Abstract
We report here on the development and characterization of a novel herpes simplex virus type 1 (HSV-1) amplicon-based vector system which takes advantage of the host range and retention properties of HSV–Epstein-Barr virus (EBV) hybrid amplicons to efficiently convert cells to retrovirus vector producer cells after single-step transduction. The retrovirus genes gag-pol and env (GPE) and retroviral vector sequences were modified to minimize sequence overlap and cloned into an HSV-EBV hybrid amplicon. Retrovirus expression cassettes were used to generate the HSV-EBV-retrovirus hybrid vectors, HERE and HERA, which code for the ecotropic and the amphotropic envelopes, respectively. Retrovirus vector sequences encoding lacZ were cloned downstream from the GPE expression unit. Transfection of 293T/17 cells with amplicon plasmids yielded retrovirus titers between 106 and 107 transducing units/ml, while infection of the same cells with amplicon vectors generated maximum titers 1 order of magnitude lower. Retrovirus titers were dependent on the extent of transduction by amplicon vectors for the same cell line, but different cell lines displayed varying capacities to produce retrovirus vectors even at the same transduction efficiencies. Infection of human and dog primary gliomas with this system resulted in the production of retrovirus vectors for more than 1 week and the long-term retention and increase in transgene activity over time in these cell populations. Although the efficiency of this system still has to be determined in vivo, many applications are foreseeable for this approach to gene delivery.
One of the main objectives of gene therapy is to achieve stable genetic modification of target cells. This means that their progeny, or themselves in the case of nondividing cells, should retain and express the newly introduced genetic material until the end of their lifespan, ideally in a regulated manner. This principle is equally valid when transgenes are introduced to correct genetic deficiencies or for treatment of nonhereditary diseases. Several viral vector systems have been developed which can achieve retention of the transgene through different mechanisms. Retrovirus and adeno-associated virus (AAV) vectors can integrate genes into the genome of infected cells, while Epstein-Barr virus (EBV) vectors are maintained by episomal replication (27, 67).
Moloney murine leukemia virus (MMLV)-derived retrovirus vectors are among the most commonly used vectors in gene therapy because of their ability to stably integrate transgenes in the genome of target cells and because of their relative safety. However, their use for direct gene delivery in vivo has been limited due to the low efficiency of gene transfer. This is the result of several limiting properties: low titer, inability to infect nondividing cells, limited tropism, and relatively short half-life. Although improvements have been made to address some of these issues, MMLV-derived retrovirus vectors are still mostly used for ex vivo protocols. These strategies involve removal of the target cells from the experimental subject or use of donor cells, genetic modification in culture by infection with retrovirus vectors carrying the transgene of interest, selection and characterization of transduced cells, and implantation of these cells in vivo (57).
An alternative to the direct injection of retroviral particles for in vivo gene delivery is the injection of retrovirus producer cells resulting in the local production of retrovirus vectors. This strategy has been used with success in the treatment of experimental brain tumors (10), but its clinical efficacy remains to be demonstrated. In the clinical setting the retrovirus packaging cells remain localized to the injection site, resulting in a very low efficiency of gene delivery to tumor cells, i.e., delivery limited to the immediate vicinity of the packaging cells (53). The fact that the retrovirus producer cells used are typically derived from mouse fibroblasts has several disadvantages and potential risks (25). First, the retrovirus particles produced are extremely sensitive to inactivation by human serum via complement activation. This occurs because nonprimate packaging cells add a Gal(α1-3)galactosyl group to the retroviral envelope and because human serum has preexisting antibodies against that sugar group (54, 60). Second, the fact that these cells are mouse fibroblasts presents two difficulties: (i) fibroblasts transplanted into a brain tumor remain largely at the injection site and do not distribute throughout the tumor (61, 62), and (ii) their xenogeneic origin is likely to exacerbate an immune response despite the immune privileged nature of the central nervous system (CNS), thus limiting their survival time. A third consideration is safety, since murine cells carry in their genome a large variety of MMLV-like genomes which can be packaged by type C Gag proteins (6, 23, 56). The copackaging of the virus vector with one of these endogenous genomes can lead to recombination events which result in the production of replication-competent retroviruses (RCRs). Several packaging cell lines derived from human cells have been developed which could be used instead of the murine versions (9). These human retrovirus packaging cell lines package fewer endogenous sequences (49), which reduces the probability of generating RCRs, and the virus particles are resistant to inactivation by human serum (9). However, there is also an increased risk for tumor formation since most of these cell lines are derived from transformed cells.
