Abstract
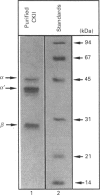
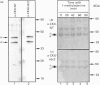
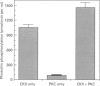
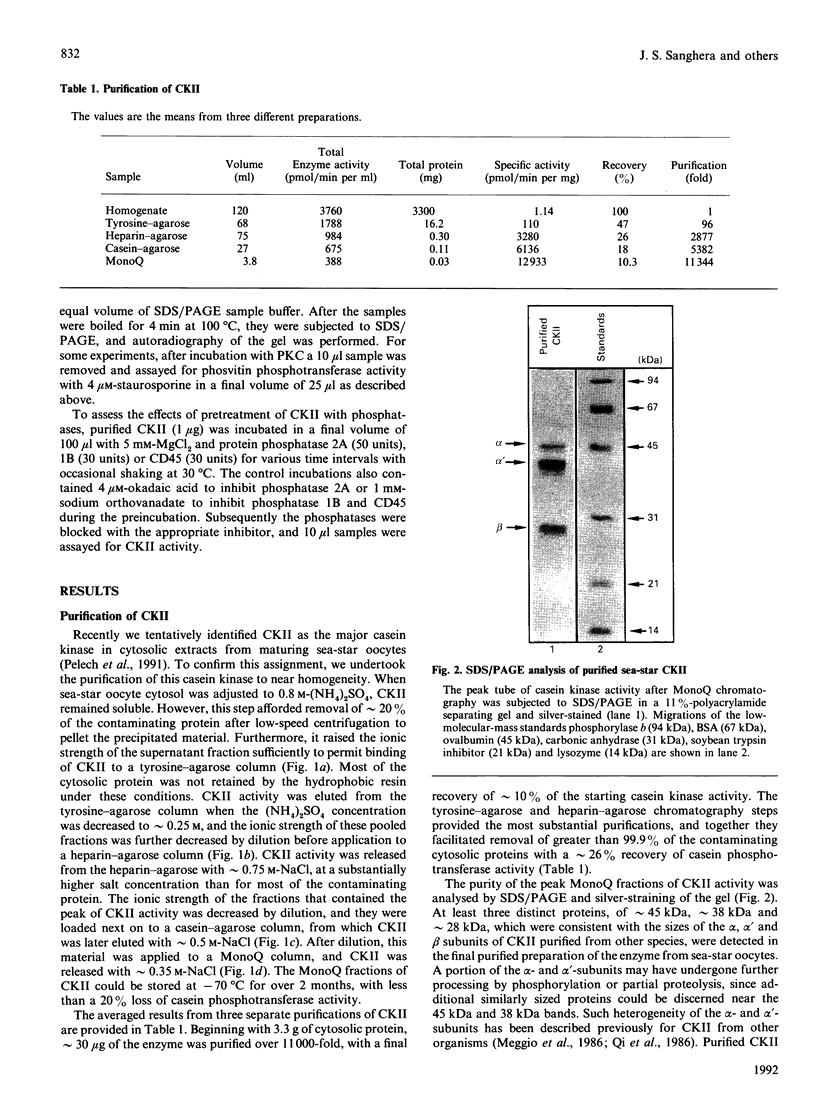
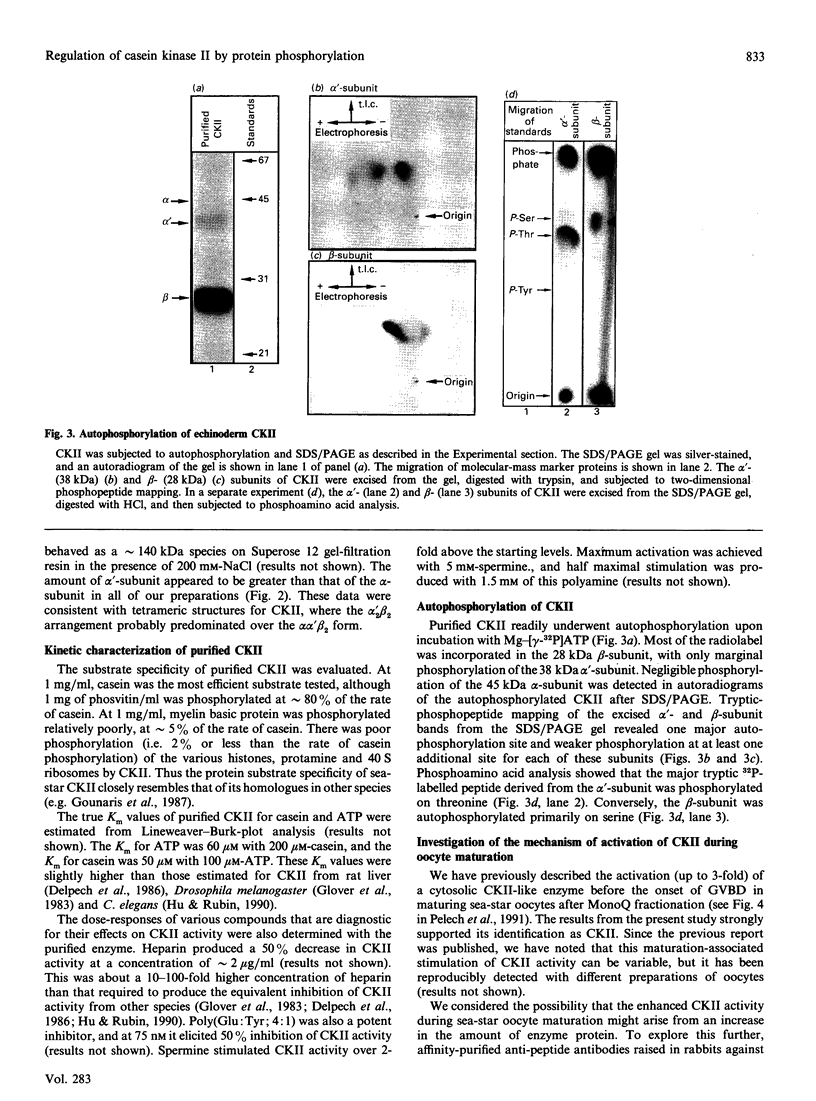
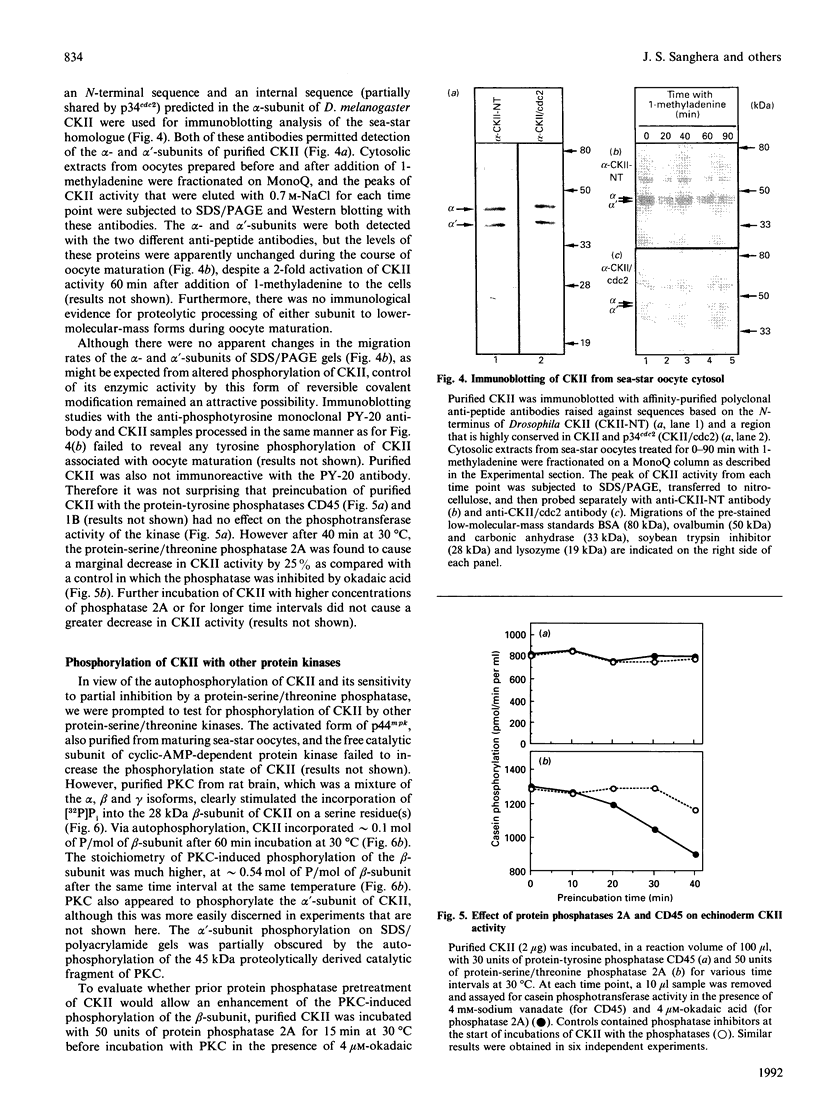
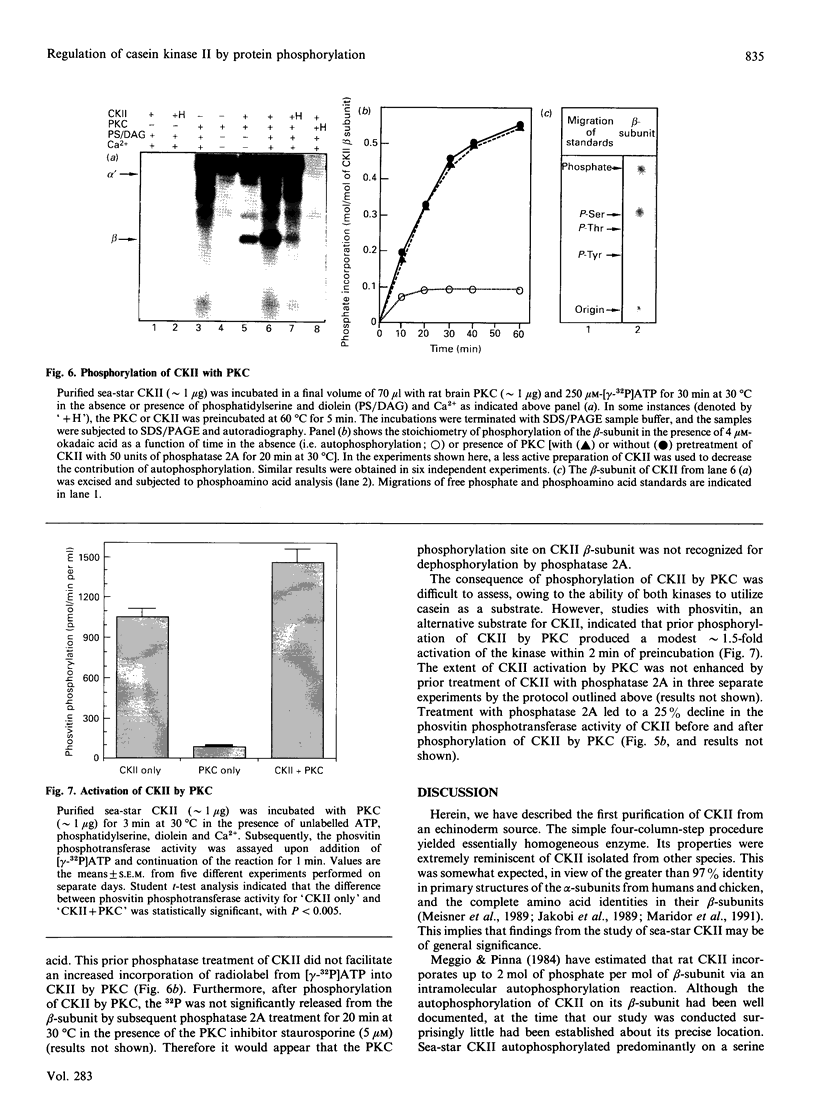
Casein kinase II (CKII) is one of several protein kinases that become activated before germinal-vesicle breakdown in maturing sea-star oocytes. Echinoderm CKII was purified over 11,000-fold with a recovery of approximately 10% by sequential fractionation of the oocyte cytosol on tyrosine-agarose, heparin-agarose, casein-agarose and MonoQ. The purified enzyme contained 45, 38 and 28 kDa polypeptides, which corresponded to its alpha, alpha' and beta subunits respectively. The beta-subunit was autophosphorylated on one major tryptic peptide on serine residues, whereas the alpha'-subunit incorporated phosphate into at least two tryptic peptides primarily on threonine residues. Western-blotting analysis of sea-star oocyte extracts with two different anti-peptide antibodies that recognized conserved regions of the alpha-subunit indicated that the protein levels of the alpha- and alpha'-subunits of CKII were unchanged during oocyte maturation. The purified CKII was partly inactivated (by 25%) by preincubation with protein-serine/threonine phosphatase 2A, but protein-tyrosine phosphatases had no effect. The beta-subunit of CKII was phosphorylated on a serine residue(s) up to 0.54 mol of P/mol of beta-subunit by purified protein kinase C, and this correlated with a 1.5-fold enhancement of its phosphotransferase activity with phosvitin as a substrate. CKII was not a substrate for the maturation-activated myelin basic protein kinase p44mpk from sea-star oocytes, nor for cyclic-AMP-dependent protein kinase. These studies point to possible regulation of CKII by protein phosphorylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit. Proc Natl Acad Sci U S A. 1990 Jan;87(2):821–825. doi: 10.1073/pnas.87.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman P., Osheroff N. Regulation of casein kinase II activity by epidermal growth factor in human A-431 carcinoma cells. J Biol Chem. 1989 Jul 15;264(20):11958–11965. [PubMed] [Google Scholar]
