Abstract
Prevalence of hypertension (HTN) and human immunodeficiency virus (HIV) are high among men while screening rates are low. Assisted partner notification service is a strategy recommended by the World Health Organization that aims to increase HIV testing and treatment uptake and may present an opportunity to offer integrated HIV/HTN screening and treatment services. In this prospective cohort study, we assessed the feasibility of integrating HTN screening for male sexual partners of females newly tested HIV-positive in 10 health facilities in Kenya. Participants were notified of the exposure and offered HIV testing and HTN screening; if they accepted and tested positive for either HTN, HIV, or both, they were referred for care. HTN was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90, or the use of antihypertensive medication. Among 1313 male partners traced, 99% accepted HIV testing and HTN screening. Overall, 4% were found to have HTN, 29% were in the pre-HTN stage, and 9% were HIV-positive. Only 75% had previously been screened for HTN compared to 95% who had previously tested for HIV. A majority preferred non-facility-based screening. The participants who refused HTN screening noted time constraints as a significant hindrance. HIV and HTN screening uptake was high in this hard-to-reach population of men aged 25 to 50. Although HTN rates were low, an integrated approach provided an opportunity to detect those with pre-HTN and intervene early. Strategic integration of HTN services within assisted partners services may promote and normalize testing by offering inclusive and accessible services to men.
Keywords: assisted partner notification service, care, HIV, hypertension, integration, Kenya, men, sub-Saharan Africa, treatment
1. Introduction
Hypertension (HTN) is a major modifiable risk factor for cardiovascular diseases. It contributes to 45% of all heart disease deaths and 51% of stroke related deaths globally.[1–3] HTN prevalence has been increasing steadily in sub-Saharan Africa (SSA), with a 41% rise seen between 2000 to 2010 which is anticipated to increase by 60% by 2030.[4,5] Significant gaps have been documented in the detection, awareness, treatment, and control of HTN in Kenya, particularly among men. Over 23.8% of Kenyan adults have HTN; however, only 1 in 5 are aware of their status and <3% of those aware have their blood pressure (BP) under control.[6]
In Kenya, men are less likely to seek screening for HTN compared to women (36% vs 63%) who have routine interactions with service providers during pregnancy, the postnatal period, and when seeking reproductive health services.[7–10] In light of the gaps in screening for HTN and human immunodeficiency virus (HIV) among men, the Kenya Ministry of Health and the World Health Organization issued a call to develop novel strategies to integrate HTN screening into existing platforms such as HIV care and treatment services.[11] Despite these recommendations, HTN screening is rarely integrated into HIV testing services.[12,13]
Assisted partner notification services (aPNS) is a global public health strategy recommended by the World Health Organization that involves tracing and offering HIV testing services to sexual partners.[14] aPNS aims to increase HIV testing and treatment uptake and presents a unique opportunity to offer HTN screening alongside HIV/acquired immunodeficiency syndrome services.[15] This pilot study sought to evaluate the feasibility of integrating HTN screening services into the aPNS by targeting male partners of females testing HIV-positive in western Kenya.
2. Methods
2.1. Project description and study setting
This prospective cohort study was nested within the ongoing aPNS scale-up project. The project was conducted by preventing and treating HIV-Kenya and the University of Washington in collaboration with the Ministry of Health Kenya; in 30 health facilities in Kisumu and Homa Bay counties in Kenya.[16] The objective of this aPNS study is to evaluate the effectiveness and acceptability of integrating aPNS into existing HIV testing services. A detailed description of the project procedures has been published.[16] In summary, the aPNS project enrolled females ≥ 15 years of age and newly diagnosed with HIV (female index client). Male sexual partners of these female index clients were contacted by HIV testing service (HTS) providers and invited to screen for HIV. The male partners who test positive for HIV received a 1-year post enrollment follow-up to assess linkage to care and treatment.[16] Simultaneously, in 10 of the 30 healthcare facilities, HTN screening was offered to all male partners ≥ 18 years who provided verbal consent. These health facilities were randomly chosen as a representative sample of the high volume versus low volume sites and urban versus rural areas in Kisumu and Homa Bay Counties.
