Abstract
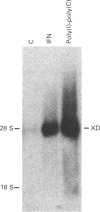
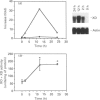
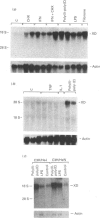
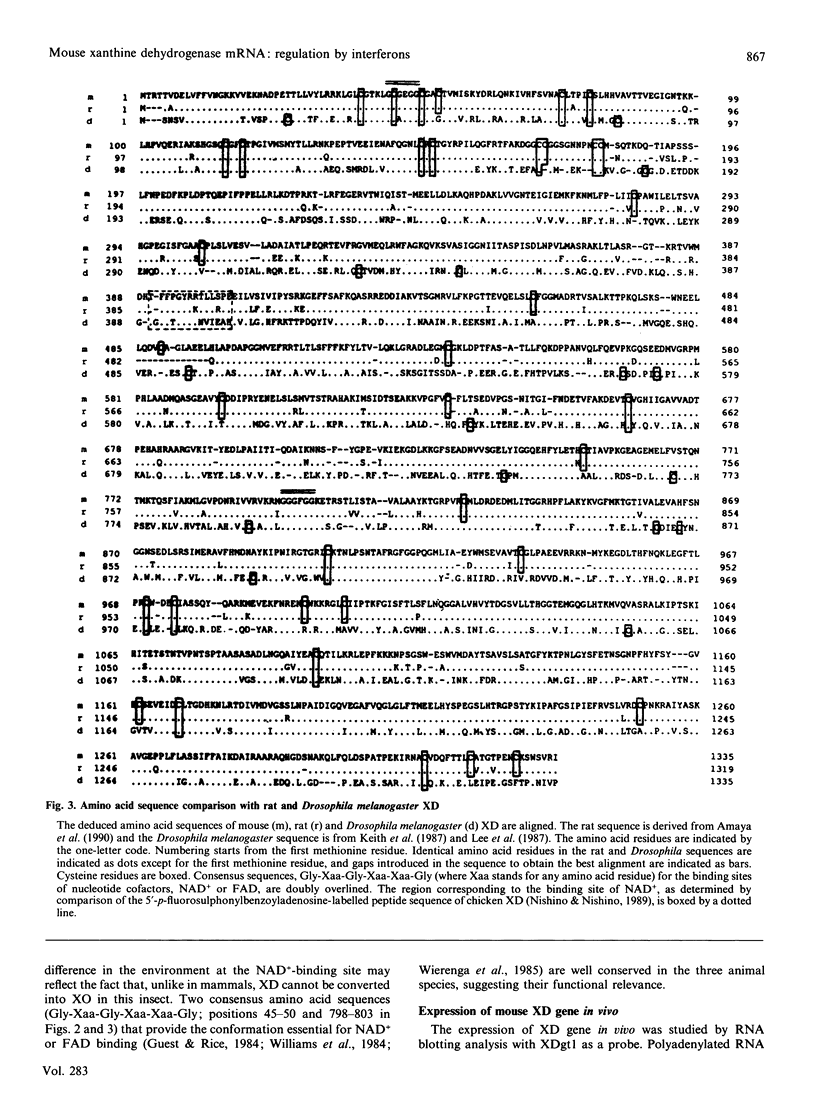
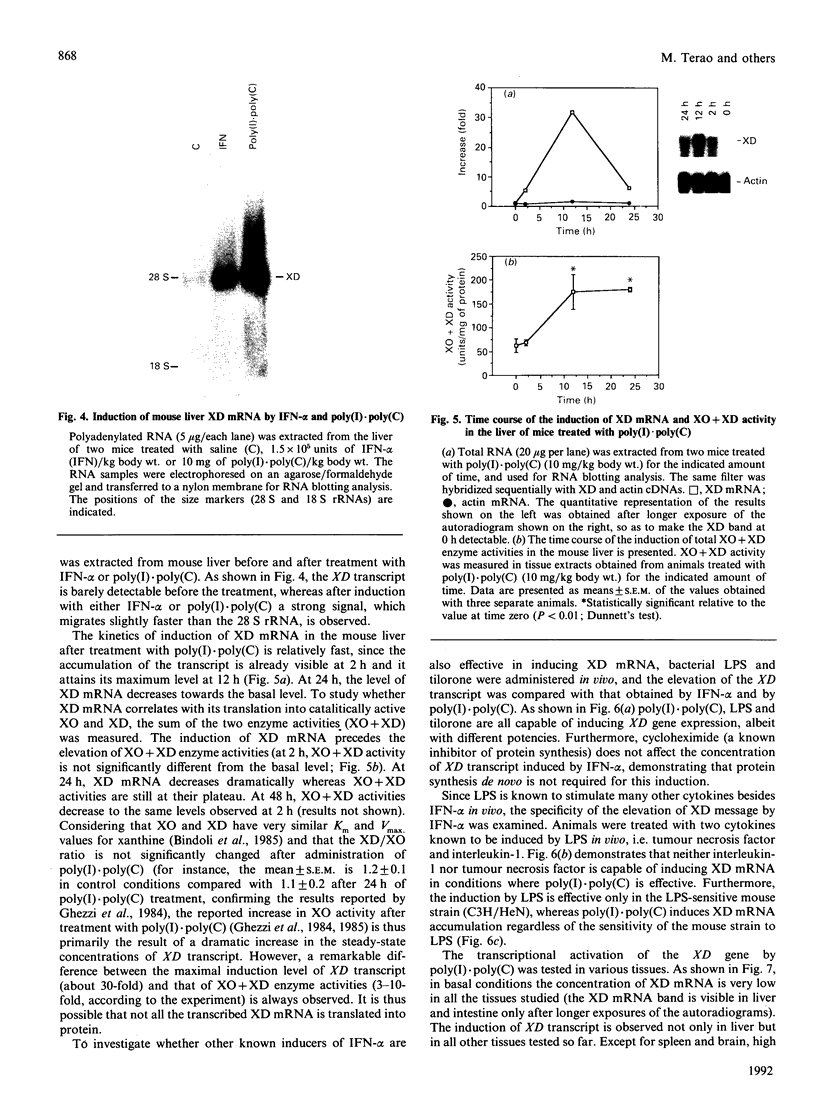
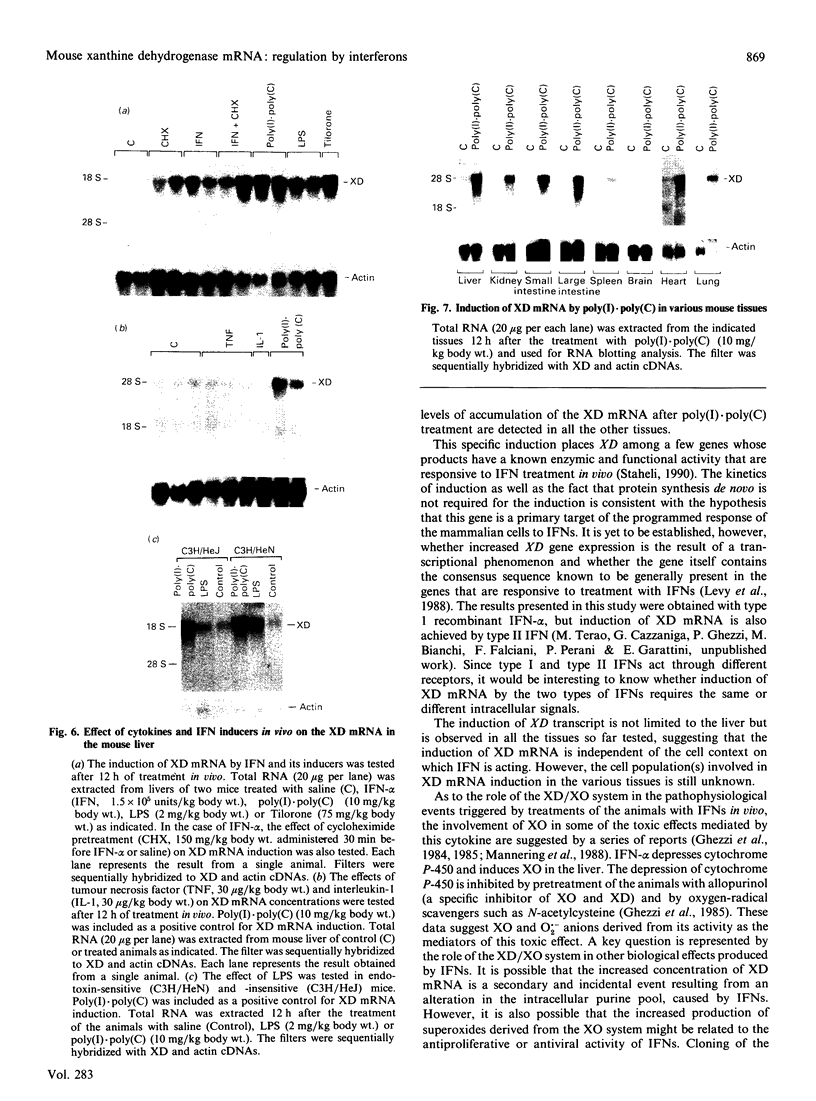
The cDNA coding for xanthine dehydrogenase (XD) is isolated from mouse liver mRNA by cross-hybridization with a DNA fragment of the Drosophila melanogaster homologue. Two lambda bacteriophage overlapping clones represent the copy of a 4538-nucleotide-residue-long transcript with an open reading frame of 4005 nucleotide residues, coding for a putative polypeptide of 1335 amino acid residues. Comparison of the deduced amino acid sequence of the mouse XD with those of the Drosophila and the rat homologues shows a high conservation of this protein (55% identity between mouse and Drosophila, and 94% identity between mouse and rat). RNA blotting analysis demonstrates that interferon-alpha (IFN-alpha) and its inducers, i.e. poly(I).poly(C), bacterial lipopolysaccharide (LPS) and tilorone (2,7-bis-[2-(diethylamino)ethoxy]fluoren-9-one), increase the expression of XD mRNA in liver. Poly(I).poly(C) also induces XD mRNA in several other tissues in vivo. Protein synthesis de novo is not required for the elevation of XD mRNA after IFN-alpha treatment, since cycloheximide does not block the induction. The elevation of XD mRNA concentration is relatively fast and precedes the induction of both XD and xanthine oxidase (XO) enzymic activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya Y., Yamazaki K., Sato M., Noda K., Nishino T., Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem. 1990 Aug 25;265(24):14170–14175. [PubMed] [Google Scholar]
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Bindoli A., Cavallini L., Rigobello M. P., Coassin M., Di Lisa F. Modification of the xanthine-converting enzyme of perfused rat heart during ischemia and oxidative stress. Free Radic Biol Med. 1988;4(3):163–167. doi: 10.1016/0891-5849(88)90024-x. [DOI] [PubMed] [Google Scholar]