- Agostinis P., Goris J., Pinna L. A., Merlevede W. Regulation of casein kinase 2 by phosphorylation/dephosphorylation. Biochem J. 1987 Dec 15;248(3):785–789. doi: 10.1042/bj2480785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B. G., Katz S. Isolation and characterization of the calcium- and phospholipid-dependent protein kinase (protein kinase C) subtypes from bovine heart. Biochemistry. 1991 Apr 30;30(17):4334–4343. doi: 10.1021/bi00231a032. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Edmonds C., Fisher C., Schiller J. T., Lowy D. R., Vousden K. H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990 Jan;9(1):153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll D., Marshak D. R. Serum-stimulated cell growth causes oscillations in casein kinase II activity. J Biol Chem. 1989 May 5;264(13):7345–7348. [PubMed] [Google Scholar]
- Carroll D., Santoro N., Marshak D. R. Regulating cell growth: casein-kinase-II-dependent phosphorylation of nuclear oncoproteins. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):91–95. doi: 10.1101/sqb.1988.053.01.014. [DOI] [PubMed] [Google Scholar]
- Chen-Wu J. L., Padmanabha R., Glover C. V. Isolation, sequencing, and disruption of the CKA1 gene encoding the alpha subunit of yeast casein kinase II. Mol Cell Biol. 1988 Nov;8(11):4981–4990. doi: 10.1128/mcb.8.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Reed L. J. Purification and properties of a protamine kinase and a type II casein kinase from bovine kidney mitochondria. Arch Biochem Biophys. 1988 May 1;262(2):574–584. doi: 10.1016/0003-9861(88)90408-0. [DOI] [PubMed] [Google Scholar]
- Delpech M., Levy-Favatier F., Moisand F., Kruh J. Rat liver nuclear protein kinases NI and NII. Purification, subunit composition, substrate specificity, possible levels of regulation. Eur J Biochem. 1986 Oct 15;160(2):333–341. doi: 10.1111/j.1432-1033.1986.tb09976.x. [DOI] [PubMed] [Google Scholar]
- Firzlaff J. M., Galloway D. A., Eisenman R. N., Lüscher B. The E7 protein of human papillomavirus type 16 is phosphorylated by casein kinase II. New Biol. 1989 Oct;1(1):44–53. [PubMed] [Google Scholar]