2.2. Study procedures and data collection
HTS providers anonymously notified male partners of the HIV exposure by phone or in person and offered HTN screening and HIV testing at the nearest hospital, home, or convenient venue. Informed consent was obtained before data collection. A questionnaire was used to collect demographic characteristics, HTN screening history, and additional details on those who declined HTN screening. Using a digital automatic BP monitor, 2 BP measurements were obtained on the right arm after the subject was seated in a resting position for at least 5 minutes. The lower reading of the 2 was recorded and used for analysis. A rapid HIV test was used to confirm HIV infection. Participants testing HIV positive and/or with elevated BP readings were referred to link to care in an HIV clinic or a regular outpatient clinic. Enrollment and engagement in care for HTN were confirmed during follow-up phone calls made by HTS providers 6 weeks post enrollment.
2.3. Outcome measures
Feasibility outcome measures included the proportion of male partners in the aPNS study that; Were offered combined HTN/HIV screening; Accepted HIV/HTN screening, and; Screened positive for HTN, HIV, or both. The proportion of individuals with HTN who were linked to care and initiated effective antihypertensive therapy was included. HTN was defined as a mean systolic BP of ≥ 140 mm Hg or a diastolic BP of ≥ 90 mm Hg, or self-report of previous HTN diagnosis by a health care provider and currently taking antihypertensive drugs within the last 2 weeks as per the Kenyan guidelines.[17] Pre HTN was defined as systolic BP of 130 to 139 mm Hg or diastolic BP of 80 to 89 mm Hg.
2.4. Data analyses
Sociodemographic characteristics were described using medians or percentages. Categorical variables were described using proportions, while continuous variables used medians and interquartile ranges. Proportions were used to quantify the feasibility of integrating HTN screening with and without HIV testing. All analyses were conducted using STATA (College Station, TX).
2.5. Ethical approval
Ethical approval was obtained from the University of Washington institutional review board and the Kenyatta National Hospital ethics review committee. Verbal informed consent was obtained from the male partners before BP screening.
3. Results
3.1. Participant’s characteristics
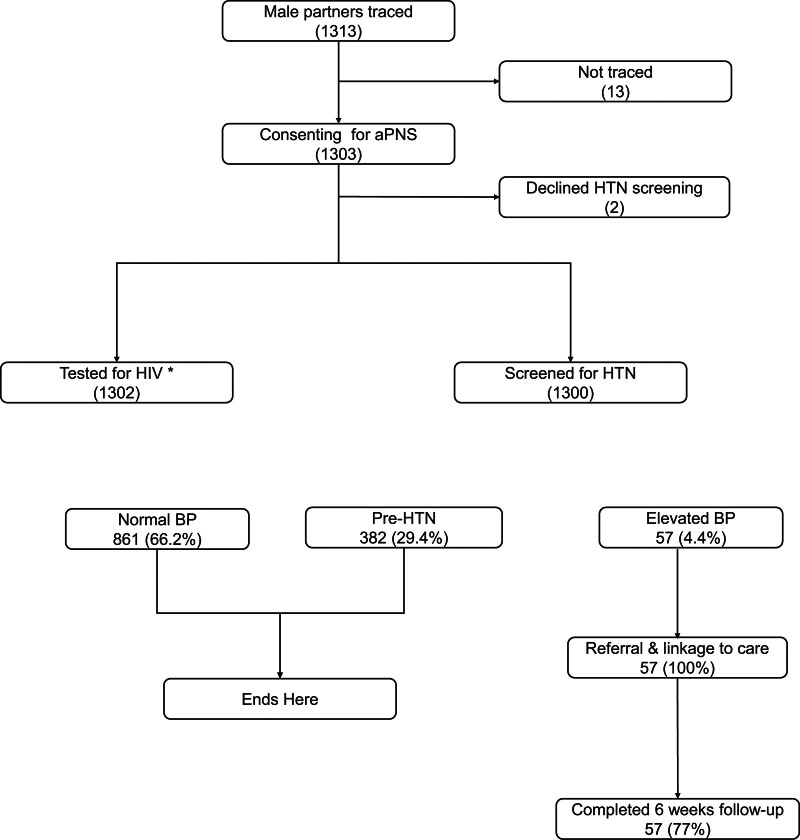
Between September 2020 and April 2021, 1313 male partners were traced and 1303 were enrolled (Fig. 1). Table 1 represents the sociodemographic characteristics of male partners. The median age was 38, and most (77%) were married. The median number of male partners for each female index was 3.
