Abstract
Epstein-Barr virus (EBV) is a herpesvirus commonly associated with several malignancies, particularly in immunocompromised hosts. As a strategy for stimulating immunity against EBV for the treatment of EBV-associated tumors, we have genetically engineered dendritic cells (DC) to express EBV antigens, such as latent membrane protein 2B (LMP2B), using recombinant adenovirus vectors. CD8+ T lymphocytes from HLA-A2.1+, EBV-seropositive healthy donors were cultured with autologous DC infected with recombinant adenovirus vector AdEGFP, encoding an enhanced green fluorescent protein (EGFP), or AdLMP2B at a multiplicity of infection of 250. After 48 h, >95% of the DC were positive for EGFP expression as assessed by fluorescence-activated cell sorting analysis, indicating efficient gene transfer. AdLMP2-transduced DC were used to stimulate CD8+ T cells. Responder CD8+ T cells were tested for gamma interferon (IFN-γ) release by enzyme-linked spot (ELISPOT) assay and cytotoxic activity. Prior to in vitro stimulation, the frequencies of T-cells directed against two HLA-A2-presented LMP2 peptides (LMP2 329-337 and LMP2 426-434) were very low as assessed by IFN-γ spot formation (T-cell frequency, <0.003%). IFN-γ ELISPOT assays performed at day 14 showed a significant (2-log) increase of the day 0 frequency of T cells reactive against the LMP2 329-337 peptide, from 0.003 to 0.3 (P < 0.001). Moreover, specific cytolytic activity was observed against the autologous EBV B-lymphoblastoid cell lines after 21 days of stimulation of T-cell responders with AdLMP2-transduced DC (P < 0.01). In summary, autologous mature DC genetically modified with an adenovirus encoding EBV antigens stimulate the generation of EBV-specific CD8+ effector T cells in vitro, supporting the potential application of EBV-based adenovirus vector vaccination for the immunotherapy of the EBV-associated malignancies.
Epstein-Barr virus (EBV) is a ubiquitous gammaherpesvirus that can establish both latent and lytic infections. EBV is commonly associated with several malignant diseases, including Burkitt’s lymphoma (BL), Hodgkin’s disease (HD), undifferentiated nasopharyngeal carcinoma (NPC), and various T-cell lymphomas (27). It is also frequently detected in immunocompromised hosts, especially in immunosuppressed allograft recipients who develop posttransplantation lymphoproliferative disorder (29, 39).
EBV elicits a strong cytotoxic T-lymphocyte (CTL) response directed against a broad range of viral antigens, including the latent EBV nuclear antigens (EBNA1, EBNA2, EBNA3, EBNA4, EBNA5, and EBNA6) and latent membrane proteins (LMP1, LMP2A, and LMP2B), that are involved in the maintenance and regulation of latency and in the induction of proliferative transformation (19). These viral antigens, processed as peptide fragments, are presented on the B-cell surface in association with HLA class I major histocompatibility complex (MHC) molecules in an immunogenic format recognized by CD8+ CTL. Several studies have demonstrated that most EBV-infected individuals generate an immunodominant CTL response specific for EBNA3B- and EBNA3C-derived epitopes (30), with typically a lower T-cell reactivity observed against EBNA2-, LMP1-, and LMP2-derived determinants. Hence, vaccines designed to elicit immunity directed against these latent proteins may prove particularly effective in the clinical setting. There is an increasing body of evidence that EBV-associated malignancies, such as NPC, BL, and HD, can escape the antiviral CTL responses by restricting the transcription of viral genes such as the EBNA1 gene in BL (28) and the LMP1 and LMP2 genes in NPC and HD (3). To date, there is no evidence that EBNA1 is properly processed to allow for immune recognition, indicating that LMPs are potentially the major target antigens for developing immunotherapies designed to induce therapeutically relevant, virus-specific CTL responses.
Dendritic cells (DC) are the most potent antigen-presenting cells (APC) and appear optimally capable of initiating and modulating the T-cell immune response (1). It has been further demonstrated that genetically modified DC can be successfully applied in tumor immunotherapy (33, 38). The clinical utility of DC is supported by evidence that antigen-pulsed or recombinant virus-infected DC promote cellular immunity both in experimental animal models and in humans (5, 6, 25, 41). In this study, we have evaluated the in vitro potential of DC transduced with a recombinant adenovirus encoding the EBV LMP2B antigen to elicit EBV-specific T cells that we believe will prove germane to the treatment of EBV malignancies. DC generated by this approach effectively induced antigen-specific T-cell responses from EBV-seropositive healthy individuals in vitro.
MATERIALS AND METHODS
Blood donors and cell lines.
All cell lines were derived from healthy donors, NS1 and NS2, of known HLA type (NS1, HLA-A2,28,B44,51; NS-2, HLA-A2,24, B36,62). As determined by Western blotting, donors NS1 and NS2 were positive for immunoglobulin G antibodies to the EBV protein EBNA (titer, 1:100 to 1:250) and negative for reactivity against EBV viral capsid antigen. B-lymphoblastoid cell lines (B-LCLs) were established by in vitro virus transformation of B cells, using the B95.8 (type 1) virus isolate (kindly provided by C. Wilson, University of Colorado Health Sciences Center). B-LCLs were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, penicillin (100 IU/ml), and streptomycin (100 μg/ml). Autologous phytohemagglutinin (PHA)-activated T blasts (PHA blasts) were prepared by stimulating peripheral blood mononuclear cells (PBMCs) with 5 μg of PHA (Sigma, St. Louis, Mo.) per ml. Cells were cultured in AIM-V medium supplemented with 5% heat-inactivated human AB serum (Sigma). A CTL clone recognizing the EBV epitope LMP2A 426-434 was kindly provided by A. B. Rickinson (University of Birmingham, Birmingham, United Kingdom). It was cultured as previously described (18). CRE8 cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% bovine calf serum and selected with neomycin (G418; 750 μg/ml; Life Technologies, Gaithersburg, Md.). All cell culture reagents were purchased from Life Technologies.
Generation and culture of DC.
DC were generated from Ficoll-Hypaque-purified PBMCs (lymphocyte separation medium [LSM]; Organon-Teknika, Durham, N.C.). Briefly, PBMCs from healthy donors were washed, resuspended in AIM-V medium, and allowed to differentially adhere to 75-cm2 tissue culture flasks (Costar, Corning, N.Y.) by culturing 108 cells in 10 ml of medium/flask for 90 min at 37°C. Nonadherent cells were then removed by gentle rinsing, and the remaining adherent cells were cultured in DC medium (AIM-V medium supplemented with 1,000 U of recombinant granulocyte-macrophage colony-stimulating factor [GM-CSF] and interleukin-4 [IL-4] [both from Schering-Plough, Kenilworth, N.J.] per ml) for 6 days. DC were used for maturation studies and as targets for gene transfer. Mature DC were obtained by adding a cytokine cocktail consisting of recombinant human tumor necrosis factor alpha (rhTNF-α; 10 ng/ml; Sigma), rhIL-1β (10 ng/ml; Genzyme, Cambridge, Mass.), rhIL-6 (1,000 IU/ml; Novartis, Basel, Switzerland), and prostaglandin E2 (PGE2; 1 μg/ml; Sigma) to DC medium cultures at day 6 as previously described (14).
Adenovirus vectors.
