Abstract
Endoscopic colorectal stenting has gained momentum over the last two decades as a viable alternative to surgical intervention in a subgroup of patients with colorectal disease. Stenting can be used as a temporizing bridge to surgical intervention or as a definitive treatment measure. Patient selection and the technical expertise of the endoscopist are of paramount importance to optimize the clinical outcome. Technical skills in therapeutic endoscopy and the choice of proper equipment including the consumables are required for the conduct of a safe and successful procedure. In this article, we share the lessons learned from a two-decade journey of the senior author with therapeutic endoscopy.
Keywords: colorectal stricture, colon obstruction, endoscopic stent, technical tips
Endoluminal stent interventions have revolutionized the field of medicine. Numerous disorders are now treated with stent placement and the spectrum of diseases encompasses the vascular system (neurovascular, cardiovascular, peripheral vascular) and the digestive tract (esophageal, biliary, enteric, colonic). Endoscopic colorectal stenting has gained momentum over the last two decades, and it has become a viable alternative for a select group of patients with colorectal disorders. 1 2 The most common indication for colorectal stenting is a malignant stricture with obstruction. 3 Traditionally patients presenting with acute malignant large bowel obstruction were treated with surgery, often requiring a diverting stoma. Significant proportions of stomas placed during emergency colorectal surgery remain for a prolonged period of time and/or are never reversed. Furthermore, emergency colorectal surgery carries significant risks for morbidity and some mortality, especially in the elderly.
Stent decompression of malignant obstruction is associated with several advantages compared with surgical intervention, including fewer complications, shorter length of stay, faster resumption of adjuvant or palliative chemotherapy when needed, and lower mortality. In addition, stent intervention is associated with a lower rate of subsequent surgical intervention and stoma formation. 1 2 Because of all the mentioned advantages, stent use is more cost-effective compared with surgical intervention.
Stenting in the setting of malignant obstruction is often performed as a definitive intervention in patients with extensive metastatic disease or in those who are high risk for surgical intervention due to severe medical comorbidities. In a subset of patients, stenting can be considered as a bridge to future intervention. 4 Bridging to definitive surgical intervention allows for medical optimization of the patient and provides an opportunity to decompress the colon for mechanical bowel preparation. Such prospect transitions the patient from the emergent to the elective setting, which is associated with fewer complications, lower stoma formation rate, and a higher rate of laparoscopic surgery.
With a growing experience with therapeutic endoscopy and the gradual introduction of new stent technologies and consumables, the use of stent has been used in some patients with benign disease. 5 While the overall technical and clinical success rate is lower in patients with benign conditions compared with those with malignant disease, properly selected patients can benefit from endoscopic stenting. Covered stents have been used successfully to treat complex colorectal fistulas and acute anastomotic complications as initially described by the senior author. 6 7
The aim of endoluminal therapy is to successfully resolve or temporize the patient's condition. As in any endoscopic or surgical intervention, three factors determine the outcome: patient-related features, disease characteristics, and type of intervention. Outcome measures include short- and long-term results. From the standpoint of endoscopic stenting, two outcome measures have been defined to determine the effectiveness of the procedure: technical and clinical success rates. Technical success is defined as the ability to successfully carry out the procedure without complications. Clinical success entails both the short- and long-term desirable outcome. The technical skills and expertise of the endoscopist do impact both the technical and clinical outcome of endoscopic interventions. Other predictors of outcome include disease- and patient-related factors.
The main aim of this article is to provide the reader with knowledge pertaining to the technical aspect of endoscopic stenting based on lessons learned during the therapeutic endoscopist journey of the senior author of over two decades. 8 The accumulated knowledge was derived from successful stenting in a large number of patients but more importantly the lessons learned from the encountered failures, some of which are illustrated in this article. The short- and long-term results of stenting as well as other aspects of endoluminal stenting are beyond the scope of this article. The reader is referred to the enclosed bibliography for additional reading.
Indication and Patient Selection
The main indication for endoscopic colorectal stenting is decompression of malignant large bowel obstruction. The use of stenting for benign disease is more limited, but it will continue to evolve as there is an increasing interest in the use of covered stents in patients with acute anastomotic complications and benign strictures. The type of malignancy and morphology of the stricture can impact outcome. Stent usage should be reserved for symptomatic patients who have imaging and endoscopic findings suggestive of a high-grade stricture with near or complete obstruction ( Fig. 1 ). While some clinicians advocate the use of stent prophylactically in patients without obstruction, we do not support this recommendation as it is not clinically indicated in most cases. Such patients can respond to chemotherapy and rarely progress to complete obstruction ( Fig. 2A and B ).
Fig. 1.
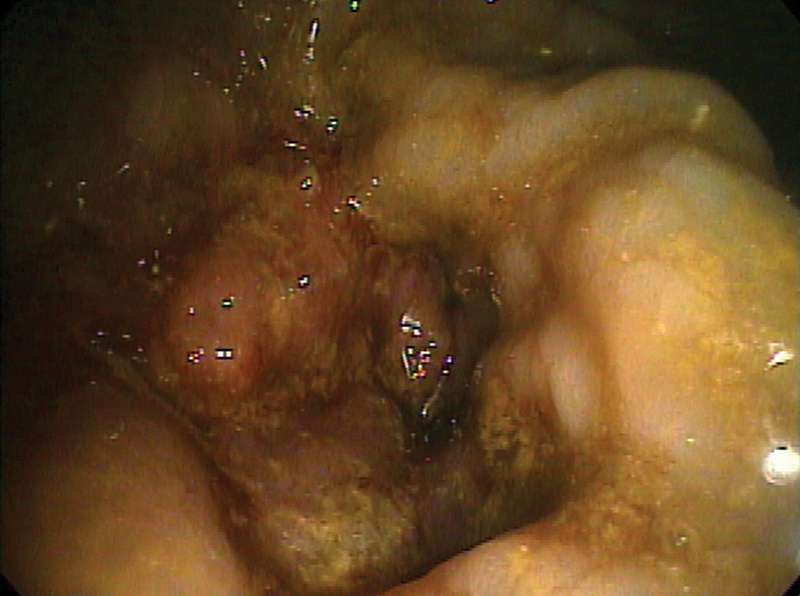
An obstructing sigmoid carcinoma with significant luminal narrowing. The patient is a good candidate for stent placement.
Fig. 2.
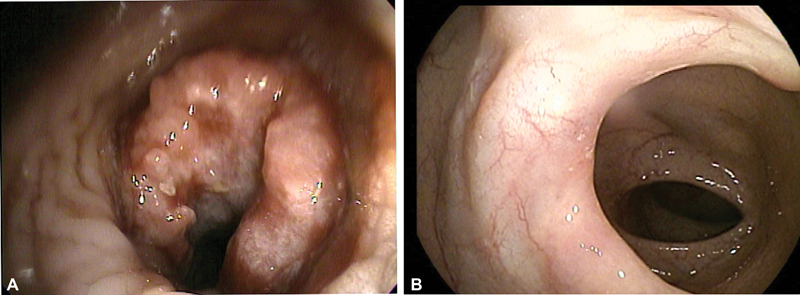
( A ) Endoscopic view of patient with descending colon carcinoma. Despite a bulky tumor, the lumen is large enough to be traversed with a pediatric colonoscope. Such patient does not require endoscopic stent as rarely there is local progression of disease while on chemotherapy. ( B ) Endoscopic view of the same patient in (A) showing significant response of the primary tumor site after 6 months of chemotherapy.
