Abstract
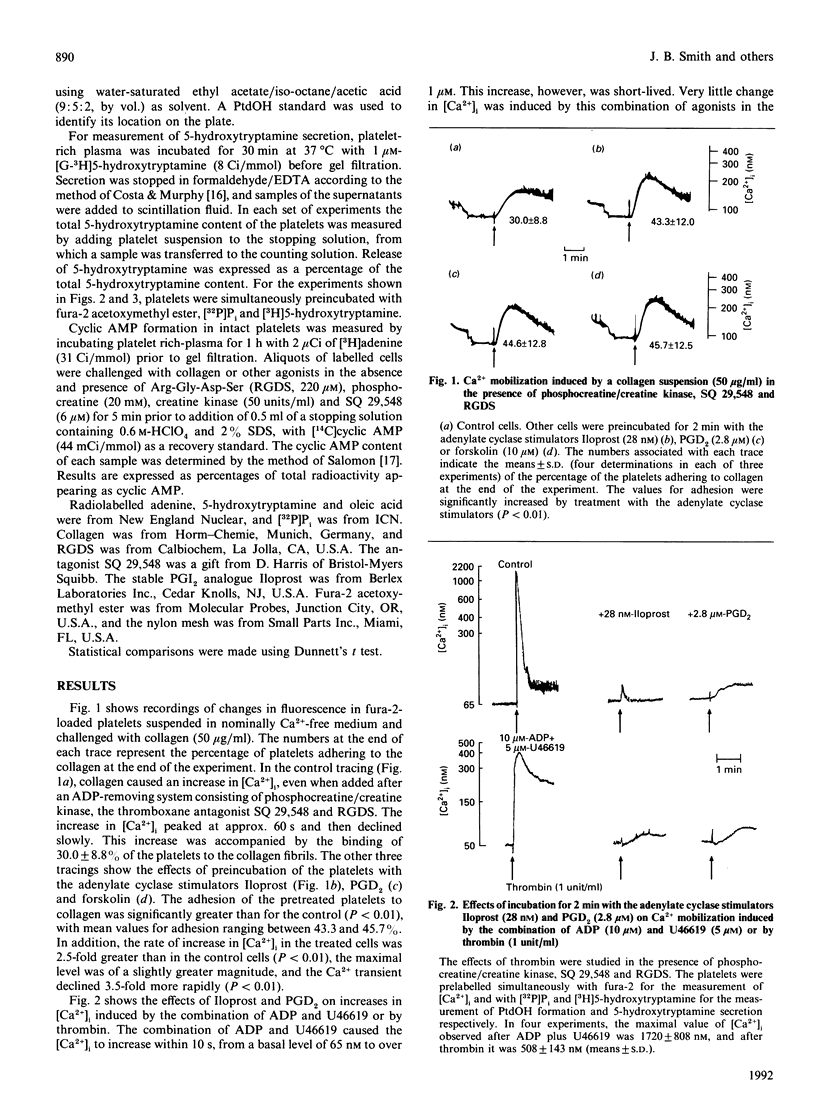
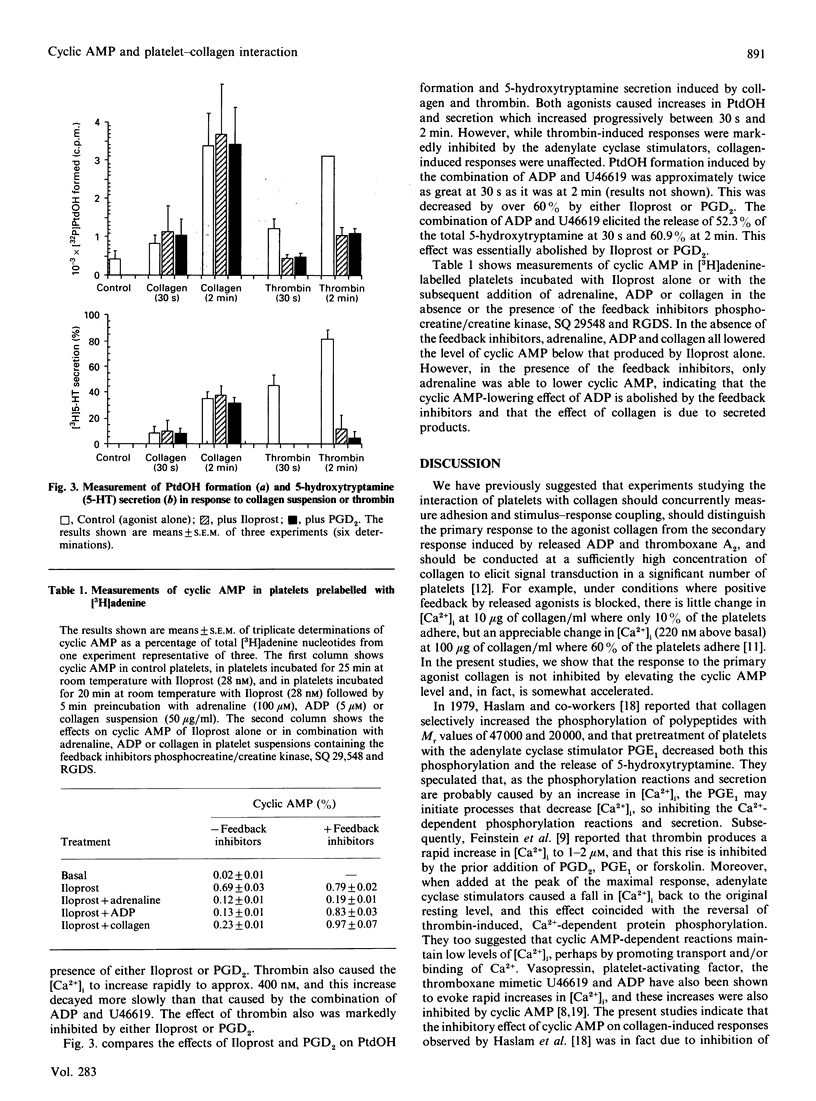
The adhesion of platelets to collagen and their activation is the primary event in haemostasis. Following adhesion, platelet aggregation mediated by ADP, thromboxane A2 and thrombin leads to the formation of a platelet plug. It is known that platelet activation by each of these agonists involves an increase in the cytosolic free Ca2+ concentration, and this has been thought to be controlled by cyclic AMP. However, we report here that while signal transduction induced by ADP plus a thromboxane mimetic (U46619), or by thrombin, is inhibited by stimulators of adenylate cyclase such as a prostaglandin I2 (PGI2) analogue (Iloprost), PGD2 and forskolin, elevation of cyclic AMP does not inhibit either platelet adhesion to collagen or the associated Ca2+ mobilization, phosphatidic acid formation or 5-hydroxytryptamine secretion. Furthermore, collagen did not lower elevated levels of cyclic AMP in platelets measured in the presence of both a thromboxane antagonist and an ADP-removing system. The present results are discussed in the context of previous findings.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., McNicol A., MacIntyre D. E. Inhibition of platelet-activating-factor-induced human platelet activation by prostaglandin D2. Differential sensitivity of platelet transduction processes and functional responses to inhibition by cyclic AMP. Biochem J. 1985 Nov 15;232(1):267–271. doi: 10.1042/bj2320267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J. P., Dejana E., Kinlough-Rathbone R. L., Richardson M., Packham M. A., Mustard J. F. Prostaglandins I2 and E1 reduce rabbit and human platelet adherence without inhibiting serotonin release from adherent platelets. Thromb Res. 1979;15(1-2):273–279. doi: 10.1016/0049-3848(79)90073-2. [DOI] [PubMed] [Google Scholar]