Figure 1.
Study flow chart highlighting screening, enrollment, and follow-up activities after partner tracing in the aPNS. *One participant declined HIV testing. HTN-BP ≥ 140/≥90 mm Hg. Pre-HTN-systolic BP of 130-139/80-89 mm Hg. aPNS = assisted partner notification services, BP = blood pressure, HIV = human immunodeficiency virus, HTN = hypertension.
Table 1.
Sociodemographic characteristics of male participants.
| Characteristics | Male partners N = 1303 | |
|---|---|---|
| Age (yr), median (IQR) | 38 | (32,42) |
| County | ||
| Homa Bay | 832 | (64) |
| Kisumu | 471 | (36) |
| Marital status | ||
| Single | 152 | (12) |
| Married monogamous/cohabiting | 1005 | (77) |
| Married polygamous | 87 | (7) |
| Divorced/separated/widowed | 59 | (4) |
| Highest Education completed | ||
| Never attended school | 5 | (0) |
| Primary school | 364 | (28) |
| Secondary school | 730 | (56) |
| postsecondary school | 204 | (16) |
| Occupation | ||
| Employed/self-employed | 1190 | (91) |
| Unemployed | 66 | (5) |
| Student | 47 | (4) |
| Monthly average income ($) | ||
| 0–100 | 596 | (46) |
| >100 | 707 | (54) |
| Screening strategy | ||
| Clinic | 392 | (30) |
| Home | 793 | (61) |
| Workplace | 109 | (8) |
| Others | 6 | (1) |
| Ever screened for HTN | ||
| Yes | 975 | (75) |
| No | 328 | (25) |
| Ever screened for HIV | ||
| Yes | 1233 | (95) |
| No | 70 | (5) |
| HIV status | ||
| Newly tested HIV positive | 128 | (10) |
| Negative | 738 | (57) |
| Persons living with HIV (known positive) | 435 | (33) |
| Unknown | 1 | (0) |
Data are reported as numbers, percentages, or median (interquartile range [IQR]).
ART = antiretroviral therapy, aPNS = assisted partner services, HIV = human immunodeficiency virus, HTN = hypertension.
3.2. HTN integration with HIV screening
Of the 1303 male partners who consented to study enrollment, 3 male partners (0.2%) declined HTN screening, citing time constraints and emotional unreadiness as the reason for screening refusal. A quarter of the participants reported having never had their BP checked before; in contrast, only about 5% had never been tested for HIV (Table 1). All participants with a previous HIV diagnosis were on antiretroviral therapy, while only 34% with a known HTN diagnosis were on treatment. A majority (70%) of participants opted for non-facility-based screening.
3.3. Elevated blood pressure burden
Of the male partners screened for HTN, 29.4% were in the pre-HTN stage and 4% were hypertensive (Fig. 1). Kisumu County had a significantly higher prevalence of both HTN (5% vs 4% and pre-HTN (39% vs 24%) compared to Homa Bay County, respectively (P < .01 for all). At the 6-week follow-up visit, 77% of those with screening readings consistent with HTN confirmed they had their BP rechecked, were linked to care, and had initiated HTN treatment (lifestyle modification and/or antihypertensive medication) at nearby health facilities.