Adenovirus was constructed through Cre-lox recombination with reagents generously provided by Somatix (Alameda, Calif.). A SnaBI fragment containing the cytomegalovirus immediate-early promoter/enhancer, the enhanced (humanized) green fluorescent protein (EGFP) cDNA, and part of the simian virus 40 poly(A) derived from pEGFP N1 (Clontech, Palo Alto, Calif.) was inserted into the shuttle vector pAdlox. A BamHI-HindIII fragment containing LMP2 cDNA derived from pML-TP2 vector (kindly provided by G. Laux, Munich, Germany), containing the open reading frame of the LMP2B isoform of LMP2, was inserted in the shuttle vector pAdlox as reported above. E1-E3-substituted recombinant adenovirus was generated by cotransduction of SfiI-digested pAdlox-EGFP N1 or pAdlox-LMP2 and ψ5 helper virus DNA into the adenovirus packaging cell line CRE8. Recombinant adenoviruses were propagated on CRE8 cells, purified by CsCl density gradient centrifugation, and subsequently dialyzed (11). The titer of viral particles was determined by optical densitometry, and recombinant adenoviruses were stored in 3% sucrose buffer at −80°C.
Transduction of DC.
For gene transfer experiments, day 5 purified DC were harvested with cold Hanks’ balanced salt solution (HBSS; Life Technologies) from the tissue culture flasks, centrifuged at 600 × g for 8 min, and resuspended in 100 μl of HBSS. One million DC were infected with the recombinant adenoviruses (Adψ5 or AdLMP2) at a multiplicity of infection (MOI) of 250 in 200 μl of HBSS for 45 min at room temperature, cultured in DC medium with or without the maturation cocktail of cytokines for 48 h, washed to remove the excess of the virus and cytokines, and used as APC to stimulate autologous T-cell responses. Wild-type E1-E3-deleted, replication-deficient adenovirus 5 (Adψ5) and EGFP-encoding adenovirus (AdEGFP) were used as controls. EGFP expression was analyzed by flow cytometry and fluorescence and confocal microscopy.
Analysis of LMP2 gene expression.
HLA-A2.1-positive DC were infected with AdLMP2 as reported above. The expression of LMP2 by AdLMP2-infected DC was analyzed by using an HLA-A2-restricted CTL clone (kindly provided by A. B. Rickinson) specific for the LMP2 426-434 epitope as an indicator reagent in a 5-h 51Cr release assay. Autologous PHA blasts loaded with LMP2 426-434 peptide at a concentration of 1 μg/ml were used as a positive control. To confirm the specificity of the observed cytotoxicity against AdLMP2-transduced DC, cells infected with Adψ5 were used as a negative control.
Flow cytometry.
A FACScan II flow cytometer (Becton Dickinson, Mountain View, Calif.) and LYSIS II software were used to assess the DC transduction efficiency with AdEGFP infection and the phenotype of DC, using a panel of well-defined commercial markers. To determine the effects of transduction on the expression of DC surface markers, Adψ5- or AdLMP2-transduced DC were cultured for 48 h and matured with a cytokine cocktail (see above) and then stained with monoclonal antibodies (MAbs) consisting of fluorescein isothiocyanate (FITC)-conjugated anti-MHC I (Serotec, Oxford, United Kingdom), phycoerythrin (PE)-conjugated anti-MHC II (Becton Dickinson), FITC-conjugated anti-B7.1 (CD80; Ancell, Bayport, Minn.), PE-conjugated anti-B7.2 (CD86; PharMingen, San Diego, Calif.), PE-conjugated anti-CD14 (Dako, Carpinteria, Calif.), FITC-conjugated anti-CD40 (Ancell), PE-conjugated anti-CD54 (Becton Dickinson), FITC-conjugated anti-CD83 (Coulter, Immunotech, Miami, Fla.), and unconjugated anti-mannose receptor (kindly provided by Russell Salter, University of Pittsburgh) and analyzed by flow cytometry. For indirect staining, FITC-conjugated goat anti-mouse immunoglobulin G F(ab)2 antibody (ICN/Cappel, Aurora, Ohio) was used as a secondary reagent. Flow cytometry was also performed on responder T-cell populations. Cells were stained with PE-conjugated anti-CD4, FITC-conjugated anti-CD8, PE-conjugated anti-CD56, and FITC-conjugated anti-CD3 (Becton Dickinson), as well as an FITC isotype control and a PE isotype control (PharMingen). Cells were resuspended in 0.1 ml of buffer (phosphate-buffered saline [PBS] supplemented with 0.1% bovine serum albumin and 0.1% sodium azide) and incubated with 20 μl of the appropriate antibody for 30 min at 4°C. After incubation, the cells were washed twice and fixed in 0.4 ml of 2% paraformaldehyde. For each marker, 10,000 cells were evaluated.
Confocal analysis.
Confocal analysis was used to examine the distribution of EGFP within DC following infection with AdEGFP. To this end, immature and mature DC seeded onto coverslips were labeled with PE-conjugated anti-CD83 antibody (Coulter) for 30 min at 4°C and gently washed three times with FACS (fluorescence-activated cell sorting) buffer. DC were lightly fixed in 2% paraformaldehyde for 10 min at room temperature and washed twice with PBS. Subsequently, DC were scanned with a Leica TCS NT confocal microscope with a 60× oil immersion objective; the fluorophores were excited with a 488-nm argon line to detect EGFP and a 567-nm krypton line to detect PE. The microscope was set up such that no bleedthrough between the two channels occurred. Cells were scanned midplane at a 1,024- by 1,024-pixel resolution, and four serial identical sections were averaged.
Induction of CD8+ T-cell responsiveness against EBV peptides.
CD8+ T cells were positively selected from PBMCs of healthy donors NS1 and NS2 with immunomagnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). In vitro sensitization (IVS) experiments were performed as previously described (36), with minor modifications. CD8+ cells (106) were cocultured with peptide-loaded autologous mature DC (3.3 × 104) in 24-well tissue culture plates (Costar) in 2 ml of RPMI 1640 medium supplemented with 1% l-glutamine, penicillin (100 IU/ml), streptomycin (100 μg/ml), 10 mM HEPES buffer, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 10% heat-inactivated human AB serum (Sigma). T cells were cultured for 7 days in the presence of rhIL-12 (kind gift of Stan Wolf, Genetic Institute, Cambridge, Mass.) and rhIL-6 (Sandoz) at final concentrations of 1 ng/ml and 1,000 IU/ml, respectively. On day 7, responder cells were restimulated with peptide-loaded DC along with IL-2 (10 IU/ml; kind gift of Chiron Corporation) and IL-7 (5 ng/ml; Sigma). Responder T cells were restimulated weekly for 14 days with autologous DC at a responder-to-stimulator ratio of 30:1.
IVS of T cells with transduced DC.
Peripheral blood lymphocytes (PBL) were isolated from heparinized blood of healthy donors NS1 and NS2 by centrifugation on Ficoll-Hypaque gradients. PBL (2 × 107) were cocultured with autologous immature or mature Adψ5- or Ad-LMP2-transduced DC (106) on six-well tissue culture plates (Costar) in 5 ml of RPMI 1640 medium supplemented with 1% l-glutamine, penicillin (100 IU/ml), streptomycin (100 μg/ml), 10 mM HEPES buffer, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 10% heat-inactivated human AB serum (Sigma). rhIL-2 was added from day 3 at a final concentration of 25 IU/ml. Responding T cells were restimulated weekly for 14 days with autologous infected DC at a responder T cell-to-stimulator DC ratio of 20:1.
Peptide selection and synthesis.
The synthetic peptides used in this study were derived from different viral proteins and known to be recognized by CTL in association with HLA-A2.1. Peptides presented in association with HLA-2.1 were EBNA2 67-76 (30), EBNA3 596-604 (5), EBNA6 284-293 (16), BMLF-1 280-288 (34), EBV LMP2 329-337 (17), and LMP2 426-434 (18). The human immunodeficiency virus (HIV) Nef 180-189 (10) and influenza virus matrix protein (FluMP) 58-66 (2) peptides were used as negative and positive controls, respectively. Peptides were synthesized by standard 9-fluorenylmethoxycarbonyl chemistry and purified by reverse-phase high-performance liquid chromatography (HPLC) in the Peptide Synthesis Facility of the University of Pittsburgh Cancer Institute (Shared Resource). Purity exceeded 90% based on HPLC profiles with mass spectrometry/mass spectrometry fragmentation, providing unambiguous sequencing data (University of Pittsburgh Biotechnology Center, Mass Spectrometry Facility). Lyophilized peptides were dissolved in PBS–10% dimethyl sulfoxide (stock concentration of 2 mg/ml) and stored at −20°C.