- Bindoli A., Valente M., Cavallini L. Inhibitory action of quercetin on xanthine oxidase and xanthine dehydrogenase activity. Pharmacol Res Commun. 1985 Sep;17(9):831–839. doi: 10.1016/0031-6989(85)90041-4. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brass C. A., Narciso J., Gollan J. L. Enhanced activity of the free radical producing enzyme xanthine oxidase in hypoxic rat liver. Regulation and pathophysiologic significance. J Clin Invest. 1991 Feb;87(2):424–431. doi: 10.1172/JCI115013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpani G., Racchi M., Ghezzi P., Terao M., Garattini E. Purification and characterization of mouse liver xanthine oxidase. Arch Biochem Biophys. 1990 Jun;279(2):237–241. doi: 10.1016/0003-9861(90)90487-j. [DOI] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. Regulation of xanthine oxidase in rat liver: modifications of the enzyme activity of rat liver supernatant on storage at 20 degrees. Biochem J. 1968 Jun;108(2):349–351. doi: 10.1042/bj1080349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloria L., Abbott V., Gooderham N., Mannering G. J. Induction of xanthine oxidase and depression of cytochrome P-450 by interferon inducers: genetic difference in the responses of mice. Biochem Biophys Res Commun. 1985 Aug 30;131(1):109–114. doi: 10.1016/0006-291x(85)91777-2. [DOI] [PubMed] [Google Scholar]
- Elsayed N. M., Tierney D. F. Hyperoxia and xanthine dehydrogenase/oxidase activities in rat lung and heart. Arch Biochem Biophys. 1989 Sep;273(2):281–286. doi: 10.1016/0003-9861(89)90485-2. [DOI] [PubMed] [Google Scholar]
- Engerson T. D., McKelvey T. G., Rhyne D. B., Boggio E. B., Snyder S. J., Jones H. P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987 Jun;79(6):1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Ghezzi P., Bianchi M., Gianera L., Landolfo S., Salmona M. Role of reactive oxygen intermediates in the interferon-mediated depression of hepatic drug metabolism and protective effect of N-acetylcysteine in mice. Cancer Res. 1985 Aug;45(8):3444–3447. [PubMed] [Google Scholar]
- Ghezzi P., Bianchi M., Mantovani A., Spreafico F., Salmona M. Enhanced xanthine oxidase activity in mice treated with interferon and interferon inducers. Biochem Biophys Res Commun. 1984 Feb 29;119(1):144–149. doi: 10.1016/0006-291x(84)91630-9. [DOI] [PubMed] [Google Scholar]
