Abstract
Several studies have reported that antioxidants exert both preventive and inhibitory effects against tumors. However, their causal effects on small-cell lung cancer (SCLC) remain controversial. Herein, we explored the causal effects of 6 antioxidants on SCLC by combining a genome-wide association study database and the Mendelian randomization (MR) approach. We obtained antioxidant genetic variance data for 6 exposure factors: carotene, vitamin A (retinol), selenium, zinc, vitamin C, and vitamin E, from the genome-wide association study database. The instrumental variables for exposure factors and SCLC outcomes were integrated by screening instrumental variables and merging data. Two-sample MR was used to analyze the causal relationship between exposure and outcomes. Finally, we examined the heterogeneity and horizontal pleiotropy of the MR analysis by performing multiple sensitivity analyses. We found a causal relationship between carotene and SCLC using two-sample MR analysis and sensitivity analysis (P = .02; odds ratio = 0.73; 95% confidence interval: 0.55–0.95). In contrast, there was no causal relationship between other examined antioxidants and SCLC. We found that diet-derived circulating antioxidants could afford protection against SCLC, and carotene is the causal protective factor against SCLC.
Keywords: antioxidants, GWAS, Mendelian randomization, small-cell lung cancer
1. Introduction
Lung cancer, known to be one of the most common cancers, exhibits high morbidity and mortality worldwide and remains a serious threat to human health.[1,2] Lung cancer can be divided into 2 categories according to its pathological type: small-cell lung cancer (SCLC) and non-small-cell lung cancer.[3] Although SCLC accounts for only approximately 15% of lung cancer cases,[4] it is extremely malignant, highly aggressive,[5] and prone to distant metastasis during early stages.[6] Despite the high sensitivity of SCLC to chemotherapy,[7] the five-year survival rate of patients is still <10%,[8] owing to its capacity for recurrence and metastasis.[9] Identified primary risk factors include smoking, environmental pollution, and exposure to toxic substances.[10] Clinical strategies for SCLC remain limited, and there is an urgent need to explore additional strategies.
Food has been well-documented to contain several antioxidant substances,[11] which exhibit specific antioxidant activity and can delay aging,[12] inhibit the occurrence and development of tumor cells,[13] as well as play antiviral, and other important roles.[14,15] Epidemiological studies have shown that the primary mechanism of antioxidants is their ability to combat free radicals,[16,17] thereby preventing cell membrane destruction,[18] DNA damage and repair disorders,[19] damage to important proteins and other biomolecules.[20] Furthermore, antioxidants diminish the probability of additional gene mutations, thereby hindering the potential for cancer induction. Therefore, diet-derived antioxidants should receive attention, especially in the field of antitumor therapy. Diet-derived antioxidants may reduce the incidence of colon cancer.[21] However, some reports indicate that certain dietary nutrients may increase the risk of lung cancer. For example, a double-blind, randomized controlled trial has implied that β-carotene may increase the risk of lung cancer in smokers.[22] Thus, the potential antitumor effects of antioxidants in food sources warrant further investigation.
Therefore, to explore alternative strategies for preventing and treating SCLC, as well as to avoid confounding bias and bias in the causality of previous studies, we used genetic variation as an instrumental variable (IV) to infer the causal effect of the exposure factor (diet-derived antioxidants) on the outcome factor (SCLC), using two-sample Mendelian randomization (MR) analysis.