IFN-γ enzyme-linked spot (ELISPOT) assay.
MultiScreen-HA plates (Millipore, Bedford, Mass.) were coated with 10 μg of anti-human gamma interferon (IFN-γ) MAb (1-D1K; MABtech, Stockholm, Sweden) per ml in PBS (Life Technologies) overnight at 4°C. After blocking of the plates with RPMI 1640–10% human serum for 1 h at 37°C, nonadherent cells were seeded at 105/well. Autologous DC (2 × 104) and peptides at a final concentration of 10 μg/ml were added. Each determination was performed in triplicate wells. Control wells contained unstimulated nonadherent cells in the presence of autologous DC. Culture medium was AIM-V. Plates were incubated at 37°C in a humidified 5% CO2 incubator for 20 h. Cells were removed with several washes in PBS–0.05% Tween 20 (Sigma), with plates subsequently incubated for 2 h at 37°C with 2 μg of biotinylated anti-human IFN-γ antibody (7-B6-1; MABtech) per ml in PBS–0.5% bovine serum albumin prior to treatment with avidin-biotin-peroxidase complex (diluted 1:100; Vectastain Elite kit; Vector Laboratories, Burlingame, Calif.) for 1 h at room temperature. After peroxidase staining with 3-aminoethylcarbazole (Sigma), spots appeared within 4 to 5 min. The enzymatic reaction was stopped by rinsing the plates under running tap water. The number of spots was automatically determined with computer-assisted video image analysis using autoimager software (KS Elispot version 4.1; Zeiss-Kontron, Jena, Germany) (12).
Chromium release assay.
A standard 5-h chromium release assay was performed to assess lytic activity of IVS responder T lymphocytes on day 21. Target cells were labeled in FCS for 1 h at 37°C with 100 μCi of Na251CrO4 (New England Nuclear DuPont, Bedford, Mass.). After being washed three times, labeled cells were resuspended in Dulbecco modified Eagle medium containing 10% FCS at 104 cells/ml, and 100 μl of the target suspension was added to individual wells of 96-well V-bottom microtiter plates (Costar). One hundred microliters of responder lymphocyte suspension was then added to relevant wells containing 51Cr-labeled target cells at various indicated effector/target (E/T) ratios. Anti-MHC class I (W6/32; American Type Culture Collection, Manassas, Va.) blocking antibody at a concentration of 40 μg/well was added to certain control wells to inhibit MHC class I recognition of target cells by responder CTL. Fifty microliters of medium alone or with an excess of unlabeled cold target inhibitor K562 cells at a 40:1 ratio with 51Cr-labeled target cells (to diminish NK-LAK reactivity) was added to each microwell containing IVS effector cells. For antigenic pulsing, HLA-A2.1+ day 3 PHA blasts were incubated for 90 min with synthetic LMP2 426-434 peptide at a concentration of 1 μg/ml. The LMP2-specific CTL clone was tested for cytolytic reactivity against AdLMP2- and Adψ5-infected DC. After the addition of 103 labeled target cells in 50 μl of complete medium to each well, the plates were incubated for 5 h at 37°C in a 5% CO2 atmosphere. After the incubation period, 100 μl of the supernatant was collected from each well for counting in a gamma counter (LKB, Helsinki, Finland). Target cells incubated in medium alone or in medium containing 5% Triton X-100 (Sigma) were used to determine spontaneous and maximum 51Cr release, respectively. Spontaneous release ranged between 10 and 20% of the maximum label incorporated into target cells. The percentage of specific 51Cr release was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Statistical analysis.
One-way analysis of variance was used to calculate the significance of differences in spot numbers observed in negative control versus antigen-stimulated responder T cells. A P value of ≤0.05 was defined as significant. Results are presented as means ± standard deviations (SD).
RESULTS
DC phenotype.
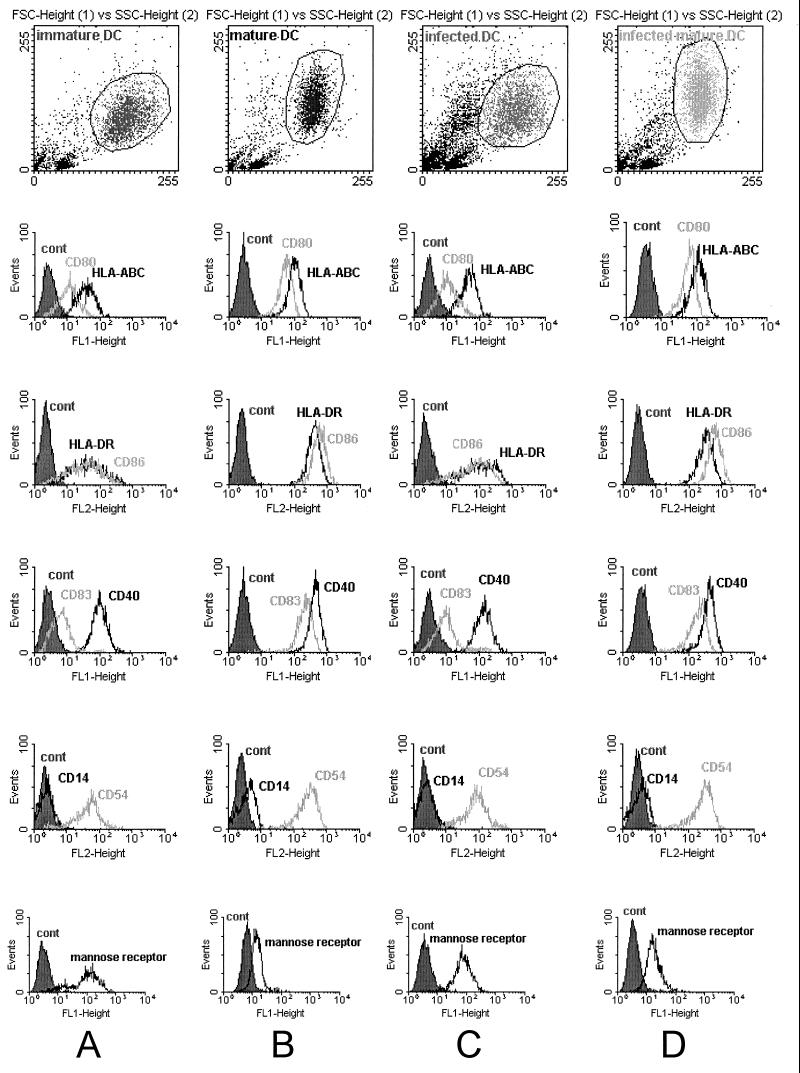
Monocyte-derived DC were harvested after 5 to 7 days of culture in the presence of IL-4 and GM-CSF and stained with specific MAbs to assess the expression of typical DC surface markers. DC generated with this procedure were defined as immature, since they expressed little or no CD83, a marker usually observed on mature DC. Flow cytometric analysis showed that these so-called immature DC expressed low or intermediate levels of HLA class I, HLA class II, CD40, CD54, CD80, CD86, and the mannose receptor and failed to express CD14 (Fig. 1A). Incubation with a maturation cocktail of cytokines (TNF-α, rhIL-1β, rhIL-6, and PGE2) for 48 h induced morphological and phenotypical changes in DC. These matured cells appeared veiled, ruffled, and clustered in large aggregates. Figure 1B depicts the results of flow cytometric analysis on these mature DC, indicating an increase in the intensity of expression of all markers except CD14, which remained negative. Consistent with maturation of these DC, mannose receptor expression was markedly reduced and CD83 expression was strongly upregulated.
