Abstract
Introduction
Gastric cancer, a significant public health concern, remains one of the most challenging malignancies to treat effectively. In the United States, survival rates for gastric cancer have historically been low, partly due to late-stage diagnosis and disparities in access to care. The Affordable Care Act (ACA) sought to address such disparities by expanding healthcare coverage and improving access to preventive and early treatment services.
Objective
This study aims to determine the causal effects of the ACA's implementation on gastric cancer survival rates, focusing on a comparative analysis between two distinct U.S. states: New Jersey, which fully embraced ACA provisions, and Georgia, which has not adopted the policy, as of 2023.
Methods
In this retrospective analysis, we utilized data from the Surveillance, Epidemiology, and End Results Program (SEER) registry to assess the impact of the ACA on cancer-specific survival (CSS) among gastric cancer patients. The study spanned the period from 2000 to 2020, divided into pre-ACA (2000-2013) and post-ACA (2016-2020) periods, with a two-year washout (2013-2015). We compared Georgia (a non-expansion state) to New Jersey (an expansion state since 2014) using a Difference-in-Differences (DiD) approach. We adjusted for patient demographics, income, metropolitan status, disease stage, and treatment modalities.
Results
Among 25,061 patients, 58.7% were in New Jersey (14,711), while 41.3% were in Georgia (10,350). The pre-ACA period included 18,878 patients (40.0% in Georgia and 60.0% in New Jersey), and 6,183 patients were in the post-ACA period (45.2% in Georgia and 54.8% in New Jersey). The post-ACA period was associated with a 20% reduction in mortality hazard among gastric cancer patients, irrespective of the state of residence (HR = 0.80, 95% CI: 0.73-0.88). Patients who were residents of New Jersey experienced a 12% reduction in mortality hazard compared to those who resided in Georgia in the post-ACA period (HR = 0.88, 95% CI: 0.78-0.99). Other factors linked to improved survival outcomes included surgery (OR = 0.30, 95% CI: 0.28-0.34) and female gender (OR=0.83, 95% CI: 0.76-0.91).
Conclusion
The study underscores the ACA's potential positive impact on CSS among gastric cancer patients, emphasizing the importance of healthcare policy interventions in improving patient outcomes.
Keywords: causal inference, difference-in-difference analysis, medicaid expansion, cancer-specific survival (css), affordable care act, gastric cancer
Introduction
Gastric cancer, also known as stomach cancer, poses a significant challenge in oncology, accounting for about 1.5% of all new cancer cases annually in the United States [1]. The American Cancer Society estimates that in 2024, there will be approximately 26,890 new cases (16,160 in men and 10,730 in women) and 10,880 deaths (6,490 men and 4,390 women) due to gastric cancer [1]. Characterized by a high mortality rate and often diagnosed at a late stage, survival rates for gastric cancer are distressingly low, with a five-year relative survival rate of 33% to 36% for all stages of gastric cancer combined [2,3]. In addition, the economic impact is also substantial, with the cost of care for gastric cancer in the United States estimated at $2.31 billion in 2024 [4]. These figures underscore the urgent need for improved detection and treatment strategies.
Significant disparities in gastric cancer incidence, treatment, and survival are evident, particularly among racial and ethnic minorities and individuals from lower socioeconomic backgrounds [4-6]. These disparities highlight a critical gap in our healthcare system's ability to provide equitable cancer care.
The Affordable Care Act (ACA), enacted in 2010, represents a landmark effort to expand healthcare access, improve health outcomes, and reduce healthcare disparities across the United States. [7,8]. While the ACA has broadly impacted healthcare, leading to earlier cancer diagnoses, increased access to treatment, and improved survival rates among cancer patients [9-11], its effects on specific cancer types, especially gastric cancer, have not been thoroughly investigated.
These disparities highlight a critical gap in our healthcare system's ability to provide equitable cancer care. Despite the potential of the ACA, particularly its Medicaid expansion component [12], to address these disparities by enhancing healthcare access for underserved populations [13-15], the specific impact of the ACA on gastric cancer survival rates remains underexplored. This study gap is significant because understanding the ACA's effects on gastric cancer can inform future policy interventions aimed at reducing healthcare disparities and improving outcomes for a cancer type that disproportionately affects vulnerable populations.
This study aims to assess the causal effects of the ACA's implementation on gastric cancer survival rates through a Difference-in-Difference (DiD) analysis of two U.S. states: New Jersey, which fully embraced ACA provisions, and Georgia, which has not adopted the policy as of 2023. By comparing gastric cancer survival rates before and after the ACA's implementation between these states, this research seeks to illuminate the potential role of the ACA in mitigating disparities in gastric cancer care and outcomes. This approach will fill a significant research gap and contribute to a more nuanced understanding of how healthcare policy can influence cancer survival rates in the United States.