2. Material and methods
2.1. Study design
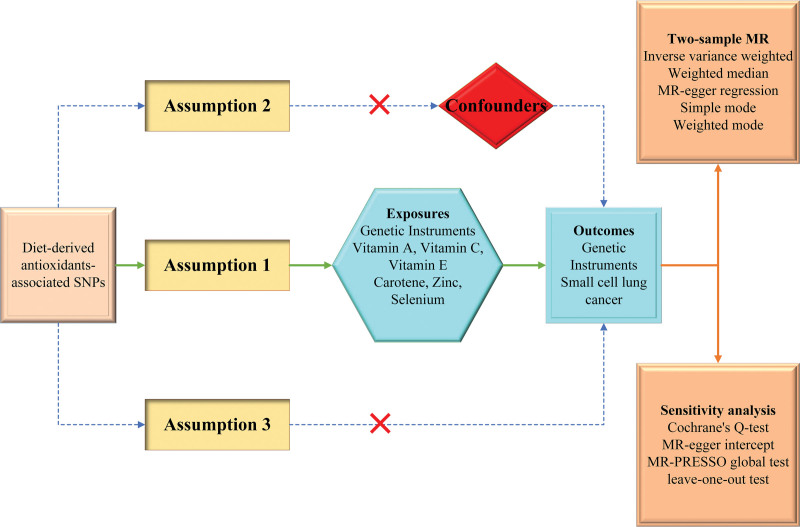
Figure 1 presents a flowchart of the study design and the 3 basic assumptions underlying the MR analysis. We first obtained exposure factors, including genetic mutation data for vitamins A, C, E, carotene, zinc, and selenium, and genetic variance data for SCLC from the genome-wide association study (GWAS) database, as well as instrumental variables (IVs) by screening. Next, we analyzed the causal association between exposure factors and SCLC using a two-sample MR study and validated the credibility of obtained results by performing heterogeneity tests and horizontal multiplicity tests for sensitivity analysis.
Figure 1.
Flow chart of the experimental design of this study.
Single nucleotide polymorphisms for dietary antioxidants (vitamin A, vitamin E, vitamin C, carotene, zinc, and selenium) were identified as genetic instrumental variables. Assumption 1: The genetic variations are strongly associated with exposure; Assumption 2: The genetic variations are not associated with either known or unknown confounders; Assumption 3: SNPs should influence risk of the outcome through the exposure, not through other pathways.
2.2. Screening and processing of exposure and outcome data
In the present study, we primarily explored the anticancer effects of antioxidants by examining genetic phenotypes, mainly of dietary origin. Six dietary sources of antioxidants from the GWAS database were considered. Next, we screened SNPs that could represent the corresponding antioxidants as IVs using the following screening criteria: (1) P-value < 5 × 106; (2) linkage disequilibrium (LD): r2 < 0:001; (3) LD distance > 10000 kb. In addition, we further calculated the strength of the correlation between the 2, expressed as an F-statistic, considering whether these IVs could represent the corresponding exposure factors; an F-value > 10 was considered a strong correlation between the 2.
Herein, we focused on SCLC, excluding the effects of all other tumor types. In total, 16,380,303 SNPs were examined from 30 relevant databases, including European participants. In addition, we compared the exposure and outcome data, and no overlapping samples were detected between the 2.
2.3. Two-sample MR analysis
To ensure the accuracy of MR analysis, we first evaluated the IVs, which were examined using PhenoScanner V2 to exclude those associated with SCLC. Next, IVs for exposure factors and SCLC outcomes were integrated, and the echo sequence was eliminated. A two-sample MR analysis was then performed using the treated IVs, in which 6 different methods were employed: inverse-variance weighted (IVW), weighted median, MR-Egger regression, simple mode, weighted mode, and MR-pleiotropy residual sum and outlier (MR-PRESSO). Among them, the IVW method is a random-effects detection method, combining the Wald ratios of each SNP; thus, this method affords the highest detection efficacy.[23]
2.4. Sensitivity analyses
Sensitivity analysis mainly includes heterogeneity analysis and the horizontal multiplicity test, the main purpose of which is to exclude bias and confounding factors in the MR analysis. In the present study, sensitivity analyses were performed using Cochrane Q test, Egger regression intercept, leave-one-out analysis, and the MR-PRESSO global test. Cochran Q test, primarily used for heterogeneity analysis, detected possible bias in the IVs, and P < .05 was considered heterogeneous. Egger regression intercepts were mainly used to detect the presence of confounding factors in IVs; horizontal multiplicity detection (P < .05) was considered as the presence of horizontal multiplicity. In addition, the MR-PRESSO global test was used to detect potentially anomalous data that caused the data to shift. A P-value of <.05 was considered as the presence of anomalous data.Data processing was performed using the R packages “TwoSampleMR” and “MR-PRESSO” (version 4.2.0).