FIG. 1.
FACS analysis of surface markers of DC. DC cultured in AIM-V medium containing GM-CSF and IL-4 were left untreated for 6 days (immature DC; A), or matured with rhTNF-α, rhIL-1β, rhIL-6, and PGE2 (mature DC; B). After 48 h, DC surface marker expression for HLA class I, HLA class II, CD80, CD86, CD83, CD40, CD14, CD54, and mannose receptor was analyzed by flow cytometry. To investigate the role of adenovirus infection in the expression of DC markers, DC were harvested and transduced (transduced DC; C) or transduced and matured at the same time (transduced and mature DC; D) at day 6, with all cells analyzed on day 8. Cells in the dot plot were gated for homogeneity of size and granularity consistent with DC.
Evaluation of gene transfer into DC with adenovirus.
In a control series of experiments, we evaluated the impact of recombinant adenovirus on the expression of cell surface markers after the infection of DC. Immature DC were transduced with AdEGFP and Adψ5 at an MOI of 250. The efficiency of adenovirus-mediated gene transfer into DC was evaluated by flow cytometry analysis of EGFP expression. In noninfected DC and in DC transduced with Adψ5, the mean fluorescence intensity, a measure of the average level of EGFP expression per cell, was low. Mean fluorescence intensity determined in DC infected with AdEGFP revealed that more than 95% of the cells expressed EGFP (Fig. 2B). The infected DC showed a pattern and intensity of marker expression similar to that observed in immature noninfected DC, with a slight upregulation of class I and class II molecules (Fig. 1C), clearly indicating that the adenovirus infection was insufficient to induce DC maturation. However, the mature DC phenotype could be readily obtained if adenovirus-infected DC were subsequently treated with the cytokine cocktail (TNF-α, rhIL-1β, rhIL-6, and PGE2) for 48 h (Fig. 1D). To determine whether cells expressing EGFP were mature DC, we analyzed CD83 versus EGFP fluorescence (Fig. 3). DC positive for CD83 were 90% positive for EGFP (data not shown). Overall, these results suggest that adenovirus infection of DC neither induces nor inhibits the subsequent capacity of these cells to be matured.
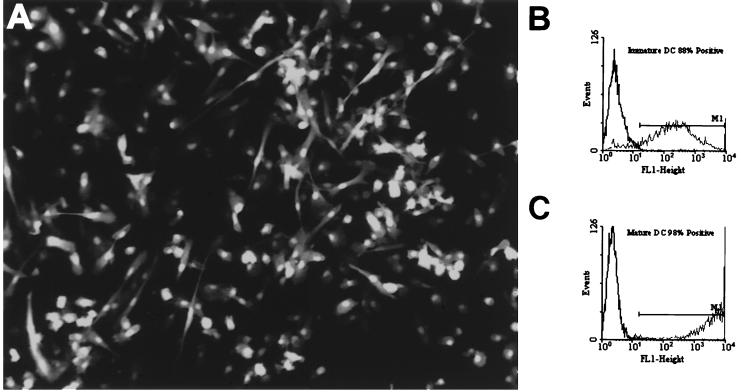
FIG. 2.
Photomicrograph of AdEGFP-transduced DC at a magnification of ×40. DC were infected with AdEGFP at an MOI of 250 and cultured for 48 h in DC medium conditioned with the maturation cytokine cocktail (A). FACS analysis on mature AdEGFP-transduced DC showed that more than 96% expressed the transgene (C), while immature DC were 88% EGFP positive (B).
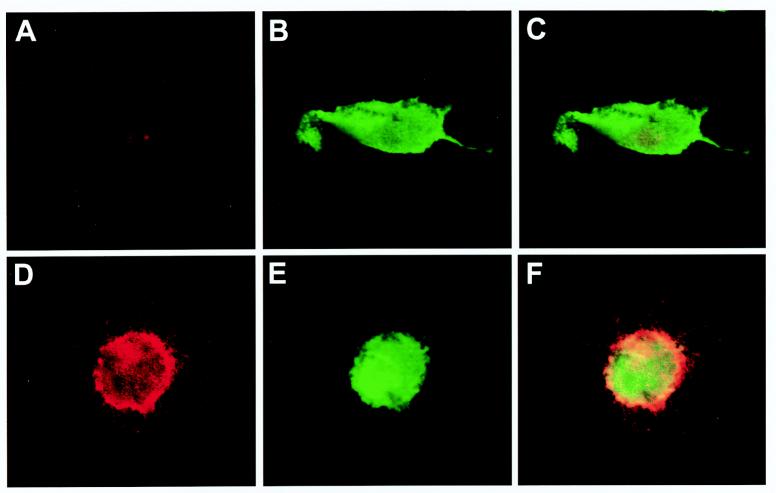
FIG. 3.
Confocal analysis of adenovirus-transduced DC. Immature (A to C) or mature (D to F) DC were transduced with AdEGFP at an MOI of 250 and stained for CD83, a marker of DC maturation. EGFP expression is shown in green (B and E), and the fluorescence for CD83 is shown in red (A and D). Colocalization appeared as yellow pixels after merging of the images recorded at the same level in each channel (C and F). As supported by the flow cytometry data, mature DC exhibited a high expression level of the CD83 surface marker that partially colocalized with EGFP within the cytoplasm of mature DC (F).
LMP2 expression in adenovirus-transduced DC.
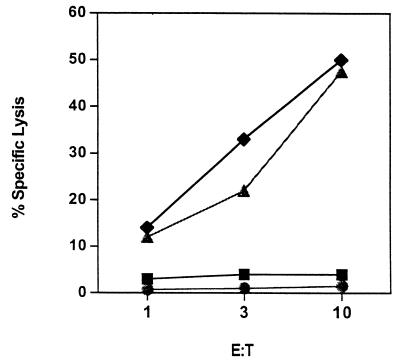
To document effective processing and presentation of virus-derived sequences, we tested HLA-A2.1+ immature DC infected with AdLMP2 for recognition by an HLA-A2-restricted CTL clone specific for the LPM2 426-434 epitope in standard cytotoxicity assays. At 48 h after infection with Ad-LPM2, DC were used as targets in a 51Cr release cytotoxicity assay, with Adψ5-transduced DC serving as a negative control target. PHA blasts loaded with and without the LMP2 426-434 peptide were also used as positive and negative controls, respectively. The CTL clone efficiently lysed transduced DC that expressed LMP2 (48%, E/T ratio = 10:1), while Adψ5-transduced DC were not recognized (Fig. 4). In addition, the CTL clone showed high specific lytic reactivity against PHA blasts loaded with the relevant LMP2 peptide, confirming the antigenic specificity of the clone used (47.5%, E/T ratio = 10:1) (Fig. 4). These data suggest that memory CTL readily recognize AdLMP2-transduced DC which may naturally occur in freshly isolated, PBMC-derived T-cell populations obtained from EBV-seropositive donors.
FIG. 4.
LMP2-specific CTL clone recognizes AdLMP2-transduced DC. An HLA-A2.1-restricted CTL clone specific for the LMP2A 426-434 epitope was used to assess the expression of this LMP2-derived epitope on the cell surface of AdLMP2-transduced DC (⧫) in a standard 5-h 51Cr release assay. Adψ5 DC were used as negative control (■). PHA blasts (autologous to the donor DC) pulsed with (▴) and without (●) the LMP2 424-436 peptide served as positive and negative control target cells, respectively. Results are expressed as percentage of specific lysis at the indicated E/T ratio.
CD8+ T cells reactive with HLA-A2.1-presented EBV peptides.