Materials and methods
Study design
This study employs a retrospective cohort design using Surveillance, Epidemiology, and End Results (SEER) registry data [16] to evaluate the impact of the ACA on cancer-specific survival (CSS) among gastric cancer patients. SEER is an authoritative source of information on cancer incidence and survival in the United States and includes demographic information, primary tumor site, tumor morphology, stage at diagnosis, first course of treatment, and follow-up for vital status, covering approximately 34.6% of the U.S. population. The analysis spans from 2000 to 2020, segmented into pre-ACA (2000-2013) and post-ACA (2014-2020) periods.
Study population
The study population consists of patients diagnosed with gastric cancer. We used the third edition of the International Classification of Diseases for Oncology (ICD-O-3), which categorizes gastric cancer as C16.0-C16.9, a classification validated by the SEER database. Patients were included if diagnosed within the study periods and were either Georgia or New Jersey residents. Georgia represents a non-expansion state, while New Jersey is an expansion state having implemented Medicaid expansion under the ACA in 2014. The selection of these states facilitated a comparative analysis of the impact of Medicaid expansion on gastric cancer outcomes.
Data collection
Data were extracted from the SEER 17 Registries Database. The SEER*Stat Database, released in April 2023, contains cancer incidence data from 17 registries covering 2000-2020, linked to county attributes from 1990 to 2021. Managed by the National Cancer Institute, this database provides insights into cancer trends and disparities across U.S. counties. Variables of interest included patient demographics (age, race, marital status), socioeconomic status indicators (household median income), clinical characteristics (disease stage at presentation), treatment modalities (surgery, chemotherapy), and outcomes, including disease stage at presentation and CSS.
Variable of interest
The implementation of the ACA was operationalized as the categorical variable representing two time periods, pre-ACA (2000-2013) and post-ACA (2016-2020), with a two-year washout period (2013-2015) to account for the transitional phase post-ACA implementation. Additionally, the interaction between the ACA implementation and the expansion status (Georgia vs. New Jersey) was examined to assess the differential impact of Medicaid expansion on gastric cancer outcomes across states.
Primary outcomes of interest
These include the disease stage at presentation and CSS.
Disease Stage at Presentation
One of the primary outcomes of interest was the disease stage at the time of gastric cancer diagnosis. The disease stage was categorized into three groups: localized (confined to the stomach), regional (spread to nearby lymph nodes or tissues), and distant metastasis (spread to distant organs or tissues).
CSS
The CSS was a crucial indicator of long-term prognosis and treatment effectiveness among cancer patients. The CSS was calculated using a Cox regression analysis. The Cox regression, also known as the proportional hazards model, is a statistical technique used to examine the association between the time until an event occurs (cancer-specific mortality) and various predictor variables (states stratified by expansion status (Georgia vs. New Jersey) and period (pre-ACA vs. post-ACA).
Covariates
Demographics, including age at diagnosis, gender, marital status, and race/ethnicity (classified as non-Hispanic White, non-Hispanic Black, Hispanic, and Others), were covariates. Socioeconomic status was assessed using the patient household median income, categorized as <$70,000 and ≥$70,000, and the metropolitan status of the area of residence. Clinical variables such as stage at diagnosis (localized, regional, distant metastasis) and initial treatment modalities (surgery, chemotherapy, radiotherapy) were also considered.
Statistical analysis
Descriptive statistics, including means, medians, and standard deviations, were used to summarize the characteristics of the study population. Specifically, we utilized Chi-square tests to examine the distribution of categorical variables across pre- and post-ACA periods. To quantify differences in gastric cancer outcomes between Georgia and New Jersey, a DiD analysis was conducted. This analysis compared changes in disease stage at presentation and CSS before and after ACA implementation, both within and between the states, while adjusting for covariates such as patients' age, race, income, disease stage, and treatment modalities. Additionally, we used margins analysis to calculate the predicted probabilities, which enhances the interpretation of the interaction term by illustrating the probabilities of each outcome across states and periods, thereby providing a clearer understanding of the impact. Statistical significance was assessed using two-tailed tests with a predetermined alpha level of p < 0.05. All analyses were performed using the STATA 16 statistical software package (StataCorp LLC, College Station, TX, USA).
Results
Table 1 highlights the baseline characteristics of the study population (N = 25,061), which showed significant differences between pre- and post-ACA implementation across several variables. The pre-ACA period spanned from 2000 to 2013, and the post-ACA period extended from 2014 to 2020. The ACA became fully operational in New Jersey in January 2014. The average age of the population was slightly lower post-ACA (64.9 ± 1.0 years) compared to pre-ACA (65.2 ± 12.5 years). There was a higher percentage of patients from Georgia in the post-ACA period (45.2%) compared to pre-ACA (40.0%), while New Jersey saw a decrease from 60.0% to 54.8% (p < 0.001). The racial composition also changed significantly, with an increase in Hispanic patients (12.8% vs. 9.3%) and a significant drop in non-Hispanic whites post-ACA (61.2% vs. 54.9%). The disease stage at presentation showed a higher percentage of localized cases post-ACA (36.4% vs. 32.0%) and fewer regional cases (26.3% vs. 31.7%, p < 0.001). Income distribution remained similar. Marital status changes included increased single and married individuals post-ACA (p < 0.001). The use of chemotherapy increased post-ACA (48.1% vs. 39.8%, p < 0.001), while surgery and radiation saw slight decreases, respectively.