2.5. Ethical considerations
Because the present study is based on published GWAS summary statistics rather than individual levels data, ethical approval is not required.
3. Results
3.1. Characteristics of IVs
The characteristics of exposure factors and selected IVs are listed in Table 1. Data sources for these variables were predominantly European, including some African Americans or Afro-Caribbean. We selected 71 SNPs for food-derived circulating oxidants as IVs. These 71 SNPs were excluded from the single SNP containing the palindrome sequence (rs2952225; selenium), and 4 SNPs were present in the exposure but did not correspond with the outcome, including rs11264455 (carotene), rs12119164 (vitamin A), rs61140500 (vitamin C), and rs113256314 (vitamin E). In addition, all 71 SNPs were screened for any direct association with the outcome. The number of samples sourced for each exposure factor ranged from 1209 to 62,991, all presenting F-statistic values ˃10, ranging from 12.28 to 60.06.
Table 1.
Characteristics of exposures’ datasets.
| Exposures | Consortium | Sample | nSNP | nIVs | F-statistic |
|---|---|---|---|---|---|
| Carotene | Pan-UKB team et al | 1209 | 15533459 | 9 | 60.06 |
| Retinol | MRC-IEU | 62,991 | 9851867 | 8 | 23.08 |
| Selenium | MRC-IEU | 11,059 | 9851867 | 7 | 12.58 |
| Vitamin C | Neale Lab | 28,536 | 10894596 | 19 | 15.66 |
| Vitamin E | MRC-IEU | 13,548 | 9851867 | 13 | 19.78 |
| Zinc | MRC-IEU | 18,826 | 9851867 | 15 | 40.93 |
IVs = instrumental variable, nIVs = number of IVs, nSNP = number of SNPs, SNP = single-nucleotide polymorphism.
3.2. MR analysis results and assessment of IVs
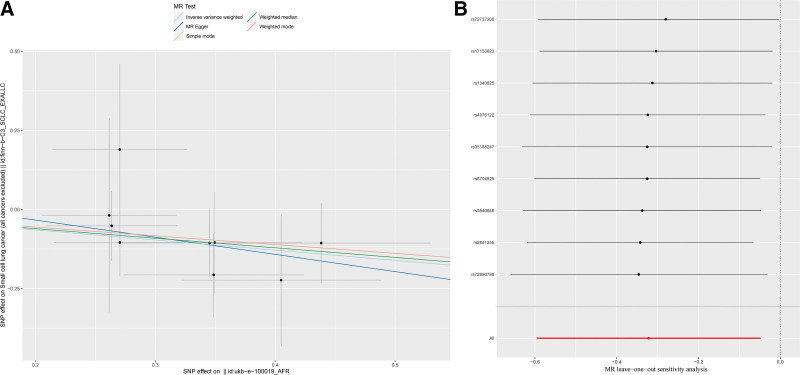
Herein, the effect of diet-derived antioxidants on SCLC was analyzed mainly using the MR approach, and the results are presented in Table 2. Based on the results of the analysis, we found that carotene exerted a causal and protective effect on SCLC, with a P-value of 0.02 for IVW and an odds ratio (OR) of 0.73. The IVs of carotene are listed in Table 3. In addition, similar conclusions were reached with MR-PRESSO, with a P-value <.01 and an OR of 0.72. Although the P-values of the other analytical methods were not statistically significant, their ORs were <1, indicating that these methods exhibit the same trend (Fig. 2A). Considering the other antioxidants, we failed to detect a causal relationship based on the analysis results, and IVs of these exposure factors are presented in Tables S1–S5, Supplemental Digital Content, http://links.lww.com/MD/L589, http://links.lww.com/MD/L590, http://links.lww.com/MD/L591, http://links.lww.com/MD/L592, http://links.lww.com/MD/L596; and Fig. S1A–J, Supplemental Digital Content, http://links.lww.com/MD/L615.
Table 2.