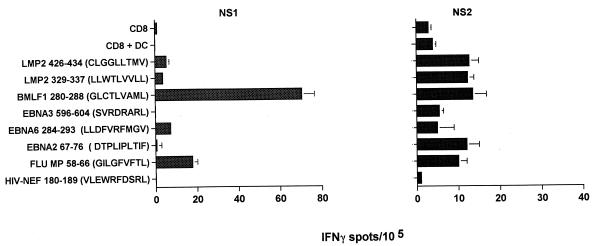
The virus-specific CD8+ T-cell precursor frequencies of two healthy HLA-A2+ EBV-seropositive donors were analyzed in IFN-γ ELISPOT assays for reactivity against six different EBV-derived peptide epitopes known to be presented by HLA-A2 (EBNA2 67-76, EBNA3 596-604, EBNA6 284-293, BMLF1 280-288, LMP2 329-337, and LMP2 426-434). Freshly isolated T lymphocytes spontaneously secreted IFN-γ at a frequency that ranged between 0.1 and 70.5 per 105 CD8+ T cells. Donor NS1 showed low reactivity to both HLA-A2-restricted LMP2 epitopes (3.6 spot-forming lymphocytes for LMP2 329-337 and 5.3 spot-forming lymphocytes for LMP2 426-434 per 105 CD8+ T cells; background = 1.5 spots/105). In contrast, higher frequencies of LMP2-specific T cells were detected in donor NS2 (10.3 and 13.7 per 105 CD8+ T cells against the LMP2 329-337 and LMP2 426-434 peptides, respectively; background = 4.5 spots/105) (Fig. 5). Of note, a strong T-cell reactivity against the BMLF1 epitope was observed in donor NS1 (70.5 spots/105 CD8+ T cells). Donor NS1 reactivity to the EBNA6 epitope was detected at 7.3 spots (above background) per 105 CD8+ T cells, and the frequencies for donor NS2 T-cells against the EBNA2 and BMLF1 peptides were 12 and 13.9 spots per 105 CD8+ T cells, respectively. Low but significant T-cell reactivity was also observed in donor NS2 against other EBV-derived peptides (i.e., EBNA3 and EBNA6). Donor reactivity against the HIV Nef peptide was not observed in these HIV-negative donors (0.1 to 1 responsive lymphocyte per 105 CD8+ T cells). The FluMP peptide was recognized by both donors’ CD8+ T cells (17.7 and 11.2 responsive lymphocytes per 105 CD8+ T cells in donors NS1 and NS2, respectively).
FIG. 5.
Basal frequencies of CD8+ T cells reactive against known HLA-A2-restricted EBV peptide epitopes in EBV-seropositive donors. The precursor frequencies of freshly isolated CD8+ T cells reactive against a series of HLA-A2-presented EBV peptides were tested in a 20-h IFN-γ ELISPOT assay. HLA-A2.1-presented FluMP and HIV Nef peptides were used as positive and negative controls, respectively. Autologous immature DC served as peptide-presenting cells. Each bar represents the mean spot number of triplicates ± SD per 105 CD8+ T cells. The numbers of antigen-reactive cells per 105 T lymphocytes are calculated by subtraction of mean spot numbers induced by DC (non-peptide loaded) seeded with CD8+ T cells from mean spot numbers of CD8+ T cells induced by peptide-loaded DC.
CD8+ T-cell responsiveness in an HLA-A2+ healthy donor after EBV peptide stimulation.
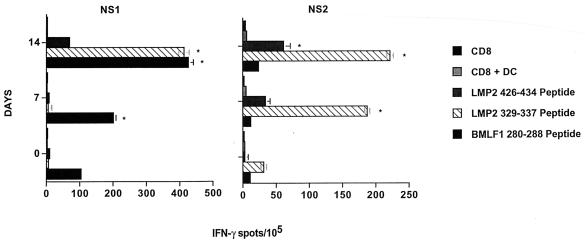
To induce enhanced CD8+ T-cell responses directed against low-frequency specificities in EBV-seropositive donors, autologous mature DC were loaded with the HLA-A2-restricted peptide LMP2 329-337 or LMP2 426-434 for use as in vitro stimulators. As a positive control, lymphocytes were stimulated with DC pulsed with the BMLF1 280-288 peptide. CD8+ T-cell responders were stimulated with peptide-loaded autologous DC at a responder-to-stimulator ratio of 15:1. IL-12 and IL-6 were added for the first 7 days of induction. The responder cells were restimulated on day 7 with peptide-loaded autologous DC and supplemented with IL-7 and IL-2. A portion of these bulk responder populations was cryopreserved for subsequent analysis by ELISPOT assay.
The precursor frequency of IFN-γ-secreting responder cells was then evaluated at days 0, 7, and 14 of culture. After 7 days of stimulation, a significant increase in the frequency of CD8+ T cells reactive against the BMLF1 280-288 peptide (201.6 spot-forming lymphocytes per 105 CD8+ T cells) was noted, while no significant reactivity was observed against the two LMP peptides, 8.3 (LMP2 329-337) and 5.5 (LMP2 424-436) spots per 105 CD8+ T cells (Fig. 6). Interestingly, on day 14, we observed a further amplification in the frequency of CD8+ T cells recognizing the BMLF1 280-288 peptide (426.5 spot-forming lymphocytes per 105 CD8+ T cells). T cells reactive against both the LMP2 426-434 and LMP2 329-337 peptides (69.9 and 413.1 spot-forming lymphocytes per 105 CD8+ T cells, respectively) also became evident in donor NS1 at this time point (Fig. 6). As was the case for donor NS1, we observed a pattern of peptide-specific T-cell induction in donor NS2, with optimal immunoreactivity noted in day 14 cultures (although the frequencies of CD8+ T cells reactive against both the LMP2 peptides and the BMLF1 peptide were less pronounced than in donor NS1).
FIG. 6.
In vitro expansion of EBV CD8+ T cells by stimulation with peptide-pulsed DC. Autologous, mature DC derived from EBV-seropositive donors were pulsed with the LMP2 424-436, LMP2 329-337, or BMLF1 280-288 peptide and cocultured with autologous CD8+ cells to expand EBV-peptide specific T-cell responses. Seven days after each of two stimulations with peptide-loaded mature DC (on days 0 and 7), responder CD8+ T cells (i.e., on days 7 and 14) were analyzed in 20-h IFN-γ ELISPOT assays for reactivity against the peptides used for stimulations. Autologous immature DC again served as peptide-presenting cells. Spots were evaluated as described in the legend to Fig. 5. Each bar represents the mean spot number of triplicate determinations ± SD per 105 CD8+ T cells. P values of <0.05 were considered significant and are indicated with asterisks. Results are representative of three experiments performed.
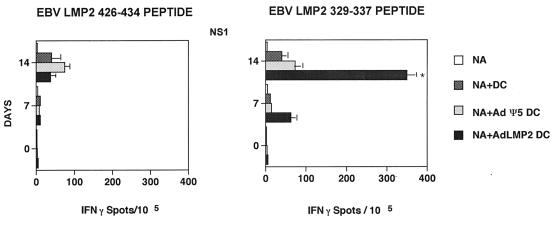
T-cell induction using adenovirus-transduced DC.
