Abstract
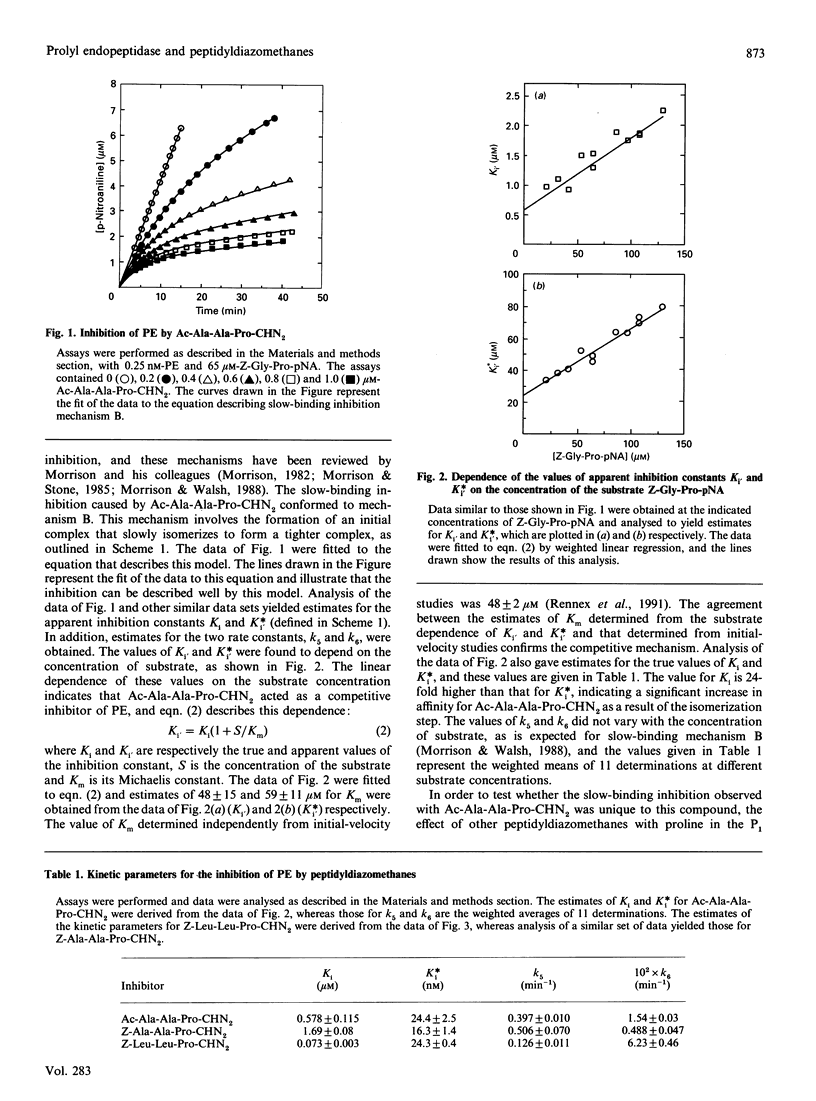
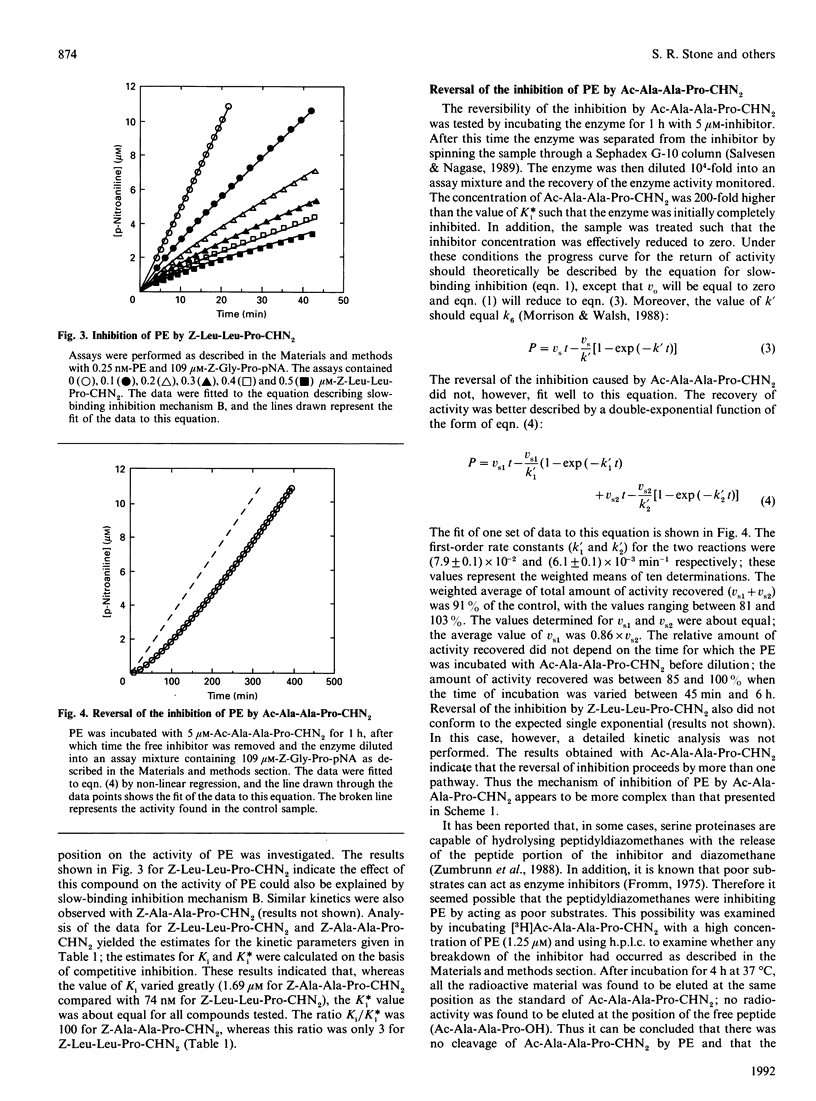
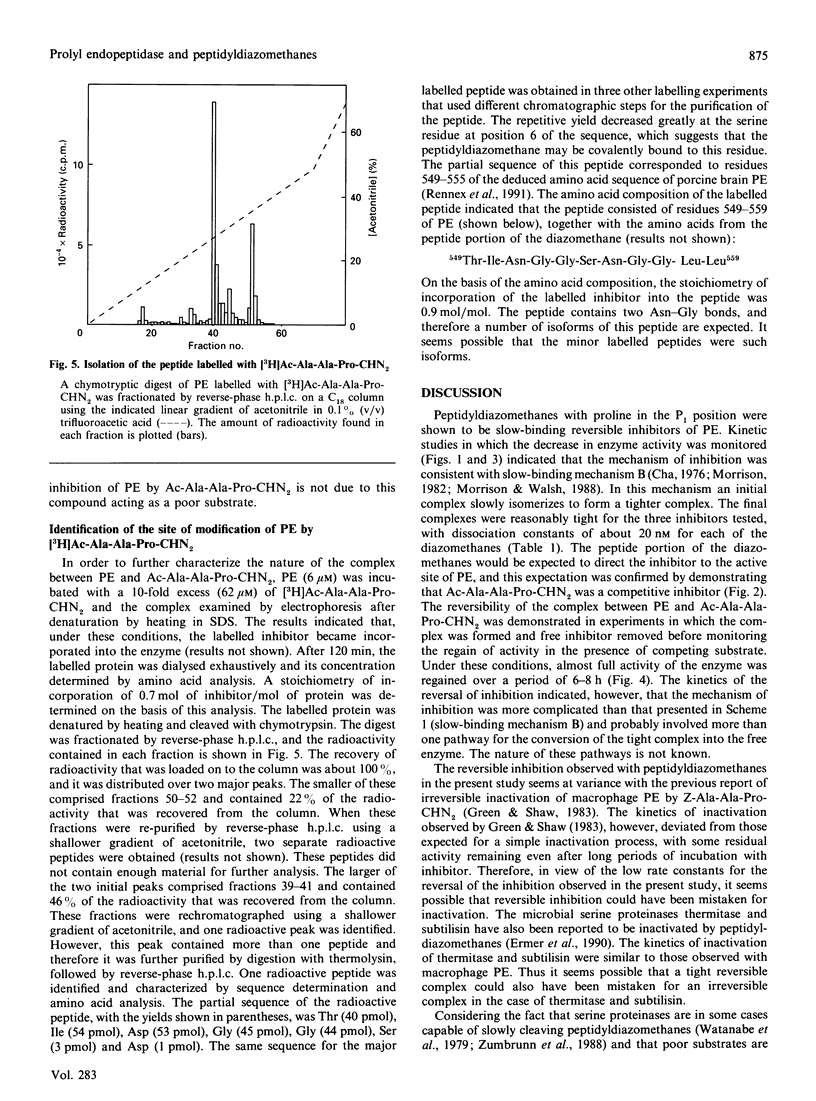
Peptidyldiazomethanes with proline in the P1 position were found to be competitive slow-binding inhibitors of prolyl endopeptidase. Progress-curve experiments monitoring the increase in the degree of inhibition with time indicated that the kinetic mechanism involved an initial complex that isomerized to form a tighter complex. Reversibility of the inhibited complex was demonstrated by monitoring the regain of enzyme activity after removal of free inhibitor and dilution into an assay containing competing substrate. The kinetics of the reversal of inhibition indicated a more complicated inhibitory mechanism involving more than one pathway for reversal of the tight complex. A slow-binding mechanism of inhibition has not been previously observed with peptidyldiazomethanes. Incorporation of [3H]Ac-Ala-Ala-Pro-diazomethane into prolyl endopeptidase was observed after denaturation of the inhibited complex. The peptide labelled with [3H]Ac-Ala-Ala-Pro-diazomethane was isolated and found to contain the active-site serine residue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brocklehurst K., Malthouse J. P. Mechanism of the reaction of papain with substrate-derived diazomethyl ketones. Implications for the difference in site specificity of halomethyl ketones for serine proteinases and cysteine proteinases and for stereoelectronic requirements in the papain catalytic mechanism. Biochem J. 1978 Nov 1;175(2):761–764. doi: 10.1042/bj1750761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors--III. A new approach for the determination of competition between tight-binding inhibitors and substrates--inhibition of adenosine deaminase by coformycin. Biochem Pharmacol. 