- Glover C. V., Shelton E. R., Brutlag D. L. Purification and characterization of a type II casein kinase from Drosophila melanogaster. J Biol Chem. 1983 Mar 10;258(5):3258–3265. [PubMed] [Google Scholar]
- Gounaris A., Trangas T. T., Tsiapalis C. M. Soluble cAMP-independent protein kinase from human spleen. Arch Biochem Biophys. 1987 Dec;259(2):473–480. doi: 10.1016/0003-9861(87)90514-5. [DOI] [PubMed] [Google Scholar]
- Grässer F. A., Scheidtmann K. H., Tuazon P. T., Traugh J. A., Walter G. In vitro phosphorylation of SV40 large T antigen. Virology. 1988 Jul;165(1):13–22. doi: 10.1016/0042-6822(88)90653-8. [DOI] [PubMed] [Google Scholar]
- Hollmann J., Niemann R., Buddecke E. Purification and characterization of a 3'-phosphoadenylylsulfate:chondroitin 6-sulfotransferase from arterial tissue. Biol Chem Hoppe Seyler. 1986 Jan;367(1):5–13. doi: 10.1515/bchm3.1986.367.1.5. [DOI] [PubMed] [Google Scholar]
- Hu E., Rubin C. S. Expression of wild-type and mutated forms of the catalytic (alpha) subunit of Caenorhabditis elegans casein kinase II in Escherichia coli. J Biol Chem. 1990 Nov 25;265(33):20609–20615. [PubMed] [Google Scholar]
- Jakobi R., Voss H., Pyerin W. Human phosvitin/casein kinase type II. Molecular cloning and sequencing of full-length cDNA encoding subunit beta. Eur J Biochem. 1989 Jul 15;183(1):227–233. doi: 10.1111/j.1432-1033.1989.tb14917.x. [DOI] [PubMed] [Google Scholar]
- Kandror K. V., Benumov A. O., Stepanov A. S. Casein kinase II from Rana temporaria oocytes. Intracellular localization and activity during progesterone-induced maturation. Eur J Biochem. 1989 Mar 15;180(2):441–448. doi: 10.1111/j.1432-1033.1989.tb14666.x. [DOI] [PubMed] [Google Scholar]
- Klarlund J. K., Czech M. P. Insulin-like growth factor I and insulin rapidly increase casein kinase II activity in BALB/c 3T3 fibroblasts. J Biol Chem. 1988 Nov 5;263(31):15872–15875. [PubMed] [Google Scholar]
- Krebs E. G., Eisenman R. N., Kuenzel E. A., Litchfield D. W., Lozeman F. J., Lüscher B., Sommercorn J. Casein kinase II as a potentially important enzyme concerned with signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):77–84. doi: 10.1101/sqb.1988.053.01.012. [DOI] [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin W. J., Tuazon P. T., Traugh J. A. Characterization of the catalytic subunit of casein kinase II expressed in Escherichia coli and regulation of activity. J Biol Chem. 1991 Mar 25;266(9):5664–5669. [PubMed] [Google Scholar]