Having established the basal and peptide-induced responses of donors NS1 and NS2 to EBV-derived epitopes, we next sought to determine if adenovirus-mediated gene expression in DC was capable of inducing an antigen-specific T-cell response in vitro. Nonadherent T cells from healthy EBV-seropositive HLA-A2+ donors NS1 and NS2 were cultured with autologous DC that had been previously infected with recombinant Adψ5 or AdLMP2. Responder T cells were restimulated on day 7 with adenovirus-infected DC identical to those used to prime cultures, and the resultant day 14 T cells were tested for the capacity to secrete IFN-γ in the presence of the HLA-A2.1-restricted LMP2 329-337 and LMP2 426-434 peptides in the ELISPOT assays. A significant increase in the frequencies of CD8+ T cells reactive against autologous DC loaded with the LMP2 329-337 peptide (350 spot-forming lymphocytes per 105 CD8+ T cells; P < 0.01) was observed in T-cell cultures that had been stimulated with AdLMP2-transduced, autologous DC. T-cell reactivity against DC transduced with Adψ5 (negative control) was comparable to the background level. These data indicated that the DC infected with AdLMP2 are capable of inducing CD8+ T-cell responses directed against known HLA-A2-restricted peptide epitopes, such as LMP2 329-337 (Fig. 7). Most important, we noted that in donor NS1, the frequency of LMP2 329-337-specific T cells resulting from stimulation by AdLMP2-transduced DC was comparable to that obtained in cultures established by LMP2 329-337 peptide-loaded DC (Fig. 6). By comparison, CTL capable of recognizing the LMP2 426-434 epitope were preferentially induced by peptide-pulsed DC (versus AdLMP2-transduced DC). Similar results were obtained for donor NS2 (22 and 64 spots per 105 CD8+ T cells reactive against LMP2 426-434 and LMP2 329-337, respectively, after 14-day stimulation with AdLMP2-transduced autologous DC [Fig. 6 and data not shown]). These data suggested that the LMP2 329-337 epitope might be immunodominant within the context of an AdLMP2-based vaccination of HLA-A2+ responders. Furthermore, the lack of specific T-cell response to Adψ5-infected DC suggests the poor immunogenicity of residual adenovirus-derived epitopes in these E1-E3-deleted viruses.
FIG. 7.
AdLMP2-transduced DC promote the expansion of anti-EBV CD8+ T cells in vitro. PBL isolated from the blood of healthy EBV-seropositive donor NS1 were stimulated twice (on days 0 and 7) with autologous Adψ5- and AdLMP2-transduced DC as described in Materials and Methods. Responder CD8+ T cells were harvested on days 0, 7, and 14 of culture and analyzed for LMP2 peptide-specific reactivity in IFN-γ ELISPOT assays. T cells were seeded at 105 cells/well and incubated with autologous immature DC pulsed with LMP2 329-337 or LMP2 424-436 peptides. Resulting spots (mean ± SD) were developed after 20 h of incubation and evaluated by computer-assisted video image analysis. Spots were evaluated as described in the legend to Fig. 5. Data are representative of three experiments performed. NA, nonadherent T cells.
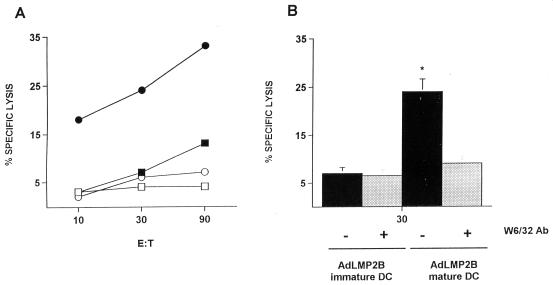
Responder T cells restimulated on day 14 with autologous AdLMP2-transduced DC were also tested for lytic reactivity against autologous EBV B-LCLs. Within the scope of this assay, we also compared the effectiveness of immature and mature transduced DC for the ability to induce CTL responses against LMP2 epitopes. AdLMP2-transduced immature DC induced CTL displaying a moderate cytotoxic activity against autologous EBV B-LCLs in donors NS1 and NS2 (16%, E/T ratio = 90:1), while T cells stimulated with Adψ5-transduced immature DC did not display any specific lytic activity. T cells stimulated with Adψ5-transduced mature DC showed only low, if any, cytotoxic activity against the target cells, while AdLMP2-transduced mature DC-stimulated T cells lysed the target cells (33%, E/T ratio = 90:1) to an extent significantly greater than that observed in cultures driven by LMP2+ immature DC (Fig. 8). Cytotoxicity was effectively inhibited by anti-MHC I blocking MAb W6/32 (31% inhibition). Cytotoxic reactivity against HLA-mismatched (A2-negative EBV B-LCL) cell lines was not observed (data not shown). Overall, these data suggest that cytotoxicity was mediated by bona fide class I-restricted CTL and was not associated with nonspecific LAK-type activity. The cytotoxicity data appeared to be corroborated by the immune phenotype analysis of the responder T-cell populations at days 0, 7, and 14, which indicated that the T-cell population was predominantly CD4+ but exhibited a time-dependent increase of CD8+ cells and concomitant decrease of NK cells (data not shown). These observations indicate a preferential expansion of CD8+ CTL triggered by chronic in vitro restimulation with AdLMP2-transduced mature DC.
FIG. 8.
AdLMP2-transduced mature DC preferentially elicit anti-EBV CTL in vitro. T cells obtained from EBV-seropositive donor NS1 were stimulated on days 0, 7, and 14 with autologous immature (□) or mature (○) Adψ5-transduced DC or immature (■) or mature (●) AdLMP2-transduced DC. Responder T cells were tested for cytolytic activity against autologous EBV B-LCL targets on day 21 in a standard 5-h 51Cr release assay (A). The HLA class I antibody (Ab) W6/32 significantly blocked the killing of the autologous EBV B-LCLs (P < 0.01) by AdLMP2-infected DC-stimulated T cells (E/T ratio = 30:1) (B). There was no background of NK activity in any of the assays performed based on the technical inclusion of nonlabeled K562 targets in all the cytolytic assessments. The lytic activity of CTL stimulated with AdLMP2-infected DC against 51Cr-labeled K562 targets was less than 5% at each E/T ratio (data not shown). Results are expressed as percentage of specific lysis at a given E/T ratio. Data are representative of three experiments performed.
DISCUSSION
EBV-related large-cell lymphomas are one of the predominant malignancies facing patients with congenital or acquired immunodeficiency who are frequently unable to establish or maintain sufficient immunosurveillance to control proliferating B cells harboring EBV. To date, conventional approaches for the treatment of EBV-related malignancies are often not curative or are associated with serious complications. New immunotherapeutic strategies, such as adoptive transfer of CTL or MAbs, are effective if the tumor cells express immunodominant proteins. HD, BL, and NPC cancers appeared to be less susceptible to immunotherapeutic intervention, presumably due to the expression of a more restricted array of subdominant EBV-encoded epitopes derived from latent proteins (EBNA1, LMP1, and LMP2). The development of protocols capable of promoting the induction and maintenance of anti-EBV-reactive T cells is therefore of significant clinical merit (26). We chose to evaluate the in vitro efficacy of DC-based genetic vaccination.
Adenovirus-mediated delivery of antigenic DNA to APC has been recently adopted as a feasible strategy for cancer vaccination (4). The immunostimulatory capability of recombinant adenovirus vectors coding for the melanoma tumor-associated antigen MART 1 in an in vitro human model has been described (6), and the polyomavirus middle T antigen (37) has been used in an in vivo murine model. In all of these systems, the adenovirus vector-delivered genes have been shown to elicit strong cellular immune responses.
This study focused on the ability of autologous DC infected with a recombinant adenovirus encoding the LMP2 protein to stimulate cellular anti-EBV immunity in vitro. We chose to initially evaluate whether the adenovirus infection per se can promote or affect maturation of human DC. To this end, we used recombinant adenovirus encoding EGFP, an easily validated marker of gene transfer to transduce DC. The efficiency of AdEGFP transduction monitored by EGFP expression on DC surface was extremely high, resulting in more than 95% of the cells expressing EGFP. In addition, the adenovirus-mediated transduction process did not promote DC maturation, as similarly reported by Zhong et al. (40). Infected DC remained phenotypically immature, as determined by low expression levels of CD83, antigen-presenting molecules (MHC I and MHC II), and adhesion and costimulatory molecules. Optimal phenotypic and functional maturation of DC occurred when DC were transduced with adenovirus and concomitantly treated with a maturational cytokine cocktail, resulting in strong augmentation in DC expression of CD83 and MHC II and coexpression of MHC I, CD80, CD86, CD40, and CD54 receptors. Thus, adenovirus infection neither promoted nor hindered our ability to generate mature DC.