Two-sample Mendelian randomization estimations showing the effects of diet-derived antioxidants on the risk of small cell lung cancer.
| Exposures | Inverse variance weighted | Weighted median | MR Egger | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Carotene | 0.73 (0.55–0.95) | .02 | 0.74 (0.51–1.06) | .10 | 0.58 (0.13–2.63) | .51 |
| Retinol | 0.16 (0.01–2.18) | .17 | 0.22 (6.6E–03–7.29) | .40 | 0.01 (2.2E–05–5.61) | .21 |
| Selenium | 3.6E–22 (9.2E–45–14.9) | .06 | 3.2E–28 (2.6E–57–39.2) | .06 | 9.9E+20 (2.9E–56–3.4E+97) | .61 |
| Vitamin C | 1.2E+05 (0.28–5.4E+10) | .08 | 1.2E+03 (1.1E–05–1.4E+11) | .45 | 2.3E–03 (1.6E–14–3.4E+08) | .65 |
| Vitamin E | 2.5E+05 (4.7E–12–1.3E+32) | .53 | 0.33 (1.3E-20–8.5E+22) | .89 | 2.5E + 03 (2.7E–52–2.3E+58) | .91 |
| Zinc | 1.1E–06 (1.9E–19–5.7E+06) | .36 | 4.8E–04 (2.6E–19–9.1E+11) | .67 | 2.7E+05 (1.3E–31–5.9E+41) | .78 |
| Exposures | Simple mode | Weighted mode | MR-PRESSO | |||
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Carotene | 0.76 (0.49–1.16) | .24 | 0.76 (0.50–1.14) | .21 | 0.72 (0.63–0.83) | <.01 |
| Retinol | 2.45 (8.9E–03–668.09) | .76 | 0.03 (3.3E–04–2.76) | .17 | 0.16 (0.02–1.15) | .15 |
| Selenium | 9.6E–19 (2.5E–62–2.8E+20) | .45 | 1.8E–20 (1.2E–60–2.8E+20) | .37 | 1.3E–24 (2.8E–42–6.4E–07) | .03 |
| Vitamin C | 4.6E+05 (4.1E–08–5.1E+18) | .41 | 4.4E+02(2.7E–08–7.3E+12) | .62 | 1.2E+5(0.75–2.0E+10) | .07 |
| Vitamin E | 0.01 (7.8E–37–1.3E+32) | .91 | 0.05 (2.7E–32–1.1E+29) | .94 | 2.5E+05 (4.7E–12–1.3E+22) | .54 |
| Zinc | 6.2E-05 (1.4E–32–2.7E+23) | .77 | 2.1E–03 (1.2E–30–3.5E+24) | .85 | 1.1E–06 (2.0E–19–5.7E+07) | .37 |
Table 3.
Harmonized dataset of Mendelian randomization for the effect of carotene on small cell lung cancer.
| SNP | Effect allele | Other allele | Chr | Exposure | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | ||||
| rs1340825 | C | T | 6 | –0.271 | 0.055 | 7.88E–07 | 0.105 | 0.107 | .328 |
| rs17153823 | G | A | 7 | 0.405 | 0.083 | 1.18E–06 | –0.224 | 0.209 | .286 |
| rs2841355 | T | C | 1 | 0.270 | 0.056 | 1.53E–06 | 0.189 | 0.271 | .484 |
| rs35188247 | A | T | 4 | 0.345 | 0.075 | 4.42E–06 | –0.106 | 0.109 | .331 |
| rs4976122 | T | C | 5 | –0.349 | 0.073 | 1.90E–06 | 0.105 | 0.161 | .513 |
| rs6704825 | T | C | 2 | 0.262 | 0.056 | 3.21E–06 | –0.018 | 0.307 | .953 |
| rs72737200 | G | A | 1 | –0.349 | 0.075 | 3.41E–06 | 0.207 | 0.134 | .122 |
| rs72890788 | A | G | 1 | –0.438 | 0.091 | 1.66E–06 | 0.106 | 0.127 | .402 |
| rs9540648 | G | A | 13 | 0.264 | 0.054 | 1.55E–06 | –0.052 | 0.110 | .639 |
Chr = chromosome.