- Gilbert D. A., Bergel F. The chemistry of xanthine oxidase. 9. An improved method of preparing the bovine milk enzyme. Biochem J. 1964 Feb;90(2):350–353. doi: 10.1042/bj0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Jung V., Jones C., Kumar C. S., Stefanos S., O'Connell S., Pestka S. Expression and reconstitution of a biologically active human interferon-gamma receptor in hamster cells. J Biol Chem. 1990 Feb 5;265(4):1827–1830. [PubMed] [Google Scholar]
- Jung V., Jones C., Rashidbaigi A., Geyer D. D., Morse H. G., Wright R. B., Pestka S. Chromosome mapping of biological pathways by fluorescence-activated cell sorting and cell fusion: human interferon gamma receptor as a model system. Somat Cell Mol Genet. 1988 Nov;14(6):583–592. doi: 10.1007/BF01535312. [DOI] [PubMed] [Google Scholar]
- Keith T. P., Riley M. A., Kreitman M., Lewontin R. C., Curtis D., Chambers G. Sequence of the structural gene for xanthine dehydrogenase (rosy locus) in Drosophila melanogaster. Genetics. 1987 May;116(1):67–73. doi: 10.1093/genetics/116.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Spector T., Hall W. W. Xanthine oxidase from human liver: purification and characterization. Arch Biochem Biophys. 1986 May 15;247(1):108–119. doi: 10.1016/0003-9861(86)90539-4. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Curtis D., McCarron M., Love C., Gray M., Bender W., Chovnick A. Mutations affecting expression of the rosy locus in Drosophila melanogaster. Genetics. 1987 May;116(1):55–66. doi: 10.1093/genetics/116.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Mannering G. J., Deloria L. B., Abbott V. Role of xanthine oxidase in the interferon-mediated depression of the hepatic cytochrome P-450 system in mice. Cancer Res. 1988 Apr 15;48(8):2107–2112. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Roy R. S. The pathophysiology of superoxide: roles in inflammation and ischemia. Can J Physiol Pharmacol. 1982 Nov;60(11):1346–1352. doi: 10.1139/y82-201. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Nishino T., Nishino T. The nicotinamide adenine dinucleotide-binding site of chicken liver xanthine dehydrogenase. Evidence for alteration of the redox potential of the flavin by NAD binding or modification of the NAD-binding site and isolation of a modified peptide. J Biol Chem. 1989 Apr 5;264(10):5468–5473. [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S., Rosenstreich D. L. Defect in macrophage effector function confers Salmonella typhimurium susceptibility on C3H/HeJ mice. Cell Immunol. 1982 Mar 1;67(2):325–333. doi: 10.1016/0008-8749(82)90224-6. [DOI] [PubMed] [Google Scholar]