4. Discussion
In this study conducted in a real-world setting, we found high HTN and HIV screening uptake among this hard-to-reach population of relatively young male partners. The preferred screening venue was home-based. Compared to HIV, a larger proportion of male partners had never been screened for HTN. Although rates of HTN were generally low, we found a high prevalence of prehypertension. Our study supports the integration of HTN screening into HIV testing through aPNS in Kenya and other low and middle income countries.
The high uptake of both HTN and HIV screening services has previously been reported in studies conducted in SSA countries. Mosha et al[18] reported high uptake of HIV/HTN screening in a community based HIV screening program in Tanzania. Drain et al[19] also demonstrated that it was feasible to integrate HTN screening in HIV voluntary testing sites in South Africa. Our study results prove that integration can be effective. We used an innovative integrated approach that leveraged aPNS to promote HIV/HTN screening among men, a population that has been defined as hard to reach and less likely to present in a hospital setting for routine screening. We found that a larger proportion of male participants had tested for HIV compared to HTN screening. This presents an opportunity to screen for HTN and capture any undiagnosed cases that would otherwise be missed and can potentially increase the number of men who routinely screen for both HTN and HIV.
We found low HTN prevalence which was expected given the younger age of our participants. Of concern was the high prevalence of pre-HTN in this relatively young population of men. This presents an opportunity for earlier intervention through lifestyle modification before progression to HTN. Studies have highlighted that approximately 40% of individuals diagnosed with pre-HTN progress to HTN within 2 years with no intervention.[20] Thus, it is critical to create opportunities for routine screening, capture pre-HTN early, and decrease or stop progression to HTN as the population ages.[21]
The current healthcare system offers better opportunities for women to interact with healthcare workers at various stages in life as they are more likely to be screened for diseases such as HTN and HIV during family planning, maternal and childcare service delivery.[22] Current service delivery creates disparities for the men who are less likely to seek healthcare services.[22] Also, hospital staffing shortages lead to long wait times, discouraging otherwise healthy individuals from routine screening. In our study, HTS providers were trained to screen for HTN and refer participants for further management. The utilization of trained nonphysician and community health care providers to screen for HTN and other noncommunicable diseases (NCD) has been successful and feasible in various studies conducted in SSA.[8,19,23,24] This may solve the staffing shortages in healthcare facilities and offer opportunities for alternative screening in the community.
A majority of men preferred home-based screening. Our study allowed flexibility in choosing a screening venue for the participants, unlike similar studies, conducted purely in the community or clinic. Verbal confirmation of linkage of care and BP recheck at 6 weeks in our study was 70% which is relatively high compared to other studies that range from 25% to 49%.[25–27] Studies with longer follow-up periods are needed to assess control of BP after linkage to care.
Our study has several strengths. We targeted a hard-to-reach population of men and screened them for both HTN and HIV, providing evidence that opportunistic screening within the hospital and community setting is feasible and potentially increases routine screening rates among men. We leveraged an existing platform and trained HTS providers to screen and offer referral services, providing evidence for integrating services and task sharing as potential solutions to increasing access to routine NCD screening within the clinical setting. Our study had several limitations. It had a short follow up period with verbal confirmation of linkage. Therefore, we cannot definitively ascertain the linkage to care following an HTN diagnosis.
As the burden of cardiovascular disease increases in SSA, integration of NCD screening services within the community setting and into other platforms such as HTS is necessary to increase the uptake of both HIV and HTN screening among men who are less likely to seek preventive services in a hospital setting yet will engage in care when diagnosed.
Acknowledgments
We thank Geoffrey Omondi, PATH HTS providers, and the study participants for their contribution.
Author contributions
Conceptualization: Harrison Lagat, George Otieno, Beatrice Wamuti, Sarah Masyuko, Monisha Sharma, Edward Kariithi, Carey Farquhar, Tecla M. Temu.