Figure 2.
(A) Scatter plot of SNPs associated with diet-derived antioxidants and risk of SCLC. The plot shows the SNP effects on diet-derived antioxidants (x-axis, SD units) as well as SCLC (y-axis, OR) with 95% CI. The MR regression slopes of the lines represent the causal estimates using 5 approaches (IVW, MR-Egger, weighted median, simple mode, and weighted mode); (B) diagram of the leave-one-out method of SNPs associated with dietary antioxidants and SCLC. Each black point indicates the log OR for SCLC per standard deviation increase in dietary antioxidants, generated utilizing each dietary antioxidants -associated SNP as an instrument. The horizontal line denotes 95% CIs of the estimates. The red point shows the combined causal estimates utilizing all SNPs as an instrument based on leave-one-out method. IVW = inverse-variance weighted, MR = Mendelian randomization, OR = odds ratio, SCLC = small-cell lung cancer, SNPs = single nucleotide polymorphisms.
3.3. Assessment of IVs
We performed heterogeneity analysis and horizontal multiplicity tests separately for selected IVs to ensure good validation (Table 4). In terms of the heterogeneity test, for the 6 exposure factors, the P-value corresponding to each exposure factor was ˃.05. For horizontal multiplicity, tests were performed using the MR-Egger intercept and MR-PRESSO global test, and the P-values for all tests were ˃.05. The results of the above analysis indicated the absence of heterogeneity or horizontal pleiotropy in the exposure data. Finally, we used the leave-one-out method for carotene data analysis. As shown in Figure 2B, we observed that each SNP had no significant effect on the overall analysis results, and all trends of effects were reflected as protective factors. Table S1, Supplemental Digital Content, http://links.lww.com/MD/L589 summarizes the results of the analysis of other exposure factors. The results suggest that the trend of effects on outcomes was non-significant, with no causal relationship currently detectable.
Table 4.
The estimations of heterogeneity and horizontal pleiotropy for MR results.
| Exposures | Inverse variance weighted | MR-Egger | MR-PRESSO | ||||
|---|---|---|---|---|---|---|---|
| Q-statistic | P | Q-statistic | P | Egger intercept | P | P for global test | |
| Carotene | 1.99 | .96 | 1.90 | .98 | 0.08 (–0.43–0.58) | .78 | 0.99 |
| Retinol | 4.96 | .66 | 4.10 | .66 | 0.13 (–0.14–0.40) | .39 | 0.68 |
| Selenium | 4.25 | .64 | 2.96 | .71 | –0.20 (–0.53–0.14) | .31 | 0.75 |
| Vitamin C | 15.39 | .64 | 12.93 | .74 | 0.11 (–0.03–0.24) | .13 | 0.65 |
| Vitamin E | 15.46 | .22 | 15.45 | .16 | 0.01 (–0.32–0.35) | .94 | 0.25 |
| Zinc | 19.61 | .14 | 18.97 | .12 | –0.08 (–0.32–0.16) | .52 | 0.15 |
4. Discussion
The MR approach is an analytical method with a high level of argumentation in evidence-based medical research,[24] which can largely avoid various errors and biases observed in clinical control experiments,[25] simultaneously minimizing the influence of confounding factors and ensuring the inference of causal relationships between exposure factors and outcomes.[26–28] In the present study, we obtained large-scale exposure and SCLC data from the GWAS database, compensating for the limited size of randomized controlled trials and increasing the credibility of data analysis. Based on the findings of the present study, we detected a negative causality of carotenoids in circulating antioxidants from dietary sources with SCLC, suggesting a protective factor for SCLC, whereas no significant causality was detected with other exposure factors.