Others have attempted to convert cells to retrovirus packaging cells by different transduction strategies. Noguiez-Hellin et al. (47) generated retrovirus-producing cells in situ by transfection with a plasmid carrying all of the necessary functions for retrovirus packaging and vector generation. These authors have shown that the transgene could be propagated in culture but to a lesser extent in a tumor model in vivo, probably due to low transfection efficiency with the plasmid (47). Other novel systems for production of retroviral vectors take advantage of the efficient gene delivery and expression mediated by virus vectors derived from herpes simplex virus (HSV) (55), Semliki Forest virus (SFV) (39), and adenovirus (13, 15, 40, 68). These systems utilize two or three different vectors to introduce the retroviral vector element and packaging functions, depending on whether the gag-pol and env genes are encoded in the same vector (15, 40, 55) or different vectors (13, 39, 40, 68). One of these adenovirus-retrovirus chimeric systems has been shown to extend the duration of transgene expression in tumors in vivo when compared to a recombinant adenovirus vector (15).
HSV amplicons are plasmid-based vectors that, in addition to the transgene of interest and corresponding expression elements, only need two noncoding HSV sequences, an origin of DNA replication (oriS) and a DNA cleavage-packaging signal (pac), to be packaged in HSV virions in the presence of helper functions (59). These virions can infect a wide range of dividing and nondividing cells and, with the development of an HSV helper virus-free packaging system (18), have essentially no toxicity. HSV virions package about 150 kb of DNA. The amplicon DNA is packaged as a concatemer of approximately that size, containing multiple copies of the plasmid repeated in tandem, due to a rolling circle mode of viral DNA replication. This presents the advantages that a single virion can transduce a cell with multiple copies of a transgene and that these vectors can carry large genes and regulatory regions (for a review, see reference 17). However, one major limitation of these vectors has been the loss of amplicon DNA from the host cell nucleus over time and therefore of gene expression, especially in dividing cells (26). Recently, two different hybrid amplicon systems have been developed that incorporate elements from other viruses that serve to increase retention of the amplicon DNA and extend transgene expression. One is an HSV-EBV hybrid amplicon which includes two EBV elements in its backbone, the latent origin of DNA replication (oriP) and the gene encoding the EBV nuclear associated antigen 1 (EBNA-1), which support nuclear replication of the amplicon DNA in dividing cells (63). The other is an HSV-AAV hybrid amplicon which incorporates the AAV ITR element and rep gene. These elements have the potential to mediate chromosomal integration of the transgene cassette (26). This principle of incorporating these AAV elements to achieve chromosomal integration of transgenes with viruses that normally do not possess this property has also been used with baculovirus (48).
The present study reports on the development and characterization of a new gene delivery system based on an HSV-EBV hybrid amplicon vector that contains the retroviral packaging functions and a retrovirus vector cassette. The ability of this amplicon vector to induce retrovirus vector production was assessed in a number of different cells. High-titer recombinant retrovirus vectors were obtained both by transfection and infection. This system can also mediate cumulative transgene delivery in cell populations starting from a small fraction of amplicon-infected cells.
MATERIALS AND METHODS
Cells.
NIH 3T3 cells, SNB-19 human glioma cells (21), and Phoenix-E cells (used with permission from Garry P. Nolan [Stanford University School of Medicine, Stanford, Calif.]) were obtained from the American Type Culture Collection (Rockville, Md.). 293T/17 cells (50) were provided by David Baltimore (Massachusetts Institute of Technology, Cambridge). These cells constitutively express the simian virus 40 (SV40) large T antigen, and clone 17 was specifically selected for its high transfectability. African green monkey kidney Vero 2-2 cells (58) were provided by Rozanne Sandri-Goldin (University of California, Irvine). T98 cells were obtained from F. S. Prado (Massachusetts General Hospital, Boston), U87.ΔEGFR cells (44) were obtained from Webster K. Cavenee (University of California at San Diego, La Jolla), and Gli-36 human primary glioma was provided by David N. Louis (Massachusetts General Hospital, Charlestown). The J3T dog glioma cell line (3) was provided by Michael E. Berens (Barrow Neurological Institute, Phoenix, Ariz.), the 9L rat gliosarcoma cell line (64) was obtained from the Brain Tumor Research Center (University of California at San Francisco), and the CNS-1 rat glioma cells (33) were obtained from C. A. Kruse and W. F. Hickey (University of Colorado Health Sciences Center, Denver). Naive Mus dunni cells (MD) and Mus dunni cells expressing LacZ (MDZ) (12) were provided by Richard Mulligan (Harvard Medical School, Boston, Mass.). NIH 3T3 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% calf serum (Sigma, St. Louis, Mo.). 293T/17, Phoenix-E, SNB-19, T98, U87.ΔEGFR, Gli-36, J3T, 9L, CNS-1, and 2-2 cells were grown in DMEM with 10% fetal bovine serum (Sigma). MD and MDZ cells were grown in DMEM–F-12 and 5% fetal bovine serum. All media were supplemented with 100 U of penicillin and 0.1 mg of streptomycin (Sigma) per ml. 2-2 and U87.ΔEGFR cell growth medium was further supplemented with 0.5 mg of G418 (GIBCO BRL, Gaithersburg, Md.) per ml. Cells were grown at 37°C and with 5% CO2 in a humidified atmosphere.