We next tested the ability of autologous mature DC to induce EBV-specific T-cell responses by using two different antigenic formats (HLA-2.1 synthetic peptides pulsed on DC and transduction of DC with an individual EBV antigen) in order to provide a basis for the development of a vaccine formulation capable of inducing potent anti-EBV immunity for the treatment of EBV-associated malignant diseases. We estimated the frequency of EBV epitope-specific memory T cells that had been freshly isolated from EBV-seropositive donors. The IFN-γ ELISPOT assay allowed us to detect peptide-specific T cells directed against a range of HLA-A2.1-presented EBV (defined lytic and subdominant latent-cycle) epitopes. Frequencies of EBV peptide-reactive freshly isolated T cells ranged between 5 and 70.5 per 105 CD8+ T cells, consistent with recent data reported for subjects with an established long-term EBV carrier status screened with the same sensitive assay (35). Using mature DC loaded with HLA-2.1-binding LMP2 and BMLF1 peptide epitopes, we then generated EBV peptide-specific T-cell lines by IVS of CD8+ T cells in short-term cultures in order to reactivate T-cell memory responses. After 2 weeks of IVS culture, we were able to induce a strong, specific CD8+ T-cell reactivity against the LMP2 329-337 HLA-A2.1-restricted epitope in both donors evaluated. We were unsuccessful in promoting a dominant response against the LMP2 426-434 peptide although anti-LMP2 424-436-specific CD8+ T cells did increase in frequency as a function of repetitive in vitro stimulation with peptide-pulsed DC. These observations suggest that LMP2 epitopes used in this study may differ in their immunogenicity (i.e., LMP2 329-337 is dominant and LMP2 426-434 is subdominant). We also analyzed T-cell responses to a known epitope of the early EBV lytic-cycle protein BMLF1. For donor NS1, IFN-γ ELISPOT analysis performed on stimulated CD8+ T cells showed an elevated frequency of T cells specific for BMLF1 peptide, although in the second donor, the anti-BMLF1 reactivity was not as apparent. Importantly, the peptide-based DC approach reactivated T-cell responses directed against the LMP2 epitopes in EBV-seropositive donors. This finding raises the possibility of reactivating and amplifying this T-cell repertoire in vaccine strategies to be applied to patients affected by EBV-associated malignancies, such as NPC, HD, and BL, or posttransplantation lymphoproliferative disorders. Although the HLA-A2-restricted response is of particular importance for a peptide-based vaccine immunotherapy since A2 is carried by approximately 50% of individuals in most world populations, other HLA alleles (i.e., A11, A24, and B40) can also present epitopes derived from EBV latent cycle antigens and will likely prove clinically important (24).
Given the limitations of the peptide-based immunotherapy in targeting only a restricted range of HLA-A/B-presented LMP2 epitopes, we developed an alternative strategy for stimulating immunity against EBV LMP2-derived epitopes by using genetically engineered DC infected with a recombinant adenovirus vector encoding LMP2B. We demonstrated that AdLMP2-transduced DC naturally process and present a known HLA-A2-restricted LMP2 peptide epitope and are able to induce EBV B-LCL-specific CTL in vitro. Indeed, HLA-A2-restricted CD8+ T-cell responses were readily induced and expanded against the LMP2 329-337 epitope (but not the LMP2 426-434 epitope) after in vitro stimulation with autologous AdLMP2-transduced DC. Our results indicate that the principal advantage of this genetic vaccine approach is the expression of a variety of known and unknown LMP2 antigenic epitopes in MHC complexes expressed on the surface of DC that are relevant to autologous responders; hence HLA haplotype information is not required (a priori) for patient treatment. This broadening of anti-LMP2 immunity, by allowing for the presentation of multiple epitopes by multiple patient MHC alleles, would conceptually minimize the potential problem caused by specific epitope-loss variants of the virus (13) that could allow for evasion of an immune response resulting from vaccination with a single defined epitope. Moreover, the direct gene transfer to DC leads to endogenous processing and presentation of the gene product in the context of MHC class I, and possibly class II, molecules, as recently reported (24). The feasibility of inducing and enhancing CD4+ T-cell responses against a defined antigen in cooperation with CD8+ T-cell responses may augment and maintain a strong memory effector T-cell response against EBV. We are currently investigating the possibility of generating CD4+ T-cell responses by using adenovirus-transduced DC.
In several recent studies, viral and nonviral vector systems have been used to achieve efficient transfer and stable gene expression in mammalian cells (7, 23). Retrovirus vectors are the most extensively used because the proviral DNA integrates into the host genome, but the inability to infect nondividing cells represents a major problem for their application in gene therapy (15). Vaccinia virus-infected LCLs have been used to induce CTL responses against EBV antigens, and recently recombinant adenoviruses encoding the EBV EBNA3C antigen (22) have been evaluated. The advantage in using an adenovirus vector is the high efficiency of infecting nondividing cells and, in the case of monocyte-derived DC, to obtain a high level of naturally processed antigenic epitopes on the cell surface of the APC. One of the major problems, however, is the immunogenicity of adenoviruses used in gene therapy and the subsequent dampening of immunity directed against the tumor-associated gene product (32). This principally occurs due to preexisting neutralizing antiadenovirus antibodies (20, 21) and to the cellular immune response to recombinant adenovirus capsid proteins (9, 31), resulting in low efficiency of gene transfer when the adenovirus is reapplied in situ (8). Despite the possibility of potent antiadenovirus CD4+ type 1 helper and CD8+ T-cell responses to adenovirus antigens (33), the engineered target antigen remains sufficiently immunogenic in transduced DC to drive the expansion of antigen-specific T cells, as recently demonstrated by Butterfield et al. (6). Despite these technical challenges, this experimental strategy appears well suited for application in the vaccine immunotherapy of infectious disease and cancer where defined antigens may be readily integrated.
In conclusion, autologous mature DC genetically modified with an adenovirus encoding EBV antigens allow for the generation of in vitro vaccines capable of promoting EBV-specific CD8+ T effector cells. These data suggest the potential clinical application of such EBV-based adenovirus vector systems for the treatment or the prevention of EBV-related malignancies. Based on our present results, such a vaccine might take the form of adenovirus (engineered to express antigens)-transduced autologous DC that have been matured ex vivo prior to injection into patients.
ACKNOWLEDGMENTS
We thank Jan Mueller-Berghaus for careful review and helpful discussion in the generation of the manuscript.
This work was supported by National Institutes of Health (NIH) grants CA 57840 (W.J.S.) and CA 143666 (P.D.R.), a Clinical Investigator Award from the Cancer Research Institute (W.J.S.), a CNR-NATO grant (216.1919; L.G.), NATO collaborative research grant CRG 973153 (L.G. and W.J.S.), a Telethon grant (A.G.), and a fellowship from the Deutsche Forschungsgemeinschaft (He 2896/1-1; W.H.).