Antioxidants mainly target the inhibition of reactive oxygen species and prevent further oxidative and carcinogenic effects on the body.[29] The main oncogenic mechanism of reactive oxygen species involves the induction of DNA damage through oxidation,[30] which results in the activation of proto-oncogenes or inactivation of oncogenes,[31] eventually leading to tumorigenesis or the induction of tumor apoptosis.[32] Several studies have highlighted the advances in cancer prevention using food-derived antioxidants. Related studies have shown that vitamin C can impact the level of oxidative stress in humans.[33] Further, it can have antimutagenic effects on plasmid and genomic DNA.[34] In addition, a meta-analysis found a reduced incidence of colorectal cancer in patients taking selenium, accompanied by an impact on overall mortality.[35] In a prospective trial using carboplatin and paclitaxel in combination with oral antioxidants (including vitamin C, vitamin E, and β-carotene) to treat ovarian cancer, the authors found that patients exhibited normal CA-125 levels and remission.[36] In addition, some antioxidants modulate the immune processes that influence tumorigenesis and progression. For example, curcumin can enhance immune surveillance mechanisms by restoring the immune cell ratio and inhibiting T cell apoptosis, thereby suppressing cancer cell growth.[37,38] Conversely, vitamin C attenuates the inflammatory response and thus plays a regulatory role in tumors exhibiting inflammation.[39] However, contradictory results have also been documented. For example, a prevention trial noted that healthy men taking vitamin E could be at high risk for prostate cancer.[40] Therefore, the effect of diet-derived antioxidants on tumors remains highly controversial, and there is a lack of data from large population samples.
Carotene, an important food source of antioxidants,[41] is widely distributed in vegetables and fruits consumed by humans. Carotene can improve cognitive function and cardiovascular health, along with its benefits for eye health.[42,43] Given its robust antioxidant properties, carotene can potentially prevent and treat several chronic diseases, including certain cancers. Several epidemiological studies have examined the benefits of carotene in gastric cancer, and experimental studies have shown that carotene can reduce the risk of gastric cancer and improve its prognosis.[44] Conversely, another meta-analysis failed to detect any significant relationship between carotenoid supplementation and lung cancer risk.[45] In the present study, we arrived at a new conclusion regarding the causal relationship between carotene and SCLC and a protective factor, indicating the ability of carotene to reduce the risk of developing SCLC.
Herein, the selected MR analysis method could overcome the limitations of some clinical control trials and analyze the effect of diet-derived antioxidants on SCLC from the perspective of a large sample of data; carotene could be a protective fact figure or against SCLC, whereas other antioxidants, although not statistically significant, may afford some potential effects. In addition, this exposure factor was associated with the IVs of SNPs, which responded to the effect of long-term antioxidant exposure on cancer in humans, potentially reducing the risk of drug use and damage to subjects themselves when compared with clinical control trials assessing short-term intake or oral administration of antioxidants. However, this study had some limitations. First, human food intake is rich, and antioxidants may mediate synergistic effects on tumor production; hence, their overall or combined effects need to be further explored. Second, given the limitations of the GWAS database, clinical and survival data for patients with SCLC are lacking, and classifying subgroups based on factors such as age or sex or determining their impact on patient prognosis can pose a considerable challenge. Finally, although no causality was detected for examined antioxidants, except for carotene, this may be related to the selected sample, and a greater sample population needs to be included to perform a larger analysis.
5. Conclusion
We performed a causal analysis of MR for certain representative food sources of antioxidants and SCLC and ultimately found that carotene was a protective factor for SCLC. Although other antioxidants failed to provide a significant causal relationship, further validation is required in future studies.
Acknowledgments
Project supported by the Education Department of Hainan Province, Project number: Hnky2021ZD-15.
Author contributions
Methodology: Li Xiao, Xiaoting Mo.
Project administration: Danxin Wang, Wei Zhang.
Writing – original draft: Li Xiao, Xiaoting Mo.
Writing – review & editing: Huiyan Li, Xiangmei Weng.
Supplementary Material
Abbreviations:
- GWAS
- genome-wide association study
- IVs
- instrumental variables
- IVW
- inverse-variance weighted
- MR
- Mendelian randomization
- MR-PRESSO
- MR-pleiotropy residual sum and outlier
- OR
- odds ratio
- SCLC
- small-cell lung cancer
- SNPs
- single nucleotide polymorphisms
LX and XM contributed equally to this work.