Stenting is most effective for primary colorectal malignancy and least effective in patients with extracolonic malignancies such as ovarian, urologic, or hepatobiliary. Patients with diffuse carcinomatosis and long strictures have lower technical and clinical success rates ( Fig. 3A and B ). While long strictures can be adequately decompressed with one or more stents, they carry lower success rate from a functional standpoint due to the lack of colonic fecal propulsion in the stented segment. Technical success with stent deployment can be achieved in such patients, but failure can be the end result with persistent functional obstruction ( Fig. 4 ). The ideal length of stricture based on personal experience is 6 cm or less ( Fig. 5 ). In particular, long fibrotic strictures in a previously operated or radiated pelvis are difficult to stent technically and are often associated with lack of expansion and/or kinking of the stent. This is due to the extraluminal fibrotic field outside the large bowel lumen.
Fig. 3.
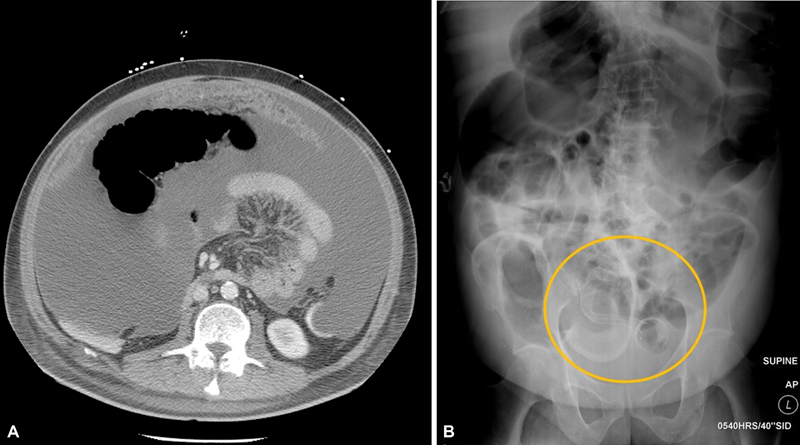
( A ) Computed tomography scan of patient with ascites and omental caking from pancreatic cancer with carcinomatosis. ( B ) Poststent placement abdominal radiograph in the same patient with pancreatic cancer with carcinomatosis shows persistent obstruction due to lack of stent expansion with kinking (circle).
Fig. 4.
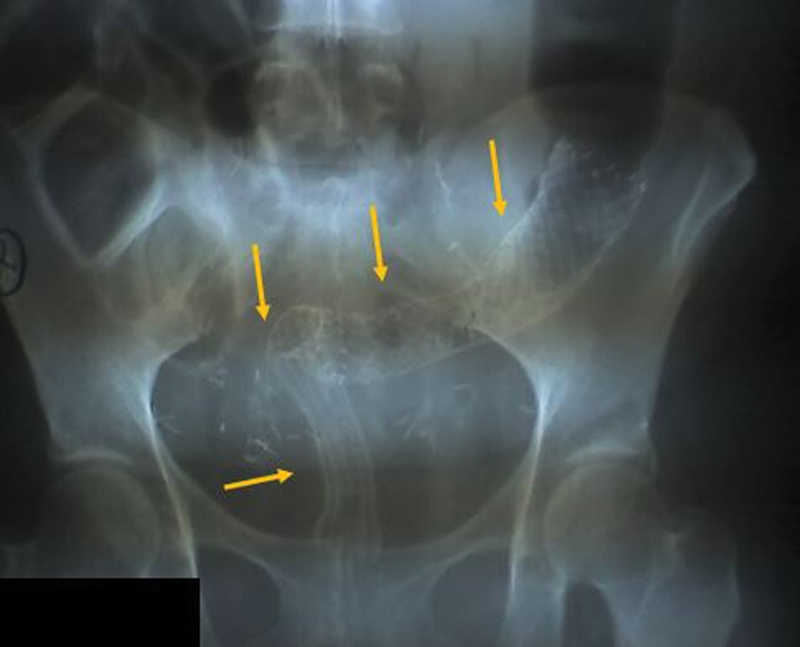
Abdominal radiograph of a patient with recurrent cervical cancer with long stricture treated with two tandem stents (arrows). Despite good positioning of the stents, the patient experienced persistent functional obstruction.
Fig. 5.
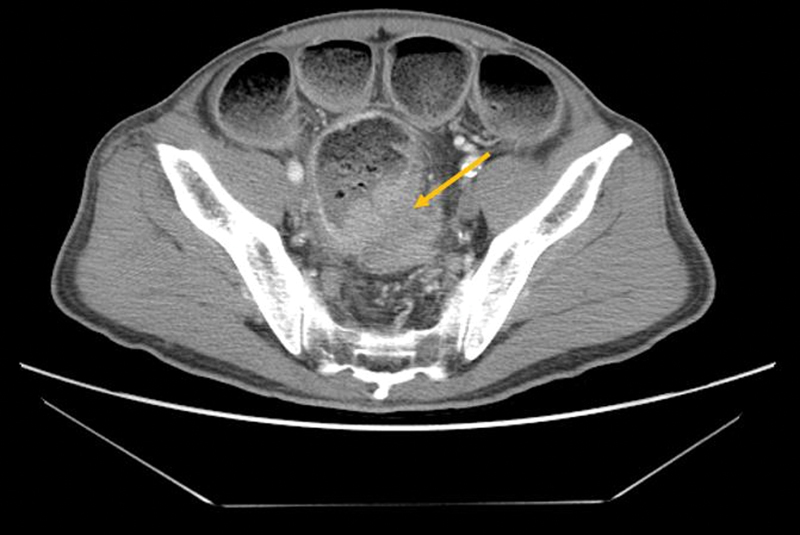
Computer tomography scan shows acute colonic obstruction from a rectosigmoid apple core lesion with pinpoint lumen (arrow). Short malignant strictures are ideal for stent placement.
Due to the concerns of worse oncologic outcome in patients with localized colorectal malignancy who are candidates for a curative resection, endoscopic stenting is not advisable as a bridge to a future surgical intervention. The main concern is systemic dissemination of tumor cells during the endoscopic manipulation of the malignancy. While the risk of endoscopic perforation is low during stent deployment, tumor perforation with intraperitoneal seeding during stenting can lead to a worse oncologic outcome. Patients who present with a primary malignant large bowel obstruction without evidence of distal metastatic disease on cross sectional imaging should be considered for resection with or without anastomosis and/or fecal diversion based on the surgeon's judgement. A bridge to surgical intervention in potentially curable patients should be considered only in high-risk patients such as malnourished patients or those with a recent cardiovascular or cerebrovascular event. Furthermore, patients who present with acute large bowel obstruction due to a benign stricture (such as in diverticulitis or inflammatory bowel disease) can be considered for a stent as a bridge to surgical intervention ( Fig. 6 ). Stenting in such patients allows for bowel preparation and nutritional optimization and shifting the operation to the elective setting.
Fig. 6.
