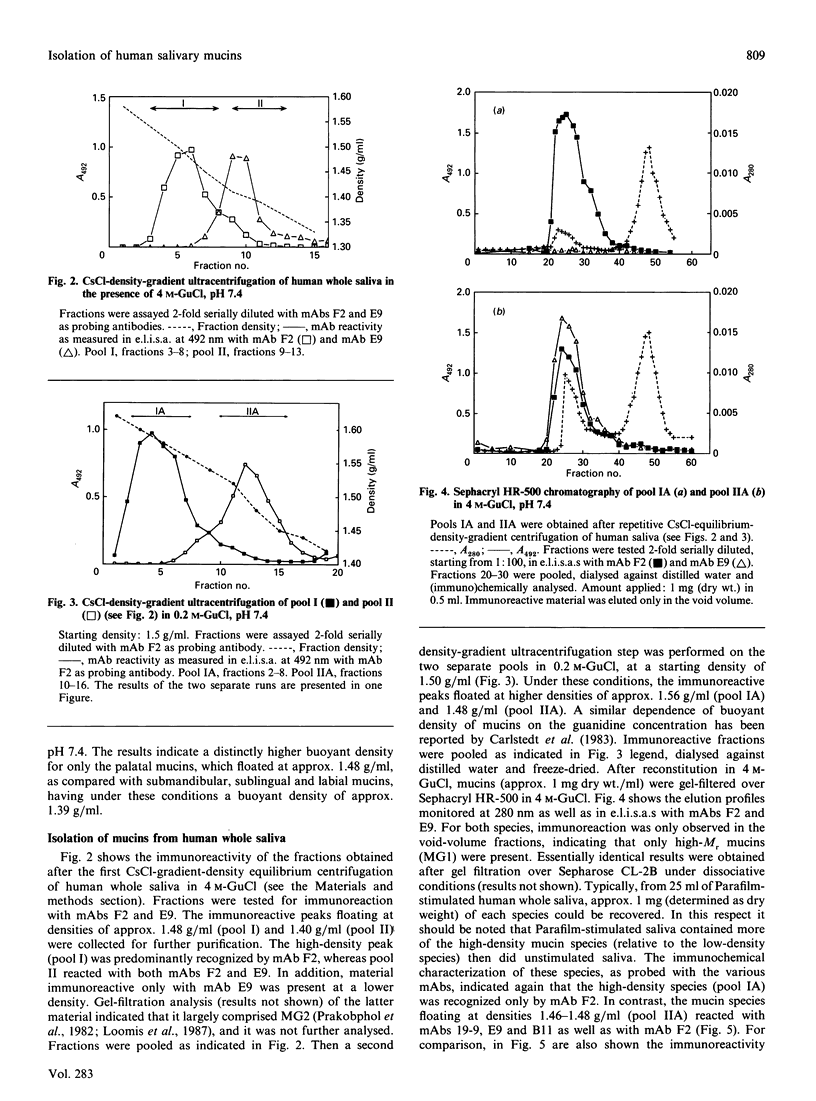
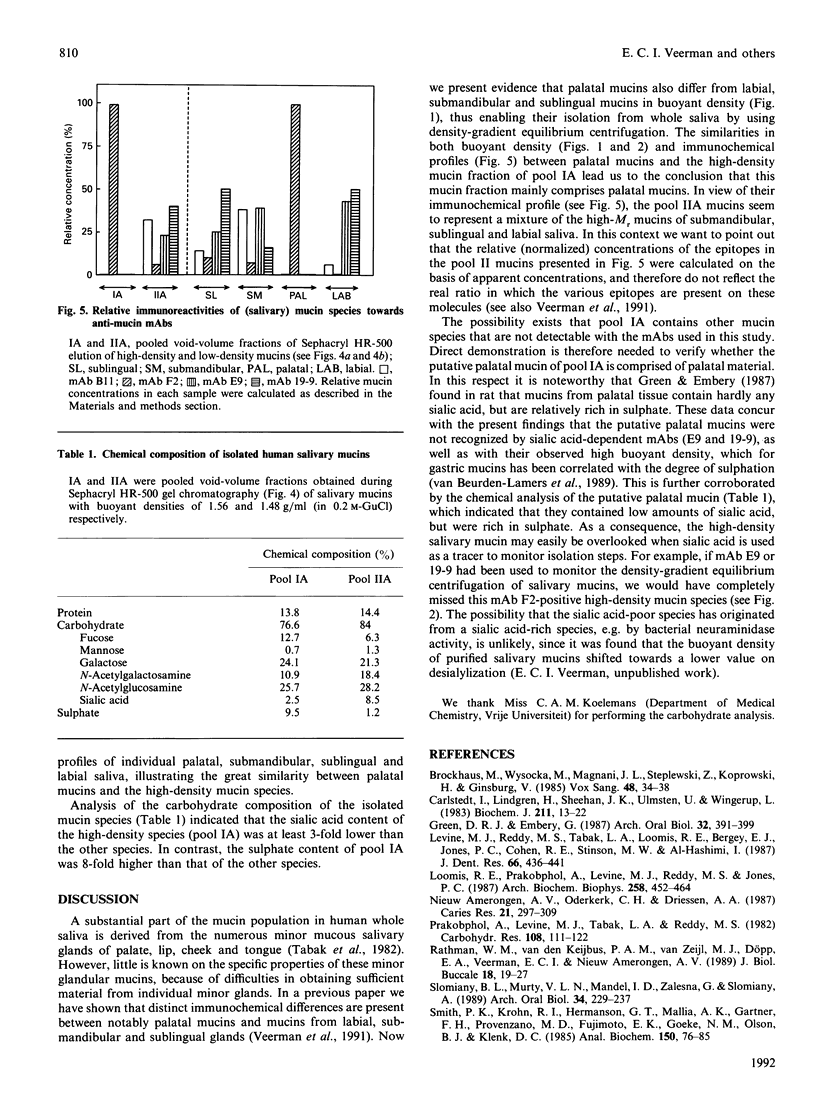
By using CsCl-density-gradient ultracentrifugation, two high-Mr mucin species were isolated from human whole saliva, having buoyant densities in 0.2 M-guanidinium chloride of approx. 1.56 g/ml (pool IA) and 1.48 g/ml (pool IIA). Analytical density-gradient centrifugation of submandibular, sublingual, labial and palatal saliva, followed by immunochemical analysis with anti-mucin monoclonal antibodies, indicated immunochemical and physicochemical similarities between the high-density mucins of pool IA and mucins from palatal salivary glands. Chemical analysis indicated that the putative palatal mucin was rich in sulphate, but poor in sialic acid. The lower-density mucins of pool IIA equated with the high-Mr mucins of submandibular-sublingual saliva, both immunochemically and physicochemically (buoyant density).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockhaus M., Wysocka M., Magnani J. L., Steplewski Z., Koprowski H., Ginsburg V. Normal salivary mucin contains the gastrointestinal cancer-associated antigen detected by monoclonal antibody 19-9 in the serum mucin of patients. Vox Sang. 1985;48(1):34–38. doi: 10.1111/j.1423-0410.1985.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Embery G. Isolation, chemical and biological characterization of sulphated glycoproteins synthesized by rat buccal and palatal minor salivary glands in vivo and in vitro. Arch Oral Biol. 1987;32(6):391–399. doi: 10.1016/0003-9969(87)90073-2. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Loomis R. E., Prakobphol A., Levine M. J., Reddy M. S., Jones P. C. Biochemical and biophysical comparison of two mucins from human submandibular-sublingual saliva. Arch Biochem Biophys. 1987 Nov 1;258(2):452–464. doi: 10.1016/0003-9861(87)90366-3. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen A. V., Oderkerk C. H., Driessen A. A. Role of mucins from human whole saliva in the protection of tooth enamel against demineralization in vitro. Caries Res. 1987;21(4):297–309. doi: 10.1159/000261033. [DOI] [PubMed] [Google Scholar]