Table 1. Baseline characteristics of the study population.
Note: Summary statistics are mean ± standard deviation (SD), or n (%)
ACA: Affordable Care Act
| Variable | Total population (N = 25,061) | Pre-ACA (n = 18,878) | Post-ACA (n = 6,183) | p-value |
| Age (years) | 65.1 ± 12.8 | 65.3 ± 12.9 | 64.6 ± 12.5 | <0.001 |
| States by policy implementation status | <0.001 | |||
| Georgia | 10,350 (41.3%) | 7,556 (40.0%) | 2,794 (45.2%) | |
| New Jersey | 14,711 (58.7%) | 11,322 (60.0%) | 3,389 (54.8%) | |
| Females | 10,057 (40.1%) | 7,558 (40.0%) | 2,499 (40.0%) | 0.596 |
| Race | <0.001 | |||
| White | 14,957 (59.7%) | 11,560 (61.2%) | 3,397 (54.9%) | |
| Black | 5,964 (23.8%) | 4,445 (23.6%) | 1,519 (24.6%) | |
| Hispanic | 2,555 (10.2%) | 1,762 (9.3%) | 793 (12.8%) | |
| Others | 1,585 (6.3%) | 1,111 (5.9%) | 474 (7.7%) | |
| Grade | <0.001 | |||
| Localized | 5,775 (33.4%) | 3,810 (32.0%) | 1,965 (36.4%) | |
| Regional | 5,190 (30.0%) | 3,770 (31.7%) | 1,420 (26.3%) | |
| Distant | 6,338 (36.6%) | 4,326 (36.3%) | 4,326 (36.3%) | |
| Household median income | 0.591 | |||
| < $70,000 | 12,484 (49.8%) | 9,385 (49.7%) | 3,099 (50.1%) | |
| ≥ $70,000 | 12,572 (50.2%) | 9,488 (50.3%) | 3,084 (49.9%) | |
| Marital status | <0.001 | |||
| Divorced | 1,898 (8.4%) | 1,393 (8.1%) | 505 (9.3%) | |
| Married | 13,026 (57.3%) | 9,856 (57.0%) | 3,170 (58.3%) | |
| Single | 3,473 (15.3%) | 2,506 (14.5%) | 967 (17.8%) | |
| Widowed | 4,342 (19.1%) | 3,544 (20.5%) | 798 (14.7%) | |
| Treatment | ||||
| Chemotherapy | 10,493 (41.9%) | 7,518 (39.8%) | 2,975 (48.1%) | <0.001 |
| Surgery | 11,775 (54.1%) | 9,096 (56.0%) | 2,679 (48.3%) | <0.001 |
| Radiation | 4,856 (19.6%) | 3,744 (20.1%) | 1,112 (18.3%) | <0.001 |
Table 2 is a Cox regression analysis predicting mortality hazard in individuals with gastric cancer in the study period. The interaction between the ACA implementation period and states based on the policy implementation status was substantive. Patients treated in New Jersey in the post-ACA implementation period of 2014-2020 had a reduced mortality hazard (HR = 0.881, 95% CI: 0.778-0.998, p = 0.047) compared to those treated in Georgia. Other significant predictors included staging, surgery, radiotherapy, gender, chemotherapy, and age. The mortality hazard was also significantly lower post-ACA implementation (HR = 0.800, 95% CI: 0.731-0.875, p < 0.001), irrespective of the states.
Table 2. Cox regression predicting the mortality hazards for patients with gastric cancer (SEER registry 2000-2020).