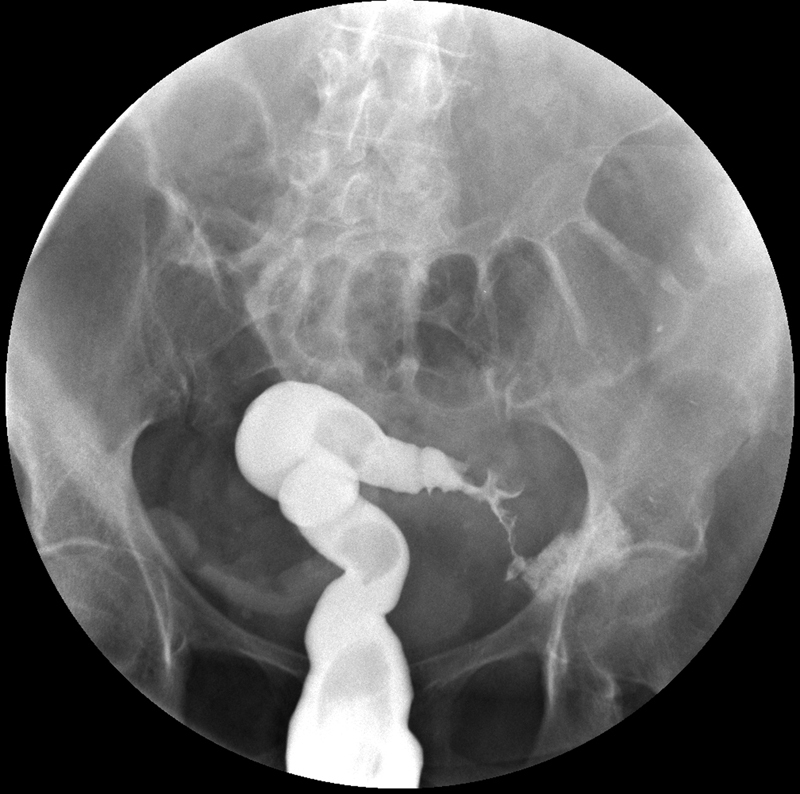
Gastrografin enema demonstrates a sigmoid stricture in a patient with long-standing history of ulcerative colitis.
Successful stent deployment can be performed in both the proximal as well as distal large bowel with few exceptions. Current stent delivery devices allow for stenting of lesions on the right or left side. Any lesion in the colon can be stented except for a distal rectal mass. To avoid the risk of stent migration and/or tenesmus, ideally 2 cm of normal rectum is required beyond the distal aspect of the stent. As a general rule, a rectal mass that is easily palpable with the examining finger is not amenable to stent deployment. Splenic flexure lesions are particularly difficult to stent due to sharp angulation leading to a higher risk for stent migration, kinking, and/or perforation ( Figs. 7 and 8 ). While an endoscopic stent procedure can be considered for such lesions, it is important to counsel the patient properly about a higher failure rate and the need for surgical intervention. Finally, patients with severe abdominal distention with evidence of localized or diffuse peritonitis are not candidates for endoscopic stenting.
Fig. 7.
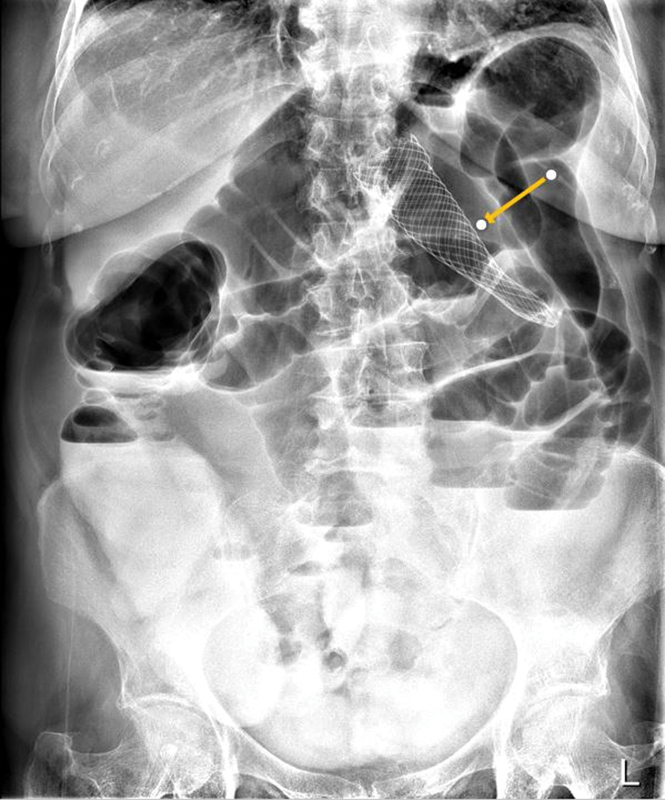
Abdominal radiograph shows proximal stent migration into the transverse colon in a patient with a splenic flexure lesion (arrow). Persistent large bowel obstruction is noted.
Fig. 8.
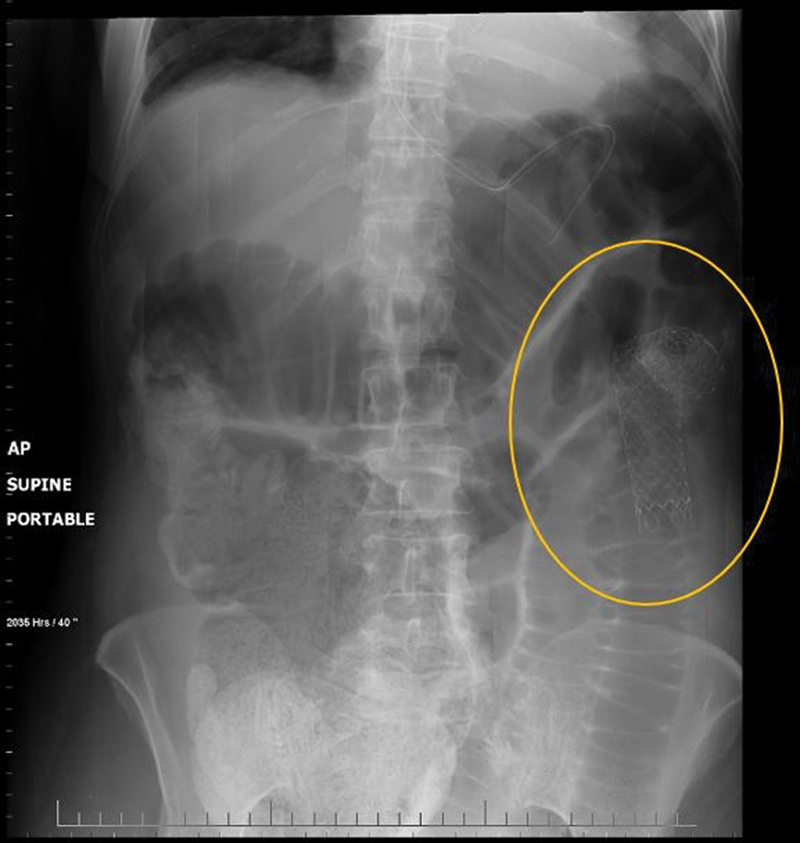
Abdominal radiograph shows stent kinking with persistent large bowel obstruction in a patient with a lesion in a redundant splenic flexure (circle).