- Prakobphol A., Levine M. J., Tabak L. A., Reddy M. S. Purification of a low-molecular-weight, mucin-type glycoprotein from human submandibular-sublingual saliva. Carbohydr Res. 1982 Oct 1;108(1):111–122. doi: 10.1016/s0008-6215(00)81896-0. [DOI] [PubMed] [Google Scholar]
- Rathman W. M., van den Keybus P. A., van Zeyl M. J., Döpp E. A., Veerman E. C., Nieuw Amerongen A. V. Characterization of monoclonal antibodies to human salivary (glyco) proteins. Cellular localization of mucin, cystatin-like 14 kD protein and 20 kD glycoprotein in the human submandibular gland. J Biol Buccale. 1990 Mar;18(1):19–27. [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Mandel I. D., Zalesna G., Slomiany A. Physico-chemical characteristics of mucus glycoproteins and lipids of the human oral mucosal mucus coat in relation to caries susceptibility. Arch Oral Biol. 1989;34(4):229–237. doi: 10.1016/0003-9969(89)90063-0. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Veerman E. C., Valentijn-Benz M., Bank R. A., Nieuw Amerongen A. V. Isolation of high molecular weight mucins from human whole saliva by ultracentrifugation. J Biol Buccale. 1989 Dec;17(4):307–312. [PubMed] [Google Scholar]
- Veerman E. C., Valentijn-Benz M., van den Keybus P. A., Rathman W. M., Sheehan J. K., Nieuw Amerongen A. V. Immunochemical analysis of high molecular-weight human salivary mucins (MG1) using monoclonal antibodies. Arch Oral Biol. 1991;36(12):923–932. doi: 10.1016/0003-9969(91)90125-e. [DOI] [PubMed] [Google Scholar]
- van Beurden-Lamers W. M., Spee-Brand R., Dekker J., Strous G. J. Sulphation causes heterogeneity of gastric mucins. Biochim Biophys Acta. 1989 Mar 24;990(3):232–239. doi: 10.1016/s0304-4165(89)80039-x. [DOI] [PubMed] [Google Scholar]