Data curation: Jerusha N. Mogaka, George Otieno, Tecla M. Temu.
Formal analysis: Jerusha N. Mogaka, George Otieno.
Funding acquisition: Monisha Sharma, Edward Kariithi, Carey Farquhar, Tecla M. Temu.
Investigation: Carey Farquhar, Tecla M. Temu.
Methodology: Beatrice Wamuti, Sarah Masyuko, Edward Kariithi, Carey Farquhar, Tecla M. Temu.
Project administration: Jerusha N. Mogaka, Harrison Lagat, George Otieno, Paul Macharia, Beatrice Wamuti, Sarah Masyuko, Edward Kariithi, Carey Farquhar, Tecla M. Temu.
Supervision: Harrison Lagat, George Otieno, Paul Macharia, Beatrice Wamuti, Sarah Masyuko, Monisha Sharma, Carey Farquhar, Tecla M. Temu.
Validation: Carey Farquhar.
Writing – original draft: Jerusha N. Mogaka.
Writing – review & editing: Jerusha N. Mogaka, George Otieno, Paul Macharia, Beatrice Wamuti, Sarah Masyuko, Monisha Sharma, Edward Kariithi, Carey Farquhar, Tecla M. Temu.
Abbreviations:
- aPNS
- assisted partners services
- BP
- blood pressure
- HIV
- human immunodeficiency virus
- HTN
- hypertension
- HTS
- HIV testing services
- SSA
- sub-Saharan Africa
CF and TMT contributed equally to this work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors have no conflicts of interest to disclose.
This project was supported by the National Institutes of Health (NIH) grant 3R01AI134130-03S1 and the European & Developing Countries Clinical Trials Partnership (EDCTP) program grant TMA-2016-1598-Kenya CVHIV. TT is supported by NIH grant K01 HL147723 from NHLBI. The funders did not participate in data collection or any activity directly related to the execution of the research.
How to cite this article: Mogaka JN, Lagat H, Otieno G, Macharia P, Wamuti B, Masyuko S, Sharma M, Kariithi E, Farquhar C, Temu TM. Descriptive study: Feasibility of integrating hypertension screening into HIV assisted partner notification services model in Kenya. Medicine 2023;102:8(e33067).
Contributor Information
Harrison Lagat, Email: hlagat@path.org.
George Otieno, Email: GOtieno@path.org.
Paul Macharia, Email: macharia.m.paul@gmail.com.
Beatrice Wamuti, Email: bwamuti@gmail.com.
Sarah Masyuko, Email: sarah.masyuko@gmail.com.
Monisha Sharma, Email: msharma1@uw.edu.
Edward Kariithi, Email: ekariithi@path.org.
Carey Farquhar, Email: cfarq@uw.edu.
Tecla M. Temu, Email: ttemu@uw.edu.
References
- [1].Yoruk A, Boulos PK, Bisognano JD. The State of Hypertension in Sub-Saharan Africa: review and Commentary. Am J Hypertens. 2018;31:387–8. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. WHO | raised blood pressure. World Heal Organ. 2011;39:40. [Google Scholar]
- [3].Gaciong Z, Siński M, Lewandowski J. Blood pressure control and primary prevention of stroke: summary of the recent clinical trial data and meta-analyses. Curr Hypertens Rep. 2013;15:559–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health. 2015;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Adeloye D, Basquill C. Estimating the prevalence and awareness rates of hypertension in Africa: a systematic analysis. PLoS One. 2014;9:e104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ministry of Health, Kenyan National Bureau of Statistics, Ministry of Health. Kenya STEPwise survey for non communicable diseases risk factors 2015 report. Public Health. 2015;5:8–210. [Google Scholar]
- [7].Mohamed SF, Mutua MK, Wamai R, et al. Prevalence, awareness, treatment and control of hypertension and their determinants: results from a national survey in Kenya. BMC Public Health 2018;18:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mecha JO, Kubo EN, Odhiambo CO, et al. Burden of pre-hypertension among adults in Kenya: a retrospective analysis of findings from the Healthy Heart Africa (HHA) programme. BMC Public Health. 2020;20:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Skeete J, Ramsey E, Battle S, et al. Sex-based differences in hypertension: understanding the trends. J Clin Hypertens. 2021;23:1129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemography Soc Biol. 2015;61:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ministry of Health, Kenya. National strategic plan for the prevention and control of non-communicable diseases (NCDs) 2021/22-2025/26. 2021. Available at: https://www.health.go.ke/wp-content/uploads/2021/07/Kenya-Non-Communicable-Disease-NCD-Strategic-Plan-2021-2025.pdf [access date January 12, 2023].