Patient Preparation
A contrast-based imaging study is advisable in all patients who are evaluated for stent placement. Imaging serves two purposes: determine the degree of obstruction and provide invaluable information about the stricture. Gastrografin enema or computed tomography scan with rectal contrast can be helpful in localizing the lesion, characterizing the morphology of the stricture (length and degree of obstruction) and providing some information about the colon anatomical configuration distal to the obstruction ( Fig. 9 ). Imaging findings assist in determining whether a patient is eligible for stent decompression and aids in the choice of stent and delivery device. Strictures with a diameter < 1 cm, especially those with evidence of proximal large bowel dilation, are good candidates for endoscopic stenting.
Fig. 9.
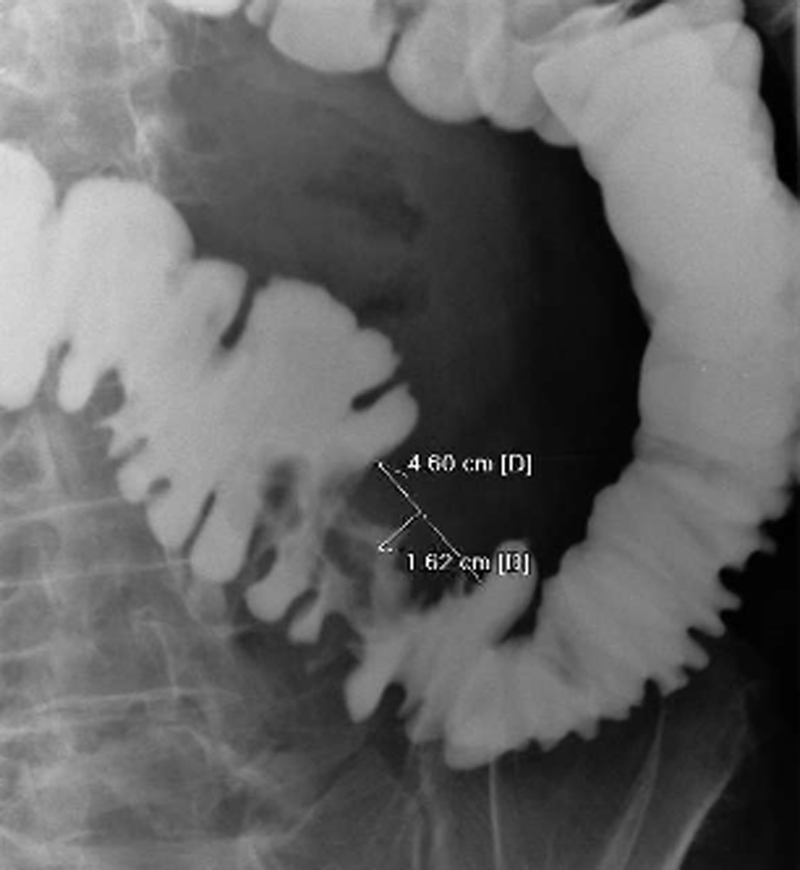
Gastrografin enema demonstrates the morphologic features of a sigmoid malignant stricture.
All patients should be counseled regarding both short- and long-term risks of stent placement including perforation, erosion, migration, impaction, and the potential need for additional endoscopic and/or surgical intervention ( Fig. 10A and B ). Informed consent should be obtained for both the endoscopic procedure and possible surgical intervention if required. Bowel preparation should be tailored to the patient's condition. In the setting of complete obstruction, two rectal enemas (saline or phosphate-based enema) are advisable to clear the rectosigmoid area. Oral bowel preparation (standard colonoscopy regimen) is recommended in patients who are passing bowel movements without significant large bowel dilation. This can be performed in patients who do not have nausea, vomiting, severe abdominal distension and do not have radiographic evidence of a dilated colon.
Fig. 10.
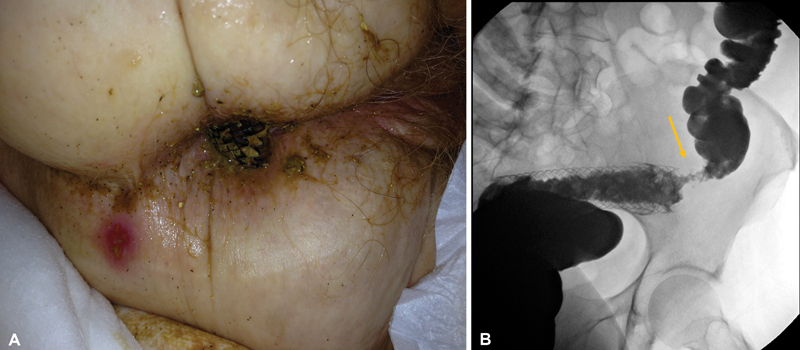
( A ) Gastrografin enema demonstrates distal migration of sigmoid stent. Arrow shows the location of the malignant stricture. ( B ) Migration of stent is followed by spontaneous passage from anus in the same patient.
Room Setup and Staff
Endoscopic stenting is advisable in a suite outfitted with endoscopic equipment and fluoroscopy. Various settings are acceptable venues for stenting including the operating room, endoscopy suite, or the interventional radiology unit. The setting is institution dependent as long as the endoscopist has access to the operating room in case surgical intervention is required.
A variety of endoscopes should be available including pediatric colonoscope, adult gastroscope, ultraslim gastroscope, and adult flexible sigmoidoscope. Fluoroscopy equipment (mobile C-arm or built-in fluoroscopy table) proved helpful during various parts of the procedures. Two experienced endoscopy nurses served the role of first and second assistants. An anesthesiologist, a nurse anesthetist, or a qualified endoscopy nurse with experience providing intravenous sedation under endoscopist supervision provided the anesthetic. The majority of colorectal stenting can be safely performed under intravenous sedation. Patient with severe abdominal distention, nausea, or emesis or imaging findings of small bowel dilation should have a nasogastric tube. Under such circumstances an anesthesiologist is needed for airway management with the consideration for a general anesthetic via endotracheal tube for airway protection and to provide adequate ventilation during the procedure. In our institution endoscopic stenting is routinely performed in the endoscopy suite. An industry representative familiar with the stent delivery devices can be helpful in supporting the endoscopist and the team. If the endoscopist has access to such support, it is highly encouraged, especially if he/she is early in his/her therapeutic endoscopy journey.
A single dose of prophylactic antibiotics (second or third generation cephalosporins with metronidazole or alternatively ciprofloxacin with metronidazole) is administered. The patient is positioned in the left lateral decubitus position with the anesthetist standing at the head of the table to the left of the endoscopist and the two endoscopy nurses to the right ( Fig. 11 ). All monitors and fluoroscopy equipment are positioned on the opposite side. If a nasogastric tube is present, it should be connected to active suction.
Fig. 11.