- Oda T., Akaike T., Hamamoto T., Suzuki F., Hirano T., Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989 May 26;244(4907):974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983 Aug;245(2):G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Rehberg E., Kelder B., Hoal E. G., Pestka S. Specific molecular activities of recombinant and hybrid leukocyte interferons. J Biol Chem. 1982 Oct 10;257(19):11497–11502. [PubMed] [Google Scholar]
- Reiners J. J., Jr, Pence B. C., Barcus M. C., Cantu A. R. 12-O-tetradecanoylphorbol-13-acetate-dependent induction of xanthine dehydrogenase and conversion to xanthine oxidase in murine epidermis. Cancer Res. 1987 Apr 1;47(7):1775–1779. [PubMed] [Google Scholar]
- Renton K. W., Singh G., Stebbing N. Relationship between the antiviral effects of interferons and their abilities to depress cytochrome P-450. Biochem Pharmacol. 1984 Dec 1;33(23):3899–3902. doi: 10.1016/0006-2952(84)90058-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Suleiman S. A., Stevens J. B. Purification of xanthine dehydrogenase from rat liver: a rapid procedure with high enzyme yields. Arch Biochem Biophys. 1987 Oct;258(1):219–225. doi: 10.1016/0003-9861(87)90338-9. [DOI] [PubMed] [Google Scholar]
- Taylor M. D., Mellert T. K., Parmentier J. L., Eddy L. J. Pharmacological protection of reoxygenation damage to in vitro brain slice tissue. Brain Res. 1985 Nov 18;347(2):268–273. doi: 10.1016/0006-8993(85)90186-6. [DOI] [PubMed] [Google Scholar]
- Terada L. S., Rubinstein J. D., Lesnefsky E. J., Horwitz L. D., Leff J. A., Repine J. E. Existence and participation of xanthine oxidase in reperfusion injury of ischemic rabbit myocardium. Am J Physiol. 1991 Mar;260(3 Pt 2):H805–H810. doi: 10.1152/ajpheart.1991.260.3.H805. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot H., Littauer A. Reoxygenation injury in isolated hepatocytes: cell death precedes conversion of xanthine dehydrogenase to xanthine oxidase. Biochem Biophys Res Commun. 1988 Aug 30;155(1):278–282. doi: 10.1016/s0006-291x(88)81080-5. [DOI] [PubMed] [Google Scholar]