Plasmids.
All DNA sequences amplified by PCR were cloned in pCR2.1 vector by using the TA cloning kit (Invitrogen, Carlsbad, Calif.) and sequenced.
(i) Construction of gag-pol-env (GPE) expression cassette.
The primers used were as follows: MOV-1, GCTAGCCCTCTTGCAGTTGCATCCGAC (the NheI site is underlined); MOV-2, AGGAGCAACTGGCGATAGTGGAC; ENVP3, TCTAGACTATGGCTCGTACTC (the XbaI site is underlined); and ENVP4, CTGTTTAACAGATCCCCTTGG.
The pMOVΨ− plasmid (41), provided by Richard Mulligan, was used as a template for all PCR reactions. Primers ENV-P3 and ENV-P4 were used to amplify a 209-bp fragment which was cloned in pCR2.1, generating the plasmid TVENVP34. A 6,114-bp XhoI-ClaI fragment from pMOVΨ− was then cloned into TVENVP34, generating the plasmid TVMOV. A 6,226-bp XhoI-EcoRI fragment from this plasmid was then cloned in pcDNA3.1Neo(−) (Invitrogen), generating the plasmid pcDNA3.1MOV. Primers MOV-1 and MOV-2 were used to amplify a 1,237-bp fragment from pMOVΨ−, and the PCR product was cloned, generating the plasmid TVMOV12. The 1,161-bp NheI-XhoI fragment derived from this plasmid was then cloned into pcDNA3.1MOV, generating pcDNA3.1MOV12.
To construct the amphotropic version, the 4070A amphotropic genome (7), provided by Sisir Chattopadhyay (National Cancer Institute, Bethesda, Md.), was digested with SalI and ClaI, and the resulting 3,936-bp fragment, containing the amphotropic envelope gene, was cloned into TVMOV, generating the plasmid TVMOVAmpho. The 6,081-bp XhoI-to-ClaI fragment was cloned in pcDNA3.1MOV12, generating pcDNA3.1MOVAmpho.
(ii) Construction of retrovirus vector with minimal overlap with GPE cassette.
The primers used were as follows: BAB-1, GCGGCCGCTGATCATTCCGCGCACATTTCCCCGAAAAG (the NotI site is underlined); BAB-2, GTCGACAGATCTCAGCAGACAAGACGCGCGGCTTCGG (the SalI-BglII site is underlined); BAB-4, ATGCATGCGGCCGCTGATCAAAATGAAAGACCCCCGCTGAC (the NsiI-NotI site is underlined); and BAB-5, ATCGATGTCGACATTAATGTCTCCAGAAAAAGGGGGGAATGAAAG (the ClaI-SalI site is underlined). The retrovirus vector elements were derived from the vector pBabe Puro (43) obtained from Jay Morgenstern (Imperial Cancer Research Fund, London, United Kingdom). BAB-1 and BAB-2 primers were used to amplify a 933-bp fragment spanning the 5′ long terminal repeat (LTR) promoter and the packaging signal. This PCR product was cloned into pCR2.1, yielding the plasmid TVBAB12. BAB-4 and BAB-5 primers were used to amplify a 654-bp fragment spanning the 3′ LTR which was cloned in pCR2.1, generating plasmid TVBAB45. The NsiI-to-SalI fragment from this plasmid containing the BAB45 sequence was cloned in TVBAB12, generating plasmid TVB. The 1,075-bp SalI-to-NheI fragment from pBabe Puro containing the SV40 early promoter and puromycin selection marker gene were cloned in TVB, generating plasmid TVBP. The Escherichia coli lacZ gene was cloned into the BglII site of this plasmid.
(iii) Construction of HERE and HERA vectors.
A 1.1-kb PacI fragment flanked by NotI sites was inserted into the unique PacI site of the pREHGCa HSV-EBV hybrid amplicon plasmid (54a), designated M12Y. The NheI-to-PmeI fragments from pcDNA3.1MOV12 and pcDNA3.1MOVAmpho encompassing the coding sequences for the retrovirus proteins were cloned into M12Y, generating the vectors pHERE and pHERA, respectively. The retrovirus vector element was derived from the TVBPlacZ plasmid after digestion with NotI and cloned into the unique NotI site of pHERE and pHERA.
Transfections and vector titer determination. (i) Retrovirus packaging.