REFERENCES
- 1.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Bednarek M A, Sauma S Y, Gammon M C, Porter G, Tamhankar S, Williamson A R, Zweerink H J. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J Immunol. 1991;147:4047–4053. [PubMed] [Google Scholar]
- 3.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brossart P, Goldrath A W, Butz E A, Martin S, Bevan M J. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158:3270–3276. [PubMed] [Google Scholar]
- 5.Burrows S R, Gardner J, Khanna R, Steward T, Moss D J, Rodda S, Suhrbier A. Five new cytotoxic T cell epitopes identified within Epstein-Barr virus nuclear antigen 3. J Gen Virol. 1994;75:2489–2493. doi: 10.1099/0022-1317-75-9-2489. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield L H, Jilani S M, Chakraborty N G, Bui L A, Ribas A, Dissette V B, Lau R. Generation of melanoma-specific cytotoxic T lymphocytes by dendritic cells transduced with a MART-1 adenovirus. J Immunol. 1998;161:5607–5613. [PubMed] [Google Scholar]
- 7.Dietz A B, Vuk-Pavlovic S. High efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood. 1998;91:392–398. [PubMed] [Google Scholar]
- 8.Gahery-Segard H, Molinier-Frenkel V, Le Boulaire C, Saulnier P, Opolon P, Lengagne R, Gautier E, Le Cesne A, Zitvogel L, Venet A, Schatz C, Courtney M, Le Chevalier T, Tursz T, Guillet J G, Farace F. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J Clin Investig. 1997;100:2218–2226. doi: 10.1172/JCI119759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahéry-Ségard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P, Guillet J-G. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas G, Plikat U, Debre P, Lucchiari M, Katlama C, Dudoit Y, Bonduelle O, Bauer M, Ihlenfeldt H G, Jung G, Maier B, Meyerhans A, Autran B. Dynamics of viral variants in HIV-1 nef and specific cytotoxic T lymphocytes in vivo. J Immunol. 1996;157:4212–4221. [PubMed] [Google Scholar]
- 11.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. Construction of adenovirus vector through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herr W, Linn B, Leister N, Wandel E, Meyer zum Bueschenfelde K H, Woelfel T. The use of computer-assisted video image analysis for the quantification of CD8+ T lymphocytes producing tumor necrosis factor α spots in response to peptide antigens. J Immunol Methods. 1997;203:141–152. doi: 10.1016/s0022-1759(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 13.Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. Int J Cancer. 1997;66:470–476. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Jonuleit H, Kuehn U, Mueller G, Steinbrink K, Paragnik L, Schmitt E, Knopp J, Enk A H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 15.Kai M A, Liu D, Hoogerbrugge P. Gene therapy. Proc Natl Acad Sci USA. 1997;94:12774–12776. doi: 10.1073/pnas.94.24.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr B M, Kienzle N, Burrows J M, Cross S, Silins S L, Buck M, Benson E M, Coupar B, Moss D J, Sculley T B. Identification of type B-specific and cross-reactive cytotoxic T-lymphocyte responses to Epstein-Barr virus. J Virol. 1996;70:8858–8864. doi: 10.1128/jvi.70.12.8858-8864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S P, Tierney R J, Thomas W A, Brooks J M, Rickinson A B. Conserved CTL epitopes within EBV latent membrane protein 2. A potential target for CTL-based tumor therapy. J Immunol. 1997;158:3325–3334. [PubMed] [Google Scholar]
- 18.Lee S P, Thomas W A, Murray R J, Khanim F, Kaur S, Young L S, Rowe M, Kurilla M, Rickinson A B. HLA A2.1-restricted cytotoxic T cells recognizing a range of Epstein-Barr virus isolates through a defined epitope in latent membrane protein LMP2. J Virol. 1993;67:7428–7435. doi: 10.1128/jvi.67.12.7428-7435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller C L, Lee J H, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molnar-Kimber K L, Sterman D H, Chang M, Kang E H, Elbash M, Lanuti M, Elshami A, Gelfand K, Wilson J M, Kaiser L R, Albelda S M. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther. 1998;9:2121–2133. doi: 10.1089/hum.1998.9.14-2121. [DOI] [PubMed] [Google Scholar]
- 21.Morgan S M, Wilkinson G W, Floettmann E, Blake N, Rickinson A B. A recombinant adenovirus expressing an Epstein-Barr virus (EBV) target antigen can selectively reactivate rare components of EBV cytotoxic T-lymphocyte memory in vitro. J Virol. 1996;70:2394–2402. doi: 10.1128/jvi.70.4.2394-2402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabel G J. Development of optimized vectors for gene therapy. Proc Natl Acad Sci USA. 1999;96:324–326. doi: 10.1073/pnas.96.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Diez A, Butterfield L H, Li L, Chakraborty N G, Economou J S, Mukherji B. Generation of CD8+ and CD4+ T-cell response to dendritic cells genetically engineered to express the MART-1/Melan-A gene. Cancer Res. 1998;58:5305–5309. [PubMed] [Google Scholar]
- 24.Redchenko I V, Rickinson A B. Assessing Epstein-Barr virus-specific T-cell memory with peptide-loaded dendritic cells. J Virol. 1999;73:334–342. doi: 10.1128/jvi.73.1.334-342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribas A, Butterfield L H, McBride W H, Jilani S M, Bui L A, Vollmer C M, Lau R, Dissette V B, Hu B, Chen A Y, Glaspy J A, Economou J S. Genetic immunization for the melanoma antigen MART-1/Melan-A using recombinant adenovirus-transduced murine dendritic cells. Cancer Res. 1997;57:2865–2869. [PubMed] [Google Scholar]
- 26.Rickinson A B, Moss D J. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 27.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2415. [Google Scholar]
- 28.Rowe M, Rowe D T, Gregory C D, Young L S, Farrell P J, Rupani H, Rickinson A B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt’s lymphoma cells. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savoie A, Perpete C, Carpentier L, Joncas J, Alfieri C. Direct correlation between the load of Epstein-Barr virus-infected lymphocytes in the peripheral blood of pediatric transplant patients and risk of lymphoproliferative disease. Blood. 1994;83:2715–2722. [PubMed] [Google Scholar]
- 30.Schmidt C, Burrows S R, Sculley T B, Moss D J, Misko I S. Nonresponsiveness to an immunodominant Epstein-Barr virus-encoded cytotoxic T-lymphocyte epitope in nuclear antigen 3A: implications for vaccine strategies. Proc Natl Acad Sci USA. 1991;88:9478–9482. doi: 10.1073/pnas.88.21.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith C A, Woodruff L S, Rooney C, Kitchingman G R. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum Gene Ther. 1998;9:1419–1427. doi: 10.1089/hum.1998.9.10-1419. [DOI] [PubMed] [Google Scholar]
- 32.Smith C A, Woodruff L S, Kitchingman G R, Rooney C M. Adenovirus-pulsed dendritic cells stimulate human virus-specific T-cell responses in vitro. J Virol. 1996;70:6733–6740. doi: 10.1128/jvi.70.10.6733-6740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song W, Kong H L, Carpenter H, Torii H, Granstein R, Rafii S, Moore M A, Crystal R G. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steven N M, Annels N E, Kumar A, Leese A M, Kurilla M G, Rickinson A B. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O’Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A B, Callan M F. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 36.van der Bruggen P, Bastin J, Gajewski T, Coulie P G, Boel P, De Smet C, Traversari C, Townsend A, Boon T. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 37.Wan Y, Bramson J, Carter R, Graham F, Gauldie J. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther. 1997;8:1355–1363. doi: 10.1089/hum.1997.8.11-1355. [DOI] [PubMed] [Google Scholar]
- 38.Wan Y, Emtage P, Foley R, Carter R, Gauldie J. Murine dendritic cells transduced with an adenoviral vector expressing a defined tumor antigen can overcome anti-adenovirus neutralizing immunity and induce effective tumor regression. Int J Oncol. 1999;14:771–776. doi: 10.3892/ijo.14.4.771. [DOI] [PubMed] [Google Scholar]
- 39.Young L, Alfieri C, Hennessy K, Evans H, O’Hara C, Anderson K C, Ritz J, Shapiro R S, Rickinson A B, Kieff E, et al. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- 40.Zhong L, Granelli-Piperno A, Choi Y, Steinman R M. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur J Immunol. 1999;29:964–972. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Zitvogel L, Mayordomo J I, Tjandrawan T, DeLeo A B, Clarke M R, Lotze M T, Storkus W J. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]