- Litchfield D. W., Arendt A., Lozeman F. J., Krebs E. G., Hargrave P. A., Palczewski K. Synthetic phosphopeptides are substrates for casein kinase II. FEBS Lett. 1990 Feb 12;261(1):117–120. doi: 10.1016/0014-5793(90)80650-8. [DOI] [PubMed] [Google Scholar]
- Litchfield D. W., Lozeman F. J., Cicirelli M. F., Harrylock M., Ericsson L. H., Piening C. J., Krebs E. G. Phosphorylation of the beta subunit of casein kinase II in human A431 cells. Identification of the autophosphorylation site and a site phosphorylated by p34cdc2. J Biol Chem. 1991 Oct 25;266(30):20380–20389. [PubMed] [Google Scholar]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Kuenzel E. A., Krebs E. G., Eisenman R. N. Myc oncoproteins are phosphorylated by casein kinase II. EMBO J. 1989 Apr;8(4):1111–1119. doi: 10.1002/j.1460-2075.1989.tb03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiori F., Meggio F., Marin O., Borin G., Calderan A., Ruzza P., Pinna L. A. Synthetic peptide substrates for casein kinase 2. Assessment of minimum structural requirements for phosphorylation. Biochim Biophys Acta. 1988 Oct 7;971(3):332–338. doi: 10.1016/0167-4889(88)90149-8. [DOI] [PubMed] [Google Scholar]
- Maridor G., Park W., Krek W., Nigg E. A. Casein kinase II. cDNA sequences, developmental expression, and tissue distribution of mRNAs for alpha, alpha', and beta subunits of the chicken enzyme. J Biol Chem. 1991 Feb 5;266(4):2362–2368. [PubMed] [Google Scholar]
- Meek D. W., Simon S., Kikkawa U., Eckhart W. The p53 tumour suppressor protein is phosphorylated at serine 389 by casein kinase II. EMBO J. 1990 Oct;9(10):3253–3260. doi: 10.1002/j.1460-2075.1990.tb07524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggio F., Grankowski N., Kudlicki W., Szyszka R., Gasior E., Pinna L. A. Structure and properties of casein kinase-2 from Saccharomyces cerevisiae. A comparison with the liver enzyme. Eur J Biochem. 1986 Aug 15;159(1):31–38. doi: 10.1111/j.1432-1033.1986.tb09829.x. [DOI] [PubMed] [Google Scholar]