1976 Dec 15;25(24):2695–2702. doi: 10.1016/0006-2952(76)90259-8. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dresdner K., Barker L. A., Orlowski M., Wilk S. Subcellular distribution of prolyl endopeptidase and cation-sensitive neutral endopeptidase in rabbit brain. J Neurochem. 1982 Apr;38(4):1151–1154. doi: 10.1111/j.1471-4159.1982.tb05362.x. [DOI] [PubMed] [Google Scholar]
- Ermer A., Baumann H., Steude G., Peters K., Fittkau S., Dolaschka P., Genov N. C. Peptide diazomethyl ketones are inhibitors of subtilisin-type serine proteases. J Enzyme Inhib. 1990;4(1):35–42. doi: 10.3109/14756369009030386. [DOI] [PubMed] [Google Scholar]
- Green G. D., Shaw E. A prolyl endopeptidase from murine macrophages, its assay and specific inactivation. Arch Biochem Biophys. 1983 Aug;225(1):331–337. doi: 10.1016/0003-9861(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Green G. D., Shaw E. Peptidyl diazomethyl ketones are specific inactivators of thiol proteinases. J Biol Chem. 1981 Feb 25;256(4):1923–1928. [PubMed] [Google Scholar]
- Jackson R. C., Handschumacher R. E. Escherichia coli L-asparaginase. Catalytic activity and subunit nature. Biochemistry. 1970 Sep 1;9(18):3585–3590. doi: 10.1021/bi00820a013. [DOI] [PubMed] [Google Scholar]
- Knecht R., Chang J. Y. Liquid chromatographic determination of amino acids after gas-phase hydrolysis and derivatization with (dimethylamino)azobenzenesulfonyl chloride. Anal Chem. 1986 Oct;58(12):2375–2379. doi: 10.1021/ac00125a006. [DOI] [PubMed] [Google Scholar]