Reference is the distant metastasis at presentation
ACA: Affordable Care Act; CI: Confidence interval; SEER: Surveillance, epidemiology, and end results
| Variable | Hazard ratio | Standard error | z | p-value | Lower CI | Upper CI |
| Age (years) | 1.01 | 0.001 | 8.5 | <0.001 | 1.008 | 1.012 |
| Female | 0.905 | 0.026 | -3.48 | 0.001 | 0.856 | 0.958 |
| Race/ethnicity | ||||||
| White | Reference | |||||
| Black | 0.944 | 0.031 | -1.74 | 0.081 | 0.885 | 1.007 |
| Hispanic | 0.979 | 0.044 | -0.47 | 0.636 | 0.897 | 1.069 |
| Asian/Pacific Islanders | 0.877 | 0.049 | -2.34 | 0.019 | 0.786 | 0.979 |
| Others | 1.638 | 0.671 | 1.21 | 0.228 | 0.734 | 3.655 |
| Marital status | ||||||
| Divorced | Reference | |||||
| Married | 0.915 | 0.042 | -1.92 | 0.055 | 0.836 | 1.002 |
| Single | 1.099 | 0.059 | 1.76 | 0.079 | 0.989 | 1.221 |
| Widowed | 0.999 | 0.057 | -0.01 | 0.992 | 0.893 | 1.119 |
| Household median income | ||||||
| < $70,000 | Reference | |||||
| ≥ $70,000 | 1.026 | 0.03 | 0.9 | 0.369 | 0.97 | 1.086 |
| Disease stage at presentation | ||||||
| Regional | 5.126 | 0.237 | 35.33 | <0.001 | 4.682 | 5.613 |
| Distant | 8.395 | 0.407 | 43.88 | <0.001 | 7.634 | 9.232 |
| Treatment | ||||||
| Surgery | 0.371 | 0.012 | -29.48 | <0.001 | 0.348 | 0.397 |
| Radiotherapy | 1.083 | 0.035 | 2.48 | 0.013 | 1.017 | 1.154 |
| Chemotherapy | 0.664 | 0.021 | -12.87 | <0.001 | 0.624 | 0.707 |
| ACA implementation period | ||||||
| Pre-ACA implementation | Reference | |||||
| Post-ACA implementation | 0.8 | 0.037 | -4.87 | <0.001 | 0.731 | 0.875 |
| States by policy implementation status | ||||||
| Georgia | Reference | |||||
| New Jersey | 0.86 | 0.028 | -4.69 | <0.001 | 0.808 | 0.916 |
| Interaction between states and policy implementation status | ||||||
| Georgia X post-ACA period | Reference | |||||
| New Jersey X post-ACA period | 0.881 | 0.056 | -1.99 | 0.047 | 0.778 | 0.998 |
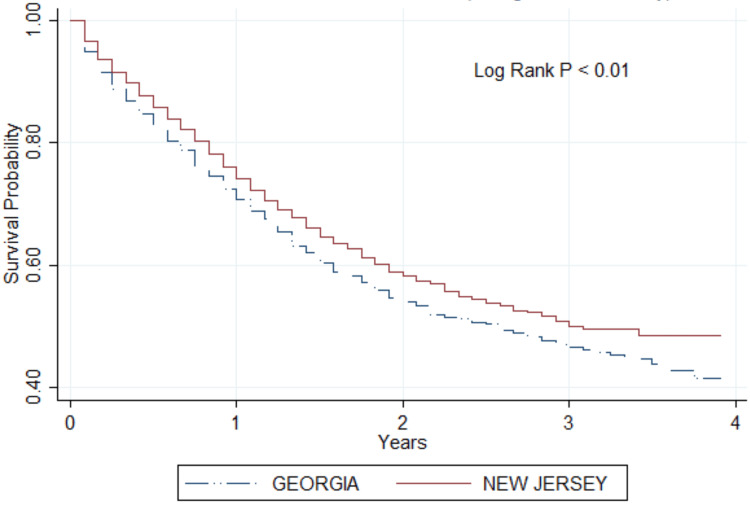
The Kaplan-Meier survival curve in Figure 1 illustrates the survival probabilities for gastric cancer patients in Georgia versus New Jersey in the post-ACA period. The graph indicates a consistent difference in survival between the two states, with New Jersey showing higher survival probabilities in the post-ACA period. At the end of a four-year follow-up period, the survival probability in New Jersey is notably higher than in Georgia. The Log Rank test, with a p-value less than 0.01, confirms that this difference is statistically significant.
Figure 1. Kaplan-Meier curve showing gastric cancer survival between Georgia and New Jersey in the post-ACA period.
p < 0.01 shows that the difference in survival between the two states in the post-ACA period is statistically significant
ACA: Affordable Care Act
The multinomial logistic regression analysis (Table 3) showed that individuals were more likely to present with localized gastric cancer in the post-ACA implementation period (RR ratio: 1.204, p = 0.025) compared to the pre-ACA period. The relative risk was lower for patients in New Jersey compared to Georgia (RR ratio: 0.847, p = 0.021). Additionally, there was a significant interaction effect between the state and policy implementation period, with individuals in New Jersey post-ACA being more likely to present with localized gastric cancer compared to those in Georgia post-ACA (RR ratio: 1.474, p = 0.001).
Table 3. Multinomial logistic regression predicting the relative risk of an individual presenting with localized gastric cancer (SEER registry 2000-2020).