- [12].Bloomfield GS, Hogan JW, Keter A, et al. Prevalence of hypertension and associated cardiovascular risk factors in an urban slum in Nairobi, Kenya: a population-based survey. BMC Public Health. 2014;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ministry of Health, Kenya. Kenya national guidelines for cardiovascular diseases management division of non-communicable diseases. Ministry of Health. 2018. Available at: www.health.go.ke/wp-content/uploads/2018/06/Cardiovascular-guidelines-2018_A4_Final.pdf [access date January 12, 2023].
- [14].World Health Organization. Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. World Heal Organ. 2016. Available at: https://apps.who.int/iris/handle/10665/251655 [access date December 25, 2022]. [PubMed] [Google Scholar]
- [15].Dzudie A, Rayner B, Ojji D, et al. Roadmap to achieve 25% hypertension control in Africa by 2025. Glob Heart. 2018;13:45–59. [DOI] [PubMed] [Google Scholar]
- [16].Kariithi E, Sharma M, Kemunto E, et al. Using assisted partner services for HIV testing and the treatment of males and their female sexual partners: protocol for an implementation science study. JMIR Res Protoc. 2021;10:e27262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Whelton PK, Williams B. The 2018 European society of cardiology/European society of hypertension and 2017 American college of cardiology/American heart association blood pressure guidelines: more similar than different. JAMA. 2018;320:1749–50. [DOI] [PubMed] [Google Scholar]
- [18].Mosha NR, Mahande M, Juma A, et al. Prevalence, awareness and factors associated with hypertension in North West Tanzania. Glob Health Action. 2017;10:1321279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Drain PK, Hong T, Hajat A, et al. Integrating hypertension screening at the time of voluntary HIV testing among adults in South Africa. PLoS One. 2019;14:e02101611–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–97. [DOI] [PubMed] [Google Scholar]
- [21].Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- [22].Njuguna B, Vorkoper S, Patel P, et al. Models of integration of HIV and non-communicable disease care in sub-Saharan Africa: lessons learned and evidence gaps. AIDS. 2018;32:S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vedanthan R, Kamano JH, DeLong AK, et al. Community health workers improve linkage to hypertension care in Western Kenya. J Am Coll Cardiol. 2019;74:1897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rachlis B, Naanyu V, Wachira J, et al. Community perceptions of community health workers (CHWS) and their roles in management for HIV, tuberculosis and hypertension in Western Kenya. PLoS One. 2016;11:e01494121–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vedanthan R, Kamano JH, Naanyu V, et al. Optimizing linkage and retention to hypertension care in rural Kenya (LARK hypertension study): study protocol for a randomized controlled trial. Trials. 2014;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Siedner MJ, Baisley K, Orne-Gliemann J, et al. Linkage to primary care after home-based blood pressure screening in rural KwaZulu-Natal, South Africa: a population-based cohort study. BMJ Open. 2018;8:e023369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Naanyu V, Vedanthan R, Kamano JH, et al. Barriers influencing linkage to hypertension care in Kenya: qualitative analysis from the LARK hypertension study. J Gen Intern Med. 2016;31:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]