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Xiao L, Mo X, Li H, Weng X, Wang D, Zhang W. Genetic overlap and causal inferences between diet-derived antioxidants and small-cell lung cancer. Medicine 2024;103:8(e37206).
Contributor Information
Li Xiao, Email: 13807577152@163.com.
Xiaoting Mo, Email: 174700659@qq.com.
Huiyan Li, Email: 593577425@qq.com.
Xiangmei Weng, Email: 463619206@qq.com.
Wei Zhang, Email: jondon741@yeah.net.
References
- [1].Shen S, Wei C, Fu J. RNA-sequencing reveals heat shock 70-kDa protein 6 (HSPA6) as a novel thymoquinone-upregulated gene that inhibits growth, migration, and invasion of triple-negative breast cancer cells. Front Oncol. 2021;11:667995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu S, Li Q, Li G, et al. The mechanism of mA methyltransferase METTL3-mediated autophagy in reversing gefitinib resistance in NSCLC cells by β-elemene. Cell Death Dis. 2020;11:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Raquel-Cunha A, Cardoso-Carneiro D, Reis R, et al. Current status of Raf kinase inhibitor protein (RKIP) in lung cancer: behind RTK signaling. Cells. 2019;8:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pan D, Chen J, Feng C, et al. Preferential localization of MUC1 glycoprotein in exosomes secreted by non-small cell lung carcinoma cells. Int J Mol Sci. 2019;20:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fiorentino F, Marchesi I, Schröder C, et al. BET-inhibitor I-BET762 and PARP-inhibitor talazoparib synergy in small cell lung cancer cells. Int J Mol Sci. 2020;21:9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Käsmann L, Janssen S, Baschnagel A, et al. Prognostic factors and outcome of reirradiation for locally recurrent small cell lung cancer-a multicenter study. Transl Lung Cancer Res. 2020;9:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Quoix E. Topotecan in the treatment of relapsed small cell lung cancer. Onco Targets Ther 2008;1:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Han F, Zhang M, Liu W, et al. SOX30 specially prevents Wnt-signaling to suppress metastasis and improve prognosis of lung adenocarcinoma patients. Respir Res. 2018;19:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jeong D, Ban S, Oh S, et al. Prognostic significance of EDIL3 expression and correlation with mesenchymal phenotype and microvessel density in lung adenocarcinoma. Sci Rep. 2017;7:8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu F, Yin Z, Yang L, et al. Smoking induced extracellular vesicles release and their distinct properties in non-small cell lung cancer. J Cancer 2019;10:3435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xia T, Zhang J, Yao J, et al. Shanxi aged vinegar protects against alcohol-induced liver injury via activating Nrf2-mediated antioxidant and inhibiting TLR4-induced inflammatory response. Nutrients. 2018;10:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tiveron A, Melo P, Bergamaschi K, et al. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int J Mol Sci. 2012;13:8943–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gao P, Zhang H, Dinavahi R, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer cell 2007;12:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Habza-Kowalska E, Gawlik-Dziki U, Dziki D. Mechanism of action and interactions between thyroid peroxidase and lipoxygenase inhibitors derived from plant sources. Biomolecules. 2019;9:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gang Y, Eom T, Marasinghe S, et al. Optimising the DPPH assay for cell-free marine microorganism supernatants. Mar Drugs. 2021;19:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li H, Zhao H, Gao Z, et al. The antioxidant and anti-aging effects of acetylated mycelia polysaccharides from Pleurotus djamor. Molecules. 2019;24:2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fruehwirth S, Egger S, Kurzbach D, et al. Ingredient-dependent extent of lipid oxidation in margarine. Antioxidants (Basel). 2021;1010:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zaghlool S, Abo-Seif A, Rabeh M, et al. Althaea officinalis gastro-protective and anti-oxidant potential of and on pyloric ligation/indomethacin-induced ulceration in rats. Antioxidants (Basel). 2019;8:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Haskins A, Buglewicz D, Hirakawa H, et al. Palmitoyl ascorbic acid 2-glucoside has the potential to protect mammalian cells from high-LET carbon-ion radiation. Sci Rep. 2018;8:13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stelmashook E, Isaev N, Genrikhs E, et al. Mitochondria-targeted antioxidants as potential therapy for the treatment of traumatic brain injury. Antioxidants (Basel). 