- Costa J. L., Murphy D. L. Platelet 5-HT uptake and release stopped rapidly by formaldehyde. Nature. 1975 May 29;255(5507):407–408. doi: 10.1038/255407a0. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Egan J. J., Sha'afi R. I., White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem Biophys Res Commun. 1983 Jun 15;113(2):598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Lynham J. A., Fox J. E. Effects of collagen, ionophore A23187 and prostaglandin E1 on the phosphorylation of specific proteins in blood platelets. Biochem J. 1979 Feb 15;178(2):397–406. doi: 10.1042/bj1780397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs E. A., Moncada S., Vane J. R., Caen J. P., Michel H., Tobelem G. Effect of prostacyclin (PGI2) on platelet adhesion to rabbit arterial subendothelium. Prostaglandins. 1978 Jul;16(1):17–22. doi: 10.1016/0090-6980(78)90197-1. [DOI] [PubMed] [Google Scholar]
- Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991 Feb 14;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Karniguian A., Simmons P., Legrand Y. J., Moncada S., Caen J. P. A comparison of the inhibitory effects of prostacyclin and carbacyclin on platelet adhesion to collagen. Prostaglandins. 1982 Dec;24(6):827–836. doi: 10.1016/0090-6980(82)90062-4. [DOI] [PubMed] [Google Scholar]
- Lages B., Scrutton M. C., Holmsen H. Studies on gel-filtered human platelets: isolation and characterization in a medium containing no added Ca2+, Mg2+, or K+. J Lab Clin Med. 1975 May;85(5):811–825. [PubMed] [Google Scholar]
- Lerea K. M., Glomset J. A., Krebs E. G. Agents that elevate cAMP levels in platelets decrease thrombin binding. J Biol Chem. 1987 Jan 5;262(1):282–288. [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C. Inhibition by ADP of prostaglandin induced accumulation of cyclic AMP in intact human platelets. J Cyclic Nucleotide Res. 1981;7(1):1–11. [PubMed] [Google Scholar]
- Mauco G., Dangelmaier C. A., Smith J. B. Inositol lipids, phosphatidate and diacylglycerol share stearoylarachidonoylglycerol as a common backbone in thrombin-stimulated human platelets. Biochem J. 1984 Dec 15;224(3):933–940. doi: 10.1042/bj2240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurov A. V., Misselwitz F., Hoffman U., Leytin V. L., Repin V. S. Effects of prostacyclin analogue (carbacyclin) on the interaction of platelets with different collagen substrates. Inhibition of cAMP increase by collagens type I, III, and IV. Prostaglandins. 