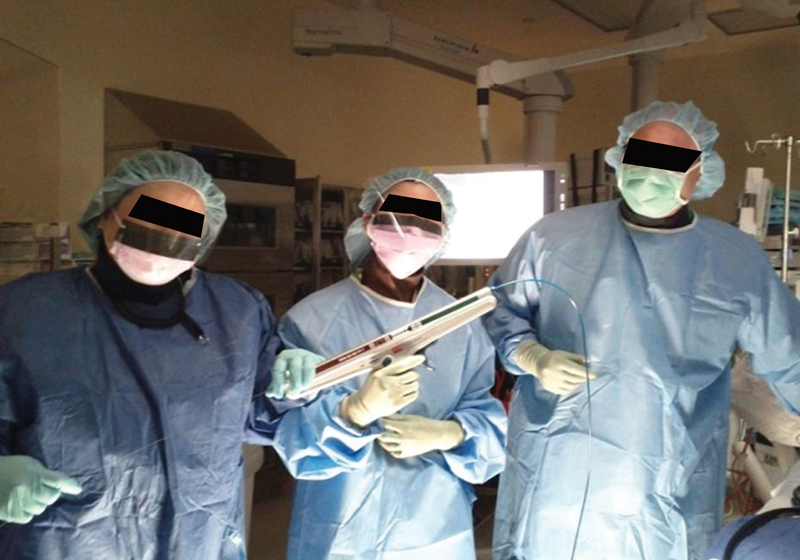
Two endoscopy nurses to the right of the endoscopist handle the stenting device and wire.
Technical Pearls
As previously noted, the outcome of endoscopic stenting is measured by immediate technical success (bowel decompression without complications) and clinical success (ability to resolve or control the symptoms without the need for surgical intervention). Clinical success is dependent on initial technical success, which is determined by the endoscopist technical expertise and skills in addition to judgement and choice of consumable items.
Choice of Endoscope and Stent Delivery Device
In patients with lesions proximal to the splenic flexure, a pediatric colonoscope is needed. In patients with left-sided lesions, an adult gastroscope or flexible sigmoidoscope can be used. An ultraslim gastroscope with smaller diameter can be helpful to traverse some tight lesions to establish wire access directly. In general, there are two types of stent delivery devices: (1) over the wire delivery system guided external to the endoscope and (2) over the wire through the working channel of endoscope stent system. We prefer the use of through the scope delivery system, which can address both right-sided as well left-sided lesions. The over the wire outside the endoscope systems are mostly effective in distal left-sided lesions.
It is important for the endoscopist to be familiar with the diameter of the stent delivery device and the working channel of the endoscope, especially when using through the scope devices. The working channel of the endoscope needs be large enough to accommodate the delivery device. A working channel of 3.2 mm is adequate for most through the scope delivery systems, which are usually 10 French in size. Such through the scope delivery devices can be accommodated by the working channel of most brands of pediatric colonoscopes, adult flexible sigmoidoscopes, or adult gastroscopes.
Choice of Stent
After determining what type of endoscope to use for the procedure, the next step is choosing the stent. The imaging findings are important in determining the proper length and diameter of the stent. Measure the length of the stricture on the imaging study. Ideally 2 to 4 cm of additional stent length is needed to cover the large bowel proximal and distal to the lesion. For example, a lesion that is 4 cm long requires a minimum stent length of 6 cm (1 cm on either margin of the lesion), preferably even 8 cm (2 cm on either margin of the lesion). This would provide adequate coverage of the entire lesion and allow for good potential for stent expansion. Most colonic stents have a proximal flare larger than the actual body diameter of the stent. A diameter of 25 to 30 mm is advisable to minimize the risk of stent migration. Such diameter provides good radial expansion and incorporation of the metal aspect of the stent into the lesion. For malignant obstruction, a noncovered stent is required. For benign disease such as colonic fistula or anastomotic leak, a partially covered metal stent is preferrable (central portion of the metal is covered, but proximal and distal part is bare metal to minimize risk of migration).
Choice of Guidewire
The use of guide wires is critical to the success of the procedure. The ideal guide wire length is 500 cm (450 cm is also available). This length is essential especially for procedures using a through the scope delivery device. A soft tip guide wire is recommended as a stiff wire can lead to microperforation. An endoscopic retrograde cholangiopancreatography or angiography catheter can be helpful in some cases to provide support to the soft tip of the wire. It is usually introduced and advanced over the wire with the tip of the catheter positioned just distal to the lesion. Such catheter support can allow the endoscopist to manipulate the tip of the wire more effectively to cannulate a very tight stricture.
Procedural Steps
Wire Access
With the patient adequately sedated or anesthetized, the endoscope is advanced to the distal aspect of the lesion. For near-obstructing or completely obstructing lesions, wire access is the first step of the stenting procedure. The guide wire is advanced through the working channel and advanced to the distal aspect of the lesion. If catheter support for the tip of the wire is needed, it is introduced with the wire in place and advanced through the working channel. Ideally, wire access should be established as efficiently as possible to minimize the amount of tumor manipulation and limit the amount of insufflation, which can lead to further dilation of the proximal colon, which can increase the risk of perforation. Excessive manipulation of the tumor can lead to edema and bleeding, which can render the procedure even more difficult and, in some cases, lead to technical failure with inability to stent the lesion. Ideally, the endoscopist should aim for an identifiable distal lumen, even if pinpoint, while avoiding blind advances of the wire ( Fig. 12 ).
Fig. 12.
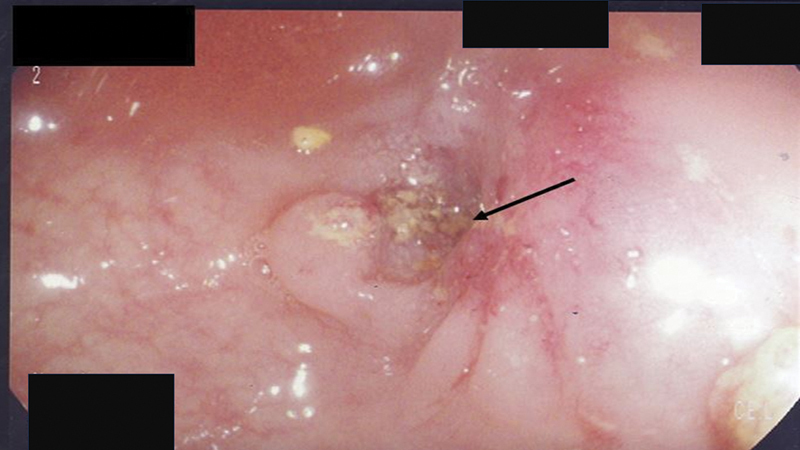
Obstructing tumor with pinpoint lumen (arrow). Endoscopist should avoid blind advancement of wire and should aim to the pinpoint opening to minimize bleeding and edema.
Once wire access is obtained and verified under fluoroscopic guidance, a significant amount of the wire is advanced into the proximal colon. Verification of the intraluminal position of the wire is obtained by noting the wire course through the colonic configuration and/or injection of contrast material via a catheter into the proximal colon to highlight the colonic wall. Contrast material that stagnates as a blush without clear colonic contour often signifies extraluminal location with perforation. An abdominal film can be obtained to determine the presence of free gas, which would confirm the perforation. Under such rare circumstances, the procedure is aborted, and the patient undergoes surgical intervention.
Balloon Dilation
Balloon dilation of the lesion is discouraged before or during stent deployment as it is associated with higher risks of perforation. In cases where the endoscopist cannot gain wire access across the lesion due to tightness of the stricture, gentle dilation of the distal aspect of the lesion (10 mm) can be attempted as a last measure before aborting the procedure with the hope of widening the distal aspect of the lesion to cross it with a wire. Again, we emphasize that this should not be done routinely and only under the circumstance when there is no other option except for surgical intervention as the next step.
