Abstract
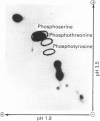
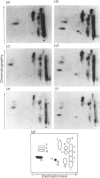
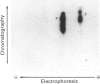
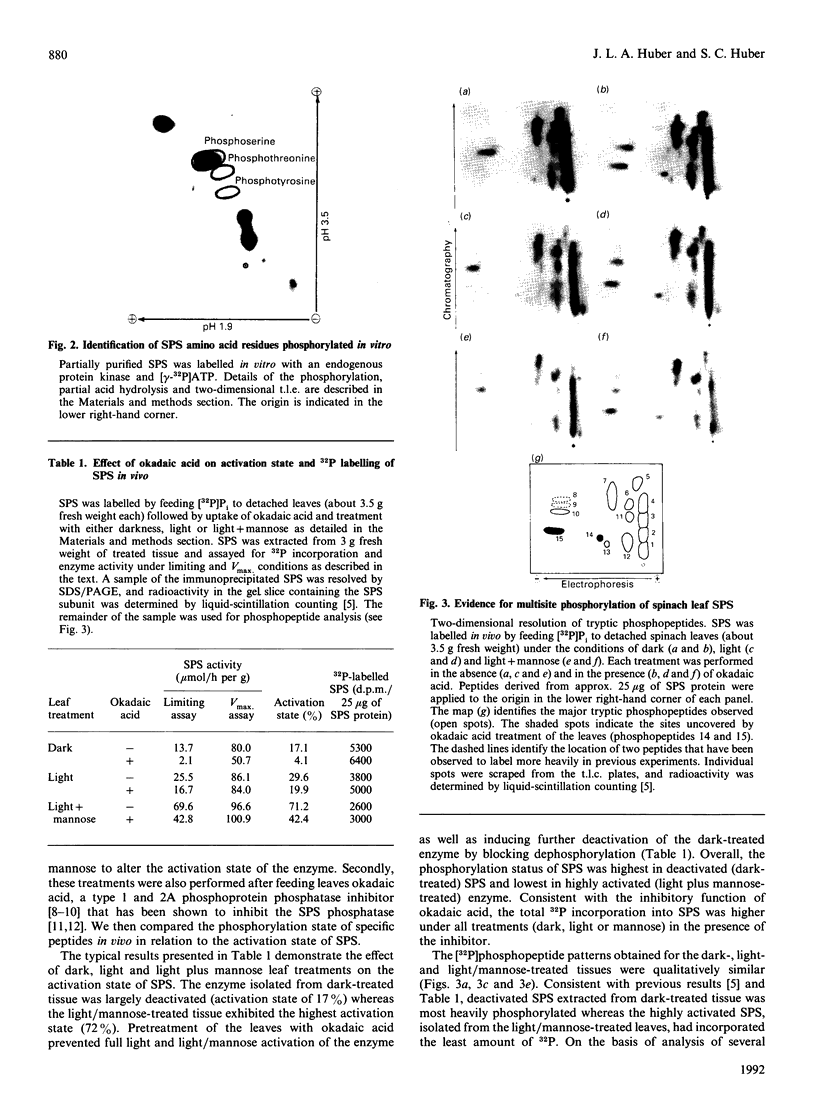
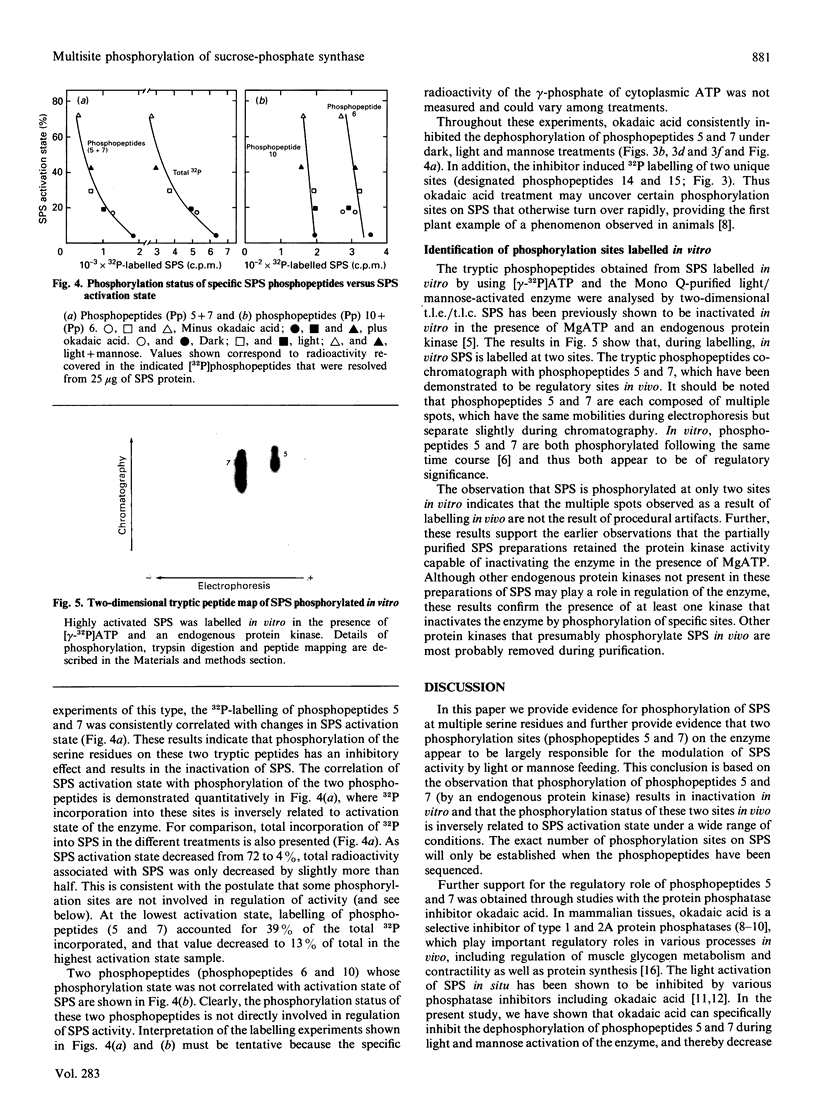
We recently reported [Huber, Huber & Nielsen (1989) Arch. Biochem. Biophys. 270, 681-690] that spinach (Spinacia oleracea L.) sucrose-phosphate synthase (SPS; EC 2.4.1.14) was phosphorylated in vivo when leaves were fed [32P]Pi. In vitro the enzyme was phosphorylated and inactivated by using [gamma-32P]ATP. We now report that SPS is phosphorylated both in vivo and in vitro on serine residues. The protein is phosphorylated at multiple sites both in vivo and in vitro as indicated by two-dimensional peptide maps of the immunopurified SPS protein. After being fed with radiolabel, leaves were illuminated or given mannose (which activates the enzyme), in the presence or absence of okadaic acid. Feeding okadaic acid to leaves decreased the SPS activation state in the dark and light and in leaves fed mannose. Across all the treatments, the activation state of SPS in situ was inversely related to the labelling of two phosphopeptides (designated phosphopeptides 5 and 7). These two phosphopeptides are phosphorylated when SPS is inactivated in vitro with [gamma-32P]ATP, and thus are designated as regulatory (inhibitory) sites [Huber & Huber (1991) Biochim. Biophys. Acta 1091, 393-400]. Okadaic acid increased the total 32P-labelling of SPS and in particular increased labelling of the two regulatory sites, which explains the decline in activation state. In the presence of okadaic acid, two cryptic phosphorylation sites became labelled in vivo that were not apparent in the absence of the inhibitor. Overall, the results suggest that light/dark regulation of SPS activity occurs as a result of regulatory serine phosphorylation. Multiple sites are phosphorylated in vivo, but two sites in particular appear to regulate activity and dephosphorylation of these sites in vivo is sensitive to okadaic acid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benore-Parsons M., Seidah N. G., Wennogle L. P. Substrate phosphorylation can inhibit proteolysis by trypsin-like enzymes. Arch Biochem Biophys. 1989 Aug 1;272(2):274–280. doi: 10.1016/0003-9861(89)90220-8. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P., Schelling D. L., Stark M. J. Remarkable similarities between yeast and mammalian protein phosphatases. FEBS Lett. 1989 Jul 3;250(2):601–606. doi: 10.1016/0014-5793(89)80804-x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of cyclic-AMP-dependent protein kinase in the regulation of glycogen metabolism in mammalian skeletal muscle. Curr Top Cell Regul. 1978;14:117–196. doi: 10.1016/b978-0-12-152814-0.50008-3. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Colburn J. C., Michnoff C. H., Hsu L. C., Slaughter C. A., Kamm K. E., Stull J. T. Sites phosphorylated in myosin light chain in contracting smooth muscle. J Biol Chem. 1988 Dec 15;263(35):19166–19173. [PubMed] [Google Scholar]
- Huber J. L., Huber S. C., Nielsen T. H. Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch Biochem Biophys. 1989 May 1;270(2):681–690. doi: 10.1016/0003-9861(89)90551-1. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Huber J. L. Activation of sucrose-phosphate synthase from darkened spinach leaves by an endogenous protein phosphatase. Arch Biochem Biophys. 1990 Nov 1;282(2):421–426. doi: 10.1016/0003-9861(90)90138-o. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Huber J. L. In vitro phosphorylation and inactivation of spinach leaf sucrose-phosphate synthase by an endogenous protein kinase. Biochim Biophys Acta. 1991 Feb 19;1091(3):393–400. doi: 10.1016/0167-4889(91)90205-c. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- King M. M., Fitzgerald T. J., Carlson G. M. Characterization of initial autophosphorylation events in rabbit skeletal muscle phosphorylase kinase. J Biol Chem. 1983 Aug 25;258(16):9925–9930. [PubMed] [Google Scholar]
- Siegl G., MacKintosh C., Stitt M. Sucrose-phosphate synthase is dephosphorylated by protein phosphatase 2A in spinach leaves. Evidence from the effects of okadaic acid and microcystin. FEBS Lett. 1990 Sep 17;270(1-2):198–202. doi: 10.1016/0014-5793(90)81267-r. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Huber S. C. Purification and preliminary characterization of sucrose-phosphate synthase using monoclonal antibodies. Plant Physiol. 1989 Feb;89(2):518–524. doi: 10.1104/pp.89.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]