- Lachman L. B., Handschumacher R. E. The active site of L-asparaginase: dimethylsulfoxide effect of 5-diazo-4-oxo-L-norvaline interactions. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1094–1100. doi: 10.1016/0006-291x(76)90235-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Walsh C. T. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- Peterson R. G., Richards F. F., Handschumacher R. E. Structure of peptide from active site region of Escherichia coli L-asparaginase. J Biol Chem. 1977 Mar 25;252(6):2072–2076. [PubMed] [Google Scholar]
- Rennex D., Hemmings B. A., Hofsteenge J., Stone S. R. cDNA cloning of porcine brain prolyl endopeptidase and identification of the active-site seryl residue. Biochemistry. 1991 Feb 26;30(8):2195–2203. doi: 10.1021/bi00222a025. [DOI] [PubMed] [Google Scholar]
- Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–347. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Rennex D., Wikstrom P., Shaw E., Hofsteenge J. Inactivation of prolyl endopeptidase by a peptidylchloromethane. Kinetics of inactivation and identification of sites of modification. Biochem J. 1991 Jun 15;276(Pt 3):837–840. doi: 10.1042/bj2760837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Green G. D., Shaw E. A comparison of the behavior of chymotrypsin and cathepsin B towards peptidyl diazomethyl ketones. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1354–1360. doi: 10.1016/0006-291x(79)92158-2. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Walter R., Tsuru D. Proline-specific endopeptidase from Flavobacterium. Purification and properties. J Biol Chem. 1980 May 25;255(10):4786–4792. [PubMed] [Google Scholar]
- Zumbrunn A., Stone S., Shaw E. Synthesis and properties of Cbz-Phe-Arg-CHN2 (benzyloxycarbonylphenylalanylarginyldiazomethane) as a proteinase inhibitor. Biochem J. 1988 Mar 1;250(2):621–623. doi: 10.1042/bj2500621. [DOI] [PMC free article] [PubMed] [Google Scholar]