Stent Deployment
Once wire access across the stricture is established, it is critical not to lose it. Establishing wire access a second time may prove very difficult, if not impossible in cases of very tight lesions with pinpoint lumen. Losing access may lead to further technical failure with the need for surgical intervention. Therefore, the importance of this part of the procedure cannot be overemphasized. It is important to secure the wire externally to the bedsheets in between active steps of the stenting procedure. A useful way is to coil the excess wire externally and secure it in place with a hemostat clamp ( Fig. 13 ). Stent delivery devices should always be advanced over a guide wire and never without. Advancing the rigid delivery device across the lesion without wire support is associated with high risk of perforation, especially in the rectosigmoid area and the flexures.
Fig. 13.
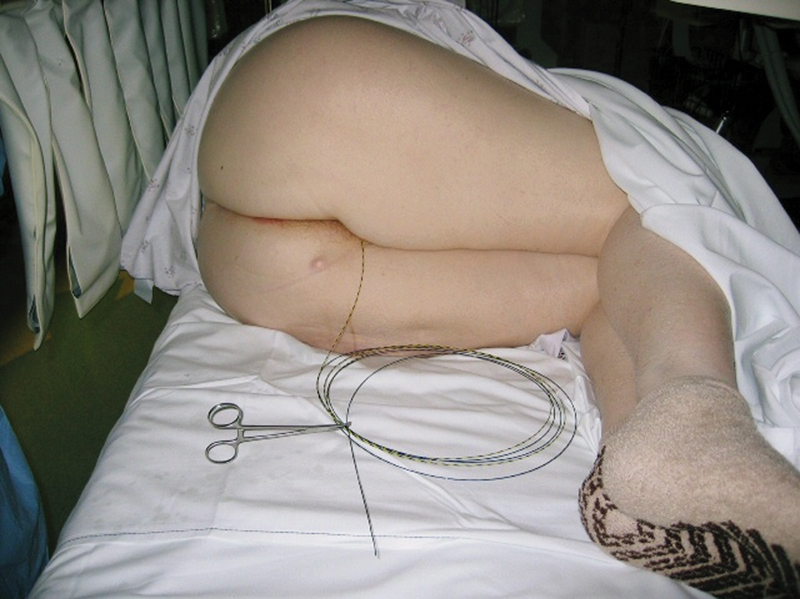
Securing the guide wire to the bed after obtaining access across the stricture.
The advantages of using through the scope delivery devices is maintaining wire access across the lesion by leaving the wire inside the working channel and advancing the delivery device over the wire. The delivery stent device is well lubricated and advanced over the wire through the endoscope. This is an active phase monitored under fluoroscopy. As the delivery device is advanced, an equal length of wire is pulled back to compensate for the forward movement of the wire with the delivery device. Under endoscopic guidance, the stent delivery device is advanced and positioned across the lesion with adequate overlap with normal colonic margins proximally and distally.
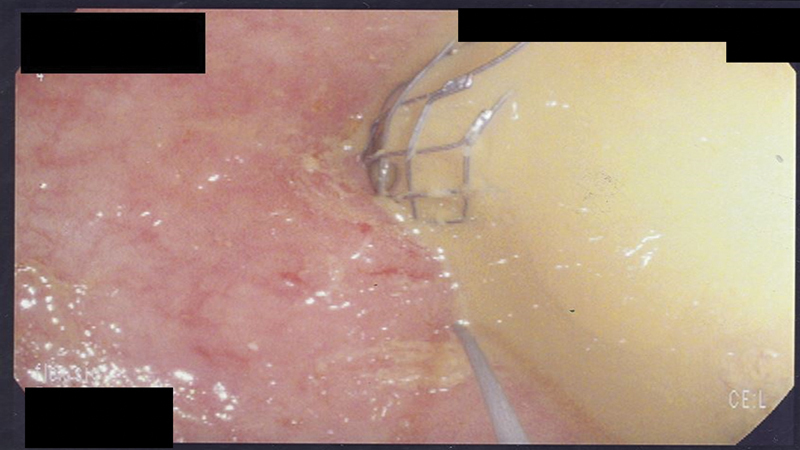
Stent deployment should be a slow and gradual process with step-by-step instruction to the first and second assistant who are handling the delivery device as the endoscopist maintains control of the endoscope and the wire. Taking incremental slow steps with frequent halts to verify the position and expansion of the stent under fluoroscopy is advisable. The endoscopist should familiarize him or herself with the type of delivery device by reading the instructions. It is important to note that for all stent delivery devices there is a “point of no return” where the stent cannot be recaptured inside the delivery device. Therefore, up to that point, the endoscopist can recapture and reposition the stent more proximal or distal, but once that “point of no return” is reached then completion of stent deployment needs to be performed. In some cases, gentle tugging on the stent can provide more distal overlap but gaining more proximal overlap is impossible; if the endoscopist pushes the stent more proximally then the risk of perforation is significantly increased. Thus, careful slow deployment of the stent with repositioning as needed is performed prior to reaching that point. Once the stent is deployed, fluoroscopy is used to confirm the position and degree of expansion. An ideally deployed stent shows a waist like an hourglass ( Fig. 14 ). The delivery device is withdrawn first while maintaining the wire access, which is removed at the end ( Fig. 15 ). In the rare occasion where a second stent is needed to overlap and bridge the first stent, maintaining wire access is critical.
Fig. 14.
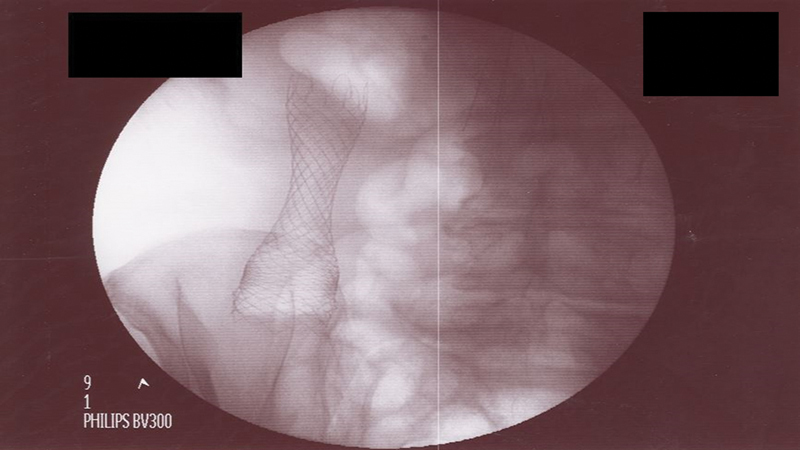
An ideally placed stent across a malignant stricture in ascending colon shows good overlap with hourglass-like appearance.
Fig. 15.
Immediately after stent deployment, guide wire access across the stent is initially maintained in case a second stent is needed.