Reference is the distant metastasis at presentation. Each relative risk ratio represents the risk of an individual presenting with localized disease versus distant metastasis
ACA: Affordable Care Act; CI: Confidence interval; SEER: Surveillance, epidemiology, and end results
| Variable | Relative risk ratio | Standard error | z | p-value | Lower CI | Upper CI |
| Age (years) | 1.007 | 0.002 | 2.86 | 0.004 | 1.002 | 1.011 |
| Female | 1.196 | 0.069 | 3.1 | 0.002 | 1.068 | 1.339 |
| Race/ethnicity | ||||||
| White | Reference | |||||
| Black | 0.889 | 0.06 | -1.73 | 0.083 | 0.778 | 1.015 |
| Hispanic | 0.898 | 0.082 | -1.17 | 0.241 | 0.75 | 1.075 |
| Asian/Pacific Islanders | 0.881 | 0.097 | -1.14 | 0.253 | 0.71 | 1.095 |
| Others | 0.392 | 0.368 | -1 | 0.319 | 0.062 | 2.469 |
| Marital status | ||||||
| Divorced | Reference | |||||
| Married | 0.923 | 0.087 | -0.85 | 0.396 | 0.766 | 1.111 |
| Single | 0.863 | 0.095 | -1.34 | 0.179 | 0.696 | 1.07 |
| Widowed | 0.873 | 0.102 | -1.16 | 0.247 | 0.694 | 1.098 |
| Household median income | ||||||
| < $70,000 | Reference | |||||
| ≥ $70,000 | 0.925 | 0.057 | -1.28 | 0.2 | 0.82 | 1.042 |
| Treatment | ||||||
| Surgery | 18.839 | 1.102 | 50.21 | <0.001 | 16.799 | 21.126 |
| Radiotherapy | 1.516 | 0.12 | 5.26 | <0.001 | 1.298 | 1.77 |
| Chemotherapy | 0.184 | 0.011 | -27.53 | <0.001 | 0.163 | 0.207 |
| ACA implementation period | ||||||
| Pre-ACA implementation | Reference | |||||
| Post-ACA implementation | 1.204 | 0.099 | 2.25 | 0.025 | 1.024 | 1.415 |
| States by policy implementation status | ||||||
| Georgia | Reference | |||||
| New Jersey | 0.847 | 0.061 | -2.31 | 0.021 | 0.735 | 0.975 |
| Interaction between states and policy implementation status | ||||||
| Georgia X post-ACA period | Reference | |||||
| New Jersey X post-ACA period | 1.474 | 0.168 | 3.41 | 0.001 | 1.179 | 1.843 |
The multinomial logistic regression analysis (Table 4) reveals that the ACA implementation period was associated with a decreased risk of individuals presenting with regional gastric cancer compared to the pre-ACA period (RR ratio: 0.789, p = 0.006). There was no significant difference in the risk between New Jersey and Georgia (RR ratio: 0.980, p = 0.777). However, the interaction between the state and policy implementation period indicates a higher risk of regional gastric cancer presentation in New Jersey post-ACA compared to Georgia post-ACA (RR ratio: 1.284, p = 0.032).
Table 4. Multinomial logistic regression predicting the relative risk of an individual presenting with regional gastric cancer (SEER registry 2000-2020).
Reference is the distant metastasis at presentation. Each relative risk ratio represents the risk of an individual presenting with localized disease versus distant metastasis
ACA: Affordable Care Act; CI: Confidence interval; SEER: Surveillance, epidemiology, and end results
| Variable | Relative risk ratio | Standard error | z | p-value | Lower CI | Upper CI |
| Age (years) | 1.02 | 0.002 | 8.37 | <0.001 | 1.015 | 1.025 |
| Female | 0.929 | 0.054 | -1.25 | 0.21 | 0.828 | 1.042 |
| Race/ethnicity | ||||||
| White | Reference | |||||
| Black | 1.103 | 0.075 | 1.44 | 0.15 | 0.965 | 1.262 |
| Hispanic | 1.147 | 0.104 | 1.52 | 0.128 | 0.961 | 1.369 |
| Asian/Pacific Islanders | 1.017 | 0.112 | 0.15 | 0.878 | 0.819 | 1.263 |
| Others | 0.264 | 0.309 | -1.14 | 0.255 | 0.027 | 2.612 |
| Marital status | ||||||
| Divorced | Reference | |||||
| Married | 0.903 | 0.087 | -1.07 | 0.287 | 0.748 | 1.09 |
| Single | 1.051 | 0.117 | 0.45 | 0.656 | 0.845 | 1.307 |
| Widowed | 1.054 | 0.126 | 0.44 | 0.657 | 0.835 | 1.332 |
| Household median income | ||||||
| < $70,000 | Reference | |||||
| ≥ $70,000 | 1.081 | 0.065 | 1.29 | 0.196 | 0.96 | 1.217 |
| Treatment | ||||||
| Surgery | 19.78 | 1.147 | 51.49 | <0.001 | 17.656 | 22.16 |
| Radiotherapy | 3.735 | 0.247 | 19.97 | <0.001 | 3.282 | 4.251 |
| Chemotherapy | 1.129 | 0.069 | 2 | 0.046 | 1.002 | 1.272 |
| ACA implementation period | ||||||
| Pre-ACA implementation | Reference | |||||
| Post-ACA implementation | 0.789 | 0.068 | -2.77 | 0.006 | 0.667 | 0.933 |
| States by policy implementation status | ||||||
| Georgia | Reference | |||||
| New Jersey | 0.98 | 0.069 | -0.28 | 0.777 | 0.853 | 1.126 |
| Interaction between states and policy implementation status | ||||||
| Georgia X post-ACA period | Reference | |||||
| New Jersey X post-ACA period | 1.284 | 0.149 | 2.15 | 0.032 | 1.022 | 1.612 |
Table 5 shows the predictive margins following a multinomial logistic regression conducted to determine the impact of the policy implementation on the disease stage at presentation. The predicted probabilities reveal significant variations in the probability of disease stage at presentation among gastric cancer patients, based on the interaction between the ACA implementation period and states' policy implementation status. Patients treated in New Jersey post-ACA implementation had the highest predicted probability of presenting with localized disease (36.1%, 95% CI: 34.1%-38.1%, p < 0.001) compared to those in Georgia in the post-ACA period (33.1%, 95% CI: 31.1%-35.2%, p < 0.001). Conversely, the probability of presenting with distant disease was relatively stable in New Jersey post-ACA (36.6%, 95% CI: 34.6%-38.6%, p < 0.001) compared to the pre-ACA period (36.7%, 95% CI: 35.4%-38.0%, p < 0.001). In Georgia, however, the predicted probability of presenting at the late stages of the disease slightly increased from 37.9% (95% CI: 36.4%-39.4%, p < 0.001) to 39.5% (95% CI: 37.4%-41.7%, p < 0.001) in the post-ACA period.