1988 Jan;35(1):51–65. doi: 10.1016/0090-6980(88)90274-2. [DOI] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A. Effects of prostaglandin I2 and forskolin on the secretion from platelets evoked at basal concentrations of cytoplasmic free calcium by thrombin, collagen, phorbol ester and exogenous diacylglycerol. Biochem J. 1984 Sep 15;222(3):833–836. doi: 10.1042/bj2220833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Santoro S. A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986 Sep 12;46(6):913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Shadle P. J., Barondes S. H. Adhesion of human platelets to immobilized trimeric collagen. J Cell Biol. 1982 Oct;95(1):361–365. doi: 10.1083/jcb.95.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Dangelmaier C. Determination of platelet adhesion to collagen and the associated formation of phosphatidic acid and calcium mobilization. Anal Biochem. 1990 May 15;187(1):173–178. doi: 10.1016/0003-2697(90)90437-e. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Dangelmaier C., Selak M. A., Daniel J. L. Facile platelet adhesion to collagen requires metabolic energy and actin polymerization and evokes intracellular free calcium mobilization. J Cell Biochem. 1991 Sep;47(1):54–61. doi: 10.1002/jcb.240470108. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Rajpara S. M., Wayner E. A., Carter W. G., Santoro S. A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989 May;108(5):1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T. Prostacyclin (prostaglandin I2, PGI2) inhibits platelet adhesion and thrombus formation on subendothelium. Blood. 1979 Feb;53(2):244–250. [PubMed] [Google Scholar]
- Yamanishi J., Kawahara Y., Fukuzaki H. Effect of cyclic AMP on cytoplasmic free calcium in human platelets stimulated by thrombin: direct measurement with quin2. Thromb Res. 1983 Oct 15;32(2):183–188. doi: 10.1016/0049-3848(83)90029-4. [DOI] [PubMed] [Google Scholar]