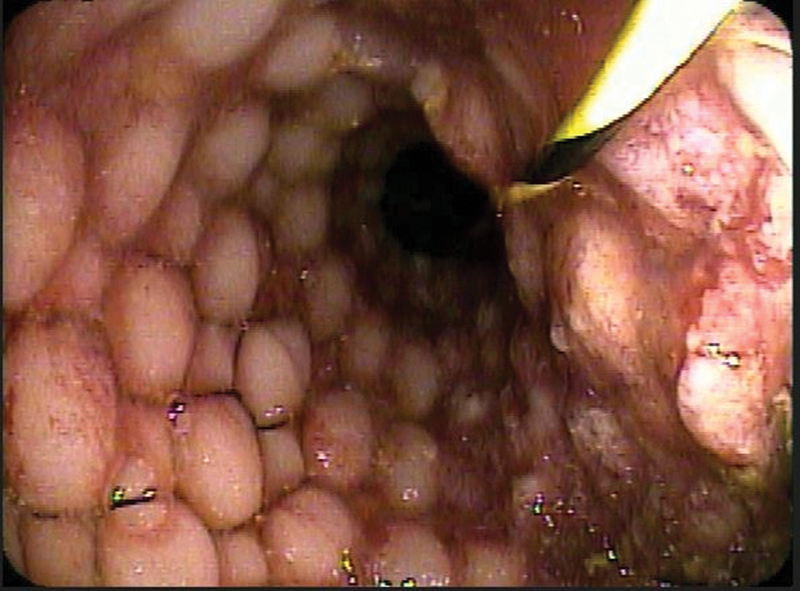
It is important not to traverse the stent with the endoscope after deployment as the endoscope can get stuck in a partially expanded stent, and it can lead to dislodging the stent upon withdrawal. Confirmation of adequate decompression is noted by fluoroscopy and often accompanied by prompt passage of stool in obstructed patients ( Fig. 16 ).
Fig. 16.
Confirmation of adequate decompression of the obstructed bowel is immediately noted by prompt passage of stool into the stent.
Postprocedural Care
Immediately after the procedure, a postprocedural abdominal radiograph is obtained to confirm the location of the stent, check for the degree of diameter expansion, and to exclude procedural related perforation, which is a rare complication ( Fig. 17 ). The radiograph is repeated 24 to 48 hours to establish a baseline for the stent location and expansion ( Fig. 18 ). The patient is transferred to the ward. A liquid diet is started once the patient recovers from the anesthetic. If a patient is completely obstructed with a nasogastric tube, it is switched to gravity drainage and if progressing well the tube is removed within 24 to 48 hours and a liquid diet is initiated. The diet is gradually advanced. Discharge from the hospital can occur anywhere from 1 to 3 days in the majority of patients. Patients are advised in the short-term setting to minimize large fiber intake (large quantity of vegetables and fruits) and if needed can use a gentle laxative such as milk of magnesium to avoid stool impaction of the stent, especially in patients with distal left-sided lesion. If a stent impaction occurs as evidenced by dilated large bowel on imaging, stent disimpaction can be accomplished by flushing laxatives endoscopically into the distal aspect of the stent. In cases of severe impaction, gentle balloon dilation of the fecal material inside the stent prior to flushing the stent can be considered.
Fig. 17.
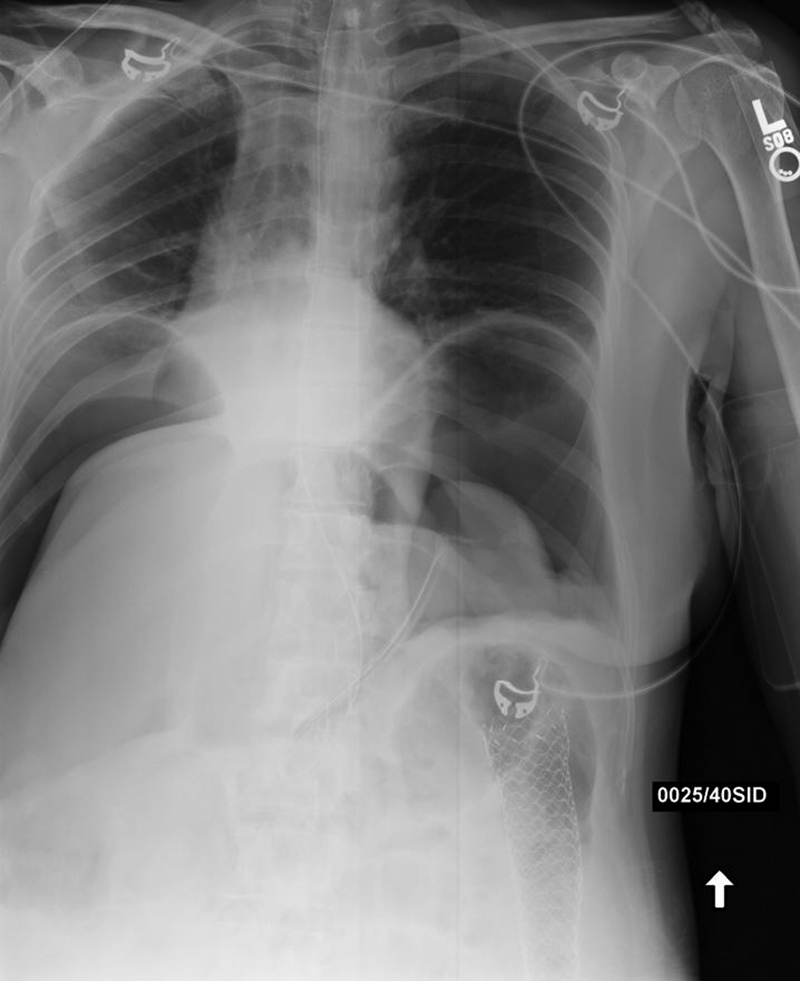
Postprocedural abdominal radiograph shows large amount of free gas from stent perforation of a splenic flexure lesion.
Fig. 18.
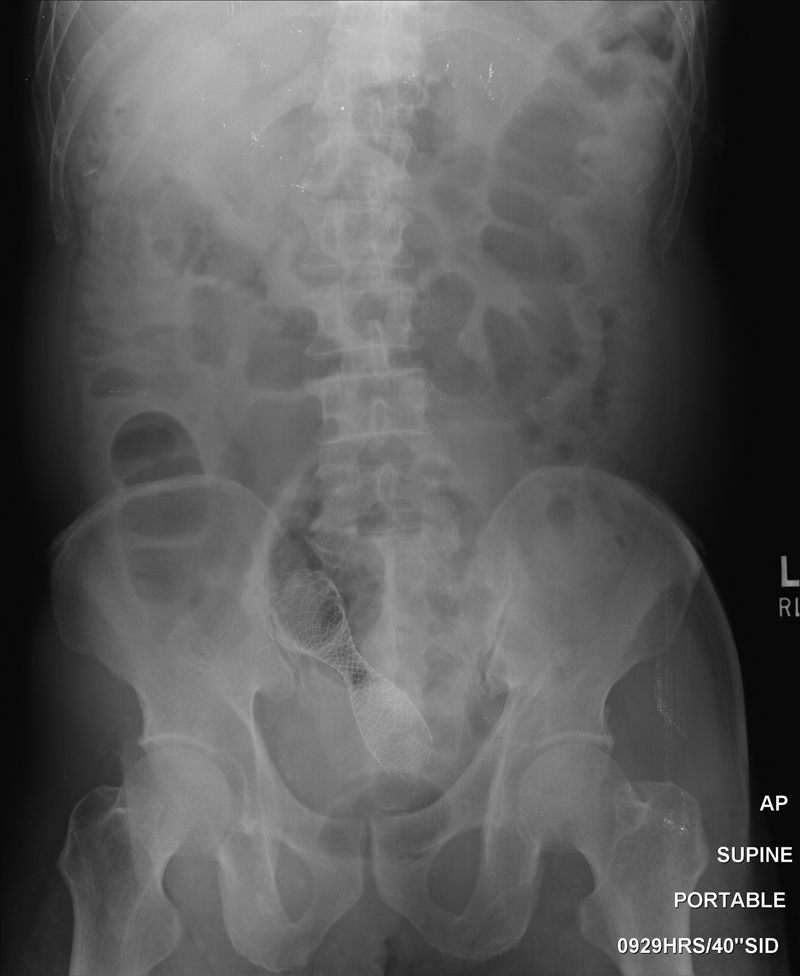
Abdominal radiography taken 24 hours after stent placement shows the location and degree of expansion in a patient with rectal cancer.