Table 5. Predicted probabilities of disease stages at presentation for patients with gastric cancer (SEER registry 2000-2020).
The predicted probabilities were generated from a multinomial logistic regression after adjusting for patients' age, sex, race/ethnicity, marital status, and household median income
ACA: Affordable Care Act; CI: Confidence interval; SEER: Surveillance, epidemiology, and end results
| Variable | Margin | Standard error | z | p-value | Lower CI | Upper CI |
| Localized | ||||||
| Georgia (pre-ACA) | 0.306 | 0.007 | 42.12 | <0.001 | 0.292 | 0.32 |
| Georgia (post-ACA) | 0.331 | 0.01 | 31.9 | <0.001 | 0.311 | 0.352 |
| New Jersey (pre-ACA) | 0.3 | 0.006 | 46.92 | <0.001 | 0.288 | 0.313 |
| New Jersey (post-ACA) | 0.361 | 0.01 | 35.3 | <0.001 | 0.341 | 0.381 |
| Regional | ||||||
| Georgia (pre-ACA) | 0.315 | 0.007 | 42.32 | <0.001 | 0.301 | 0.33 |
| Georgia (post-ACA) | 0.273 | 0.01 | 27.46 | <0.001 | 0.254 | 0.293 |
| New Jersey (pre-ACA) | 0.333 | 0.007 | 50.65 | <0.001 | 0.32 | 0.346 |
| New Jersey (post-ACA) | 0.273 | 0.009 | 29.16 | <0.001 | 0.255 | 0.292 |
| Distant metastasis | ||||||
| Georgia (pre-ACA) | 0.379 | 0.008 | 49.22 | <0.001 | 0.364 | 0.394 |
| Georgia (post-ACA) | 0.395 | 0.011 | 36.46 | <0.001 | 0.374 | 0.417 |
| New Jersey (pre-ACA) | 0.367 | 0.007 | 54.52 | <0.001 | 0.354 | 0.38 |
| New Jersey (post-ACA) | 0.366 | 0.01 | 35.71 | <0.001 | 0.346 | 0.386 |
Discussion
Our study found a substantive association between the implementation of the ACA and higher odds of early disease stage at presentation and higher survival (reduced mortality hazards) among individuals diagnosed with gastric cancers in New Jersey. While the mortality hazard decreased significantly post-ACA implementation in New Jersey and Georgia, the most substantial reduction was observed in New Jersey. Patients in New Jersey post-ACA were also more likely to present with localized disease compared to those in Georgia post-ACA.
Our findings align with several studies that have documented improved cancer outcomes following the implementation of the ACA [15,17-19]. For instance, Jemal et al. [16] found that Medicaid expansion under the ACA was associated with increased early-stage diagnosis and reduced mortality in various cancers. Our results are consistent with these findings, particularly in the context of localized disease presentation and survival improvement in New Jersey.
However, other studies have reported mixed outcomes regarding the ACA's impact. A study by Salazar et al. [20] did not find significant differences in cancer survival rates between Medicaid expansion and non-expansion states. The discrepancies between our findings and those of Salazar et al. could be attributed to differences in study populations, cancer types examined, and methodological approaches [21].
Mechanisms and implications
The observed improvement in survival and early disease presentation in New Jersey post-ACA can be attributed to several mechanisms. The ACA facilitated increased insurance coverage, particularly through Medicaid expansion, which likely improved access to healthcare services [7,22]. This improved access may have led to earlier diagnosis and timely treatment, crucial factors in cancer prognosis. Additionally, New Jersey's healthcare infrastructure and policies may have better leveraged ACA provisions, enhancing patient outcomes.
The ACA's emphasis on preventive care and early detection programs [23-25] may also explain the higher rates of localized disease presentation in New Jersey post-ACA. Enhanced access to regular screenings and primary care services could lead to earlier detection of gastric cancer, which is typically more treatable in its early stages.