- Meggio F., Perich J. W., Johns R. B., Pinna L. A. Partially dephosphorylated phosphopeptide AcSer(P)-Ser(P)-Ser(P) is an excellent substrate for casein kinase-2. FEBS Lett. 1988 Sep 12;237(1-2):225–228. doi: 10.1016/0014-5793(88)80206-0. [DOI] [PubMed] [Google Scholar]
- Meggio F., Pinna L. A. Subunit structure and autophosphorylation mechanism of casein kinase-TS (type-2) from rat liver cytosol. Eur J Biochem. 1984 Dec 17;145(3):593–599. doi: 10.1111/j.1432-1033.1984.tb08598.x. [DOI] [PubMed] [Google Scholar]
- Meijer L., Pelech S. L., Krebs E. G. Differential regulation of histone H1 and ribosomal S6 kinases during sea star oocyte maturation. Biochemistry. 1987 Dec 1;26(24):7968–7974. doi: 10.1021/bi00398a063. [DOI] [PubMed] [Google Scholar]
- Meisner H., Heller-Harrison R., Buxton J., Czech M. P. Molecular cloning of the human casein kinase II alpha subunit. Biochemistry. 1989 May 2;28(9):4072–4076. doi: 10.1021/bi00435a066. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mulner-Lorillon O., Cormier P., Labbé J. C., Dorée M., Poulhe R., Osborne H., Bellé R. M-phase-specific cdc2 protein kinase phosphorylates the beta subunit of casein kinase II and increases casein kinase II activity. Eur J Biochem. 1990 Oct 24;193(2):529–534. doi: 10.1111/j.1432-1033.1990.tb19368.x. [DOI] [PubMed] [Google Scholar]
- Mulner-Lorillon O., Marot J., Cayla X., Pouhle R., Belle R. Purification and characterization of a casein-kinase-II-type enzyme from Xenopus laevis ovary. Biological effects on the meiotic cell division of full-grown oocyte. Eur J Biochem. 1988 Jan 15;171(1-2):107–117. doi: 10.1111/j.1432-1033.1988.tb13765.x. [DOI] [PubMed] [Google Scholar]
- Padmanabha R., Chen-Wu J. L., Hanna D. E., Glover C. V. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabha R., Glover C. V. Casein kinase II of yeast contains two distinct alpha polypeptides and an unusually large beta subunit. J Biol Chem. 1987 Feb 5;262(4):1829–1835. [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S., Daya-Makin M. Protein kinase cascades in meiotic and mitotic cell cycle control. Biochem Cell Biol. 1990 Dec;68(12):1297–1330. doi: 10.1139/o90-194. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S., Paddon H. B., Quayle K. A., Brownsey R. W. Identification of a major maturation-activated acetyl-CoA carboxylase kinase in sea star oocytes as p44mpk. Biochem J. 1991 Mar 15;274(Pt 3):759–767. doi: 10.1042/bj2740759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Pyerin W., Burow E., Michaely K., Kübler D., Kinzel V. Catalytic and molecular properties of highly purified phosvitin/casein kinase type II from human epithelial cells in culture (HeLa) and relation to ecto protein kinase. Biol Chem Hoppe Seyler. 1987 Mar;368(3):215–227. doi: 10.1515/bchm3.1987.368.1.215. [DOI] [PubMed] [Google Scholar]
- Qi S. L., Yukioka M., Morisawa S., Inoue A. Heterogeneity of protein kinase NII. Multiple subunit-polypeptides. FEBS Lett. 1986 Jul 14;203(1):104–108. doi: 10.1016/0014-5793(86)81446-6. [DOI] [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Bader S. A., Pelech S. L. Purification and characterization of a maturation-activated myelin basic protein kinase from sea star oocytes. J Biol Chem. 1990 Jan 5;265(1):52–57. [PubMed] [Google Scholar]
- Saxena A., Padmanabha R., Glover C. V. Isolation and sequencing of cDNA clones encoding alpha and beta subunits of Drosophila melanogaster casein kinase II. Mol Cell Biol. 1987 Oct;7(10):3409–3417. doi: 10.1128/mcb.7.10.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommercorn J., Mulligan J. A., Lozeman F. J., Krebs E. G. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternbach H., Küntzel H. Purification of a yeast protein kinase sharing properties with type I and type II casein kinases. Biochemistry. 1987 Jul 14;26(14):4207–4212. doi: 10.1021/bi00388a005. [DOI] [PubMed] [Google Scholar]
- Takio K., Kuenzel E. A., Walsh K. A., Krebs E. G. Amino acid sequence of the beta subunit of bovine lung casein kinase II. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4851–4855. doi: 10.1073/pnas.84.14.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugh J. A., Lin W. J., Takada-Axelrod F., Tuazon P. T. Importance of subunit interactions in regulation of casein kinase II. Adv Second Messenger Phosphoprotein Res. 1990;24:224–229. [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]