Long-term Surveillance
There are no standard guidelines of how to monitor patients with endoscopic stents. With recent advances in chemotherapeutic and biologic agents, long-term survival is increasing even in patients with stage 4 colorectal cancer. In our practice, we advise patients to undergo endoscopic evaluation of the stent every 4 to 6 months ( Fig. 19 ). If tumor ingrowth occurs, endoscopic fulguration or placement of a stent through a stent can be considered ( Fig. 20A and B ).
Fig. 19.
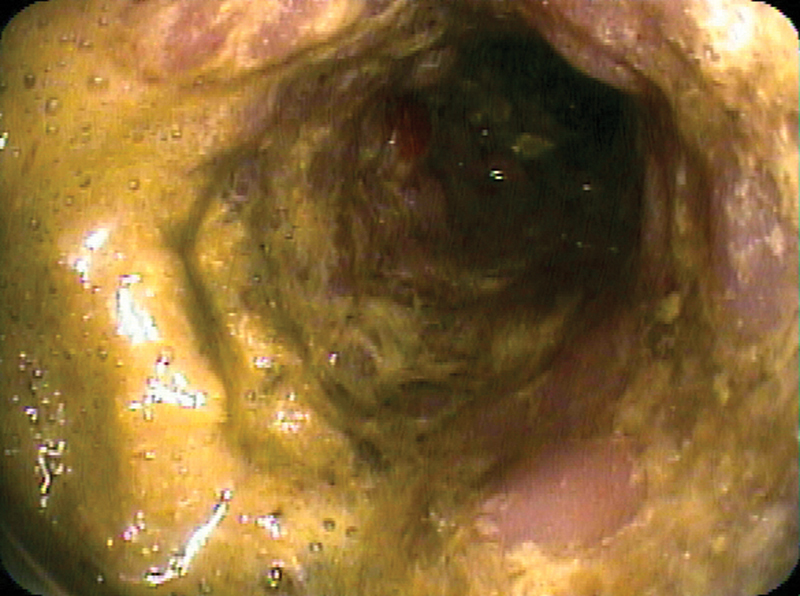
Endoscopic view of a patent stent across sigmoid cancer 4 months after deployment.
Fig. 20.
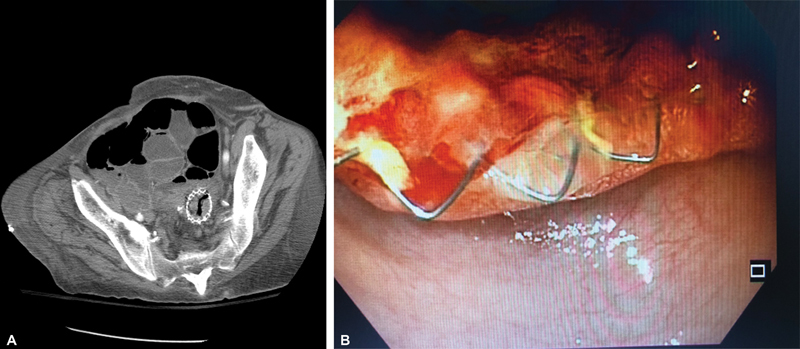
( A ) Computed tomography scan shows tumor ingrowth into a rectosigmoid stent 6 months after deployment. ( B ) Endoscopic view of tumor ingrowth into a rectosigmoid stent in the same patient.
Other than routine endoscopic stent surveillance, the patient needs to be monitored clinically for any signs of long-term stent migration (recurrent obstruction, tenesmus for distal stent) or stent erosion/perforation (pain, fever). Should any signs develop, repeat imaging is warranted for investigation and further endoscopic or surgical intervention is undertaken based on findings. Furthermore, tumor response to additional chemotherapy or biologic treatment should prompt further discussion with the oncologist and patient regarding possibility of surgical resection. If surgical resection is undertaken under such circumstances, the surgeon needs to be aware that an intense inflammatory reaction surrounds long-term in situ stents. This can render the operation more challenging, especially in the presence of rectal stents. Consideration for ureteral catheter placement at the time of surgery should be considered in such cases.
Finally, it is important to note that there is a lack of data on the consequences of delivering radiotherapy to the area of metal stents. While some radiation therapists are willing to deliver radiotherapy to a stented tumor, it is important for the surgeon to be aware that the long-term risks of stent erosion and severe inflammatory changes around the bowel are often increased. From personal experience, subsequent surgical intervention in such scenarios can be extremely challenging and risks of ureteral laceration increased.
Conclusion
Endoscopic colorectal stenting is now an established treatment intervention in a select group of patients. Stent technology has expanded the armamentarium of interventions and provided the colorectal surgeon/endoscopist with additional options for some patients with colorectal disease. Undoubtedly, this area of medicine will continue to evolve and expand with the introduction of new technologies and tools. Proper patient selection and safe conduct of the procedure are critical to optimize both short- and long-term outcomes. Our hope is that this article has provided the reader with the fundamental knowledge and framework to grow their expertise in this area of therapeutic endoscopy, which is beneficial to the patient. The information shared in this article is based on the two-decade experience and journey of the senior author.
Footnotes
Conflict of Interest None declared.
References
- 1.Halabi W, Abbas M A. Elsevier; 2019. Enteral stents in the treatment of colonic obstruction. [Google Scholar]
- 2.Lackberg Z, Abbas M A. Springer; 2017. Endoluminal colorectal stenting. [Google Scholar]
- 3.Lackberg Z, Abbas M A. Colonic stenting: when and how. Semin Colon Rectal Surg. 2017;28:34–40. [Google Scholar]
- 4.Darr H, Abbas M A. Stenting as a bridge to surgery or a palliative treatment. Clin Colon Rectal Surg. 2020;33(05):279–286. doi: 10.1055/s-0040-1713745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayhanabad J A, Abbas M A. Long-term outcome of endoscopic stenting for benign and malignant colorectal disorders. Am Surg. 2009;75:897–900. [PubMed] [Google Scholar]
- 6.Abbas M A, Falls G N. Endoscopic stenting of colovaginal fistula: the transanal and transvaginal “kissing” wire technique. JSLS. 2008;12(01):88–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas M A. Endoscopic management of acute colorectal anastomotic complications with temporary stent. JSLS. 2009;13(03):420–424. [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas M A, Kharabadze G, Ross E M, Abbass M A. Predictors of outcome for endoscopic colorectal stenting: a decade experience. Int J Colorectal Dis. 2017;32(03):375–382. doi: 10.1007/s00384-016-2696-1. [DOI] [PubMed] [Google Scholar]