Future direction
While our study provides valuable insights into the positive impacts of the ACA, further research is needed to explore the long-term effects of the policy on various cancers across different states. Future studies should consider longitudinal designs to assess the sustained impact of the ACA on cancer outcomes. Additionally, research should investigate the specific components of the ACA that are most effective in improving cancer prognosis, which could inform future health policies.
Comparative studies between states with different levels of Medicaid expansion and healthcare infrastructure could also shed light on the contextual factors that enhance or hinder the ACA's effectiveness. Understanding these nuances will be crucial for policymakers aiming to optimize cancer care and outcomes across diverse populations.
Limitations and strengths
This study, which utilizes the SEER registry for a DiD analysis, has several limitations. First, the SEER database may not capture all relevant variables, such as detailed socioeconomic factors or individual health behaviors, which could confound the results. Second, variations in healthcare access and quality within states might not be fully accounted for, potentially affecting the generalizability of the findings. Lastly, the study period may not be long enough to capture the full impact of the ACA on long-term survival outcomes.
Despite these limitations, the study has notable strengths. The large, population-based SEER registry provides a robust sample size and comprehensive cancer data, enhancing the study's statistical power and external validity. The DiD design effectively controls for temporal trends, allowing for a clearer assessment of the ACA's impact. These strengths contribute to a meaningful evaluation of policy effects on cancer outcomes.
Conclusions
Our study demonstrates that the ACA has had a significant positive impact on gastric cancer outcomes, particularly in states like New Jersey that fully embraced Medicaid expansion. The increased likelihood of early-stage disease presentation and improved survival rates highlight the importance of accessible healthcare services. As health policies continue to evolve, it is imperative to build on these findings to ensure equitable and effective cancer care for all patients. Future research should continue to evaluate the long-term benefits of the ACA and identify strategies to further enhance cancer care delivery. We recommend that policymakers prioritize the continuation and expansion of Medicaid services, as our findings suggest that such policies significantly enhance early cancer detection and improve survival rates, thereby underscoring the critical role of sustained governmental support in healthcare.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Oluwasegun A. Akinyemi, Miriam Micheal, Terhas Asfiha Weldeslase, Mojisola E. Fasokun, Eunice Odusanya, Oluranti O. Babalola, Funmilola M. Belie, Oluwatobi Lasisi, Derek Ugwendum, Oluwatayo Awolumate
Acquisition, analysis, or interpretation of data: Oluwasegun A. Akinyemi, Terhas Asfiha Weldeslase, Oluwatayo Awolumate
Drafting of the manuscript: Oluwasegun A. Akinyemi, Miriam Micheal, Mojisola E. Fasokun, Eunice Odusanya, Oluranti O. Babalola, Funmilola M. Belie, Oluwatobi Lasisi, Derek Ugwendum, Oluwatayo Awolumate
Critical review of the manuscript for important intellectual content: Oluwasegun A. Akinyemi, Miriam Micheal, Terhas Asfiha Weldeslase, Mojisola E. Fasokun, Eunice Odusanya, Oluranti O. Babalola, Funmilola M. Belie, Oluwatobi Lasisi, Derek Ugwendum, Oluwatayo Awolumate
Supervision: Oluwasegun A. Akinyemi, Miriam Micheal, Mojisola E. Fasokun
References
- 1.Impact of Medicaid expansion on pregnancy outcomes among women with gestational diabetes. Akinyemi O, Ogundare T, Fasokun M, et al. Int J Gynaecol Obstet. 2024;165:519–525. doi: 10.1002/ijgo.15439. [DOI] [PubMed] [Google Scholar]
- 2.The impact of the affordable care act on access to bariatric surgery in Maryland. Akinyemi OA, Weldeslase TA, Fasokun M, et al. Am J Surg. 2023 doi: 10.1016/j.amjsurg.2023.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Causal effects of the Affordable Care Act (ACA) implementation on non-Hodgkin’s lymphoma survival: a difference-in-differences analysis. Akinyemi OA, Weldeslase TA, Fasokun ME, et al. Cureus. 2024;16:0. doi: 10.7759/cureus.52571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evaluation of racial disparities in quality of care for patients with gastrointestinal tract cancer treated with surgery. Bakkila BF, Kerekes D, Nunez-Smith M, et al. JAMA Netw Open. 2022;5:0. doi: 10.1001/jamanetworkopen.2022.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer.Net. (2023. The affordable care act: how it helps people with cancer and their families. [ Apr; 2024 ]. 2023. https://www.cancer.net/navigating-cancer-care/financial-considerations/affordable-care-act-and-cancer https://www.cancer.net/navigating-cancer-care/financial-considerations/affordable-care-act-and-cancer