2019;8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang Z, Chen H, Li M, et al. Association of dietary carrot/carotene intakes with colorectal cancer incidence and mortality in the prostate. Front Nutr. 2022;9:888898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Middha P, Weinstein S, Männistö S, et al. β-Carotene supplementation and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: the role of tar and nicotine. Nicotine Tob Re. 2019;21:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burgess S, Bowden J, Fall T, et al. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beeghly-Fadiel A, Khankari N, Delahanty R, et al. A Mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int J Epidemiol. 2020;49:1117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fall T, Hägg S, Ploner A, et al. Age- and sex-specific causal effects of adiposity on cardiovascular risk factors. Diabetes. 2015;64:1841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nowak C, Sundström J, Gustafsson S, et al. Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes. 2016;65:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dimou N, Papadimitriou N, Gill D, et al. Sex hormone binding globulin and risk of breast cancer: a Mendelian randomization study. Int J Epidemiol. 2019;48:807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pickrell J, Berisa T, Liu J, et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li H, Wang L, Luo Y. Composition analysis by UPLC-PDA-ESI (-)-HRMS and antioxidant activity using Saccharomyces cerevisiae model of herbal teas and green teas from Hainan. Molecules. 2018;23:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Skała E, Sitarek P, Różalski M, et al. Antioxidant and DNA repair stimulating effect of extracts from transformed and normal roots of rhaponticum carthamoides against induced oxidative stress and DNA damage in CHO cells. Oxid Med Cell Longevity. 2016;2016:5753139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zamkova M, Khromova N, Kopnin B, et al. Ras-induced ROS upregulation affecting cell proliferation is connected with cell type-specific alterations of HSF1/SESN3/p21Cip1/WAF1 pathways. Cell Cycle. 2013;12:826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aggarwal V, Tuli H, Varol A, et al. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sram R, Binkova B, Rossner P, Jr. Vitamin C for DNA damage prevention. Mutat Res. 2012;733:39–49. [DOI] [PubMed] [Google Scholar]
- [35].Pais R, Dumitraşcu DL. Do antioxidants prevent colorectal cancer? A meta-analysis. Rom J Intern Med. 2013;51:152–63. [PubMed] [Google Scholar]
- [36].Drisko J, Chapman J, Hunter VJ. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J Am Coll Nutr. 2003;22:118–23. [DOI] [PubMed] [Google Scholar]
- [37].Bose S, Panda A, Mukherjee S, et al. Curcumin and tumor immune-editing: resurrecting the immune system. Cell Div. 2015;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bhattacharyya S, Md Sakib Hossain D, Mohanty S, et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sorice A, Guerriero E, Capone F, et al. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev Med Chem. 2014;14:444–52. [DOI] [PubMed] [Google Scholar]
- [40].Ledesma M, Jung-Hynes B, Schmit T, et al. Selenium and vitamin E for prostate cancer: post-SELECT (Selenium and Vitamin E Cancer Prevention Trial) status. Mol Med. 2011;17:134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vassilopoulou E, Konstantinou G, Dimitriou A, et al. The impact of food histamine intake on asthma activity: a Pilot Study. Nutrients. 2020;12:3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hirsch C, Hirsch C, Felcher K, et al. Retrospective view of North American potato (Solanum tuberosum L.) breeding in the 20th and 21st centuries. G3 (Bethesda). 2013;3:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;652:18–26. [DOI] [PubMed] [Google Scholar]
- [44].Chen Q, Wu B, Pan D, et al. Beta-carotene and its protective effect on gastric cancer. World J Clin Cases. 2021;9:6591–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kordiak J, Bielec F, Jabłoński S, et al. Role of beta-carotene in lung cancer primary chemoprevention: a systematic review with meta-analysis and meta-regression. Nutrients. 2022;14:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.