- 6.Association of the patient protection and affordable care act with insurance coverage for head and neck cancer in the SEER database. Cannon RB, Shepherd HM, McCrary H, et al. JAMA Otolaryngol Head Neck Surg. 2018;144:1052–1057. doi: 10.1001/jamaoto.2018.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center Center, C. U. I. M. (2021. Tackling disparities in stomach cancer. [ Apr; 2024 ]. 2021. https://www.cancer.columbia.edu/news/tackling-disparities-stomach-cancer https://www.cancer.columbia.edu/news/tackling-disparities-stomach-cancer
- 8.The effect of the affordable care act on cancer detection among the near-elderly. Duarte F, Kadiyala S, Kominski GF, Riveros A. Health Aff (Millwood) 2021;40:258–265. doi: 10.1377/hlthaff.2020.00369. [DOI] [PubMed] [Google Scholar]
- 9.Impact of the Affordable Care Act (ACA) Medicaid expansion on cancer admissions and surgeries. Eguia E, Cobb AN, Kothari AN, Molefe A, Afshar M, Aranha GV, Kuo PC. Ann Surg. 2018;268:584–590. doi: 10.1097/SLA.0000000000002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medicaid expansion increases survival for patients with cancer. Fillon M. CA Cancer J Clin. 2022;72:407–408. doi: 10.3322/caac.21751. [DOI] [PubMed] [Google Scholar]
- 11.Impact of the affordable care act on colorectal cancer screening, incidence, and survival in Kentucky. Gan T, Sinner HF, Walling SC, et al. J Am Coll Surg. 2019;228:342–353. doi: 10.1016/j.jamcollsurg.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association between Medicaid expansion under the Affordable Care Act and survival among newly diagnosed cancer patients. Han X, Zhao J, Yabroff KR, Johnson CJ, Jemal A. J Natl Cancer Inst. 2022;114:1176–1185. doi: 10.1093/jnci/djac077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HealthMatch. (2022. Stomach cancer statistics: survival rates and prevalence. [ Apr; 2024 ]. 2022. https://healthmatch.io/stomach-cancer/stomach-cancer-survival-rates-and-statistics https://healthmatch.io/stomach-cancer/stomach-cancer-survival-rates-and-statistics
- 14.The impact of Medicaid expansion on patients with cancer in the United States: a review. Hotca A, Bloom JR, Runnels J, Salgado LR, Cherry DR, Hsieh K, Sindhu KK. Curr Oncol. 2023;30:6362–6373. doi: 10.3390/curroncol30070469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute Institute, N. C. National cancer institute: stomach (gastric) cancer prevention (PDQ®)-health professional version. [ Apr; 2024 ]. 2024. https://www.cancer.gov/types/stomach/hp/stomach-prevention-pdq https://www.cancer.gov/types/stomach/hp/stomach-prevention-pdq
- 16.Changes in insurance coverage and stage at diagnosis among nonelderly patients with cancer after the Affordable Care Act. Jemal A, Lin CC, Davidoff AJ, Han X. J Clin Oncol. 2017;35:3906–3915. doi: 10.1200/JCO.2017.73.7817. [DOI] [PubMed] [Google Scholar]
- 17.The burden of stomach cancer mortality by county, race, and ethnicity in the USA, 2000-2019: a systematic analysis of health disparities. Kendrick P, Kelly YO, Baumann MM, et al. Lancet Reg Health Am. 2023;24:100547. doi: 10.1016/j.lana.2023.100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Changes in cancer mortality by race and ethnicity following the implementation of the Affordable Care Act in California. Martinez ME, Gomez SL, Canchola AJ, et al. Front Oncol. 2022;12:916167. doi: 10.3389/fonc.2022.916167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The ACA and cancer screening and diagnosis. Sabik LM, Adunlin G. Cancer J. 2017;23:151–162. doi: 10.1097/PPO.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baseline evaluation of cancer mortality in US states that expanded Medicaid vs nonexpansion states. Salazar MC, Kaminski MF, Canavan ME, Maduka RC, Li AX, Ermer T, Boffa DJ. JAMA Oncol. 2021;7:1394–1395. doi: 10.1001/jamaoncol.2021.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer statistics, 2017. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 22.Society Society, A. C. American cancer society: the affordable care act: how it helps people with cancer and their families. [ Apr; 2024 ]. 2023. https://www.cancer.org/cancer/financial-insurance-matters/health-insurance-laws/the-health-care-law.html https://www.cancer.org/cancer/financial-insurance-matters/health-insurance-laws/the-health-care-law.html
- 23.Society Society, A. C. (2024a. American cancer society: key statistics about stomach cancer. [ Apr; 2024 ]. 2024. https://www.cancer.org/cancer/types/stomach-cancer/about/key-statistics.html https://www.cancer.org/cancer/types/stomach-cancer/about/key-statistics.html
- 24.Society Society, A. C. (2024b. American cancer society: stomach cancer survival rates. [ Apr; 2024 ]. 2024. https://www.cancer.org/cancer/types/stomach-cancer/detection-diagnosis-staging/survival-rates.html https://www.cancer.org/cancer/types/stomach-cancer/detection-diagnosis-staging/survival-rates.html
- 25.Surveillance epidemiology and end result program. [ Apr; 2024 ]. 2024. https://seer.cancer.gov/ https://seer.cancer.gov/