293T/17 and Phoenix-E cells were transfected essentially as described previously (50). The day before transfection, 2 × 106 293T/17 cells were plated in 4 ml of medium on 60-mm-diameter dishes. The following day the medium was replaced with medium containing 25 μM chloroquine (Sigma) for 5 to 10 min before the addition of the DNA-calcium phosphate coprecipitates. Then, 15 μg of each plasmid was mixed with 62 μl of 2 M CaCl2 (Sigma) and water to a final volume of 500 μl. This solution was added to 500 μl of HEPES-buffered saline (50 mM HEPES, 10 mM KCl, 12 mM dextrose, 280 mM NaCl, 1.5 mM Na2HPO4; final pH, 7.05) and mixed by vigorous bubbling for 1 min. The resulting suspension was immediately added to the cells, and the plate was gently rocked to achieve a uniform distribution of the precipitates. Cells were returned to the 37°C incubator for 8 h. Then the medium was replaced with 4 ml of growth medium. The following day, the medium was changed to 3 ml of fresh medium, and the cells were incubated at 37°C for 24 h before the supernatants were harvested.
(ii) Retrovirus titer determination.
For all retrovirus titers determined in this study the media from retrovirus vector-producing cells were centrifuged at 500 × g for 5 min, and the supernatants were stored at −80°C. One day prior to infection, NIH 3T3 cells were plated in 12-well dishes at a density of 5 × 104 cells/well. Cells were infected in a total volume of 0.5 ml containing different volumes of supernatant in the presence of 4 μg of Polybrene (Sigma) per ml. The titer of each vector stock was determined in triplicate. One day later the total volume was brought up to 1 ml with fresh medium and incubated for 24 h before fixation in 4% paraformaldehyde (Sigma) in phosphate-buffered saline (PBS; Sigma) for 5 to 7 min at room temperature. Cells were washed twice with PBS and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Fisher) solution [1 mg of X-Gal per ml, 2 mM MgCl2, 5 mM K4Fe(CN)6, and 5 mM K3Fe(CN)6 in PBS] overnight. Positive cells were counted by using an Argus-20 image processor (Hamamatsu Photonics, Hamamatsu City, Japan) at ×100 magnification. Three fields per well were counted, and the vector titer was determined by calculating the total number of blue cells/well divided by the total volume of supernatant used and expressed as transducing units (TU) per milliliter.
(iii) Helper virus assay.
Retroviral stocks were assayed for replication-competent retrovirus by a LacZ mobilization assay via Mus dunni cells. One day prior to infection, MDZ cells, 2 × 105/100-mm-diameter plate, were infected with 1 ml of retrovirus vector stocks in 9 ml of media with 8 μg of Polybrene per ml. The following day, the medium was replaced, and the cells were incubated for 48 h. Supernatants were then passed through 0.45-μm-pore-size filters and used to infect 3 × 105 MD cells in 100-mm plates in the presence of 8 μg of Polybrene per ml. The following day the medium was changed to normal medium, and cells were incubated for an additional 24 h before being stained with X-Gal as described above. Plates were entirely scanned with a light microscope for the presence or absence of LacZ-positive cells (blue cells). To determine the sensitivity of the assay, titered wild-type 4070A amphotropic virus stocks, kindly provided by Richard Mulligan, were diluted to final concentrations of 1, 2, 10, and 100 viral particles per ml and used in place of the test virus. The assay was shown to be able to detect one particle of 4070A virus per milliliter of infectious medium. All retrovirus stocks used in the present study, including those generated by transfection and infection of various cell types, were negative for RCR by this assay.
(iv) Amplicon packaging.
Plasmids were packaged as HSV amplicons by using the helper virus-free packaging system developed by Fraefel et al. (18). For this purpose 2-2 cells were transfected by using Lipofectamine (GIBCO BRL) with a mixture of plasmid to be packaged and a set of five cosmids which spans the entire HSV genome (11) with the sequences containing the DNA cleavage-packaging signals (a sequences containing pac signals) deleted (18). Amplicon stocks were harvested 60 h later, freeze-thawed three times, sonicated, and purified by brief centrifugation at 1,000 × g for 10 min. Amplicon titers (TU/milliliter) were determined by infecting 3 × 105 293T/17 cells/well in 24-well plates and then counting the GFP-positive cells at ×100 magnification at 18 h postinfection.
Retrovirus production after infection with amplicons. (i) Retrovirus production in 293T/17 cells.
One day prior to infection, 2 × 106 cells were plated on 60-mm-diameter dishes. Cells were infected at different multiplicities of infection (MOIs) in a maximum volume of 3 ml. The following day the medium was replaced with 3 ml of fresh medium, and the cells were incubated for 24 h before the medium was harvested to determine the retrovirus titers. Each MOI was determined in triplicate. Retrovirus titers were determined as described above.
(ii) Amplicon neutralization experiment.
For amplicon neutralization, 293T/17 cells were plated as described above. On the day of infection, the appropriate number of TU to achieve an MOI of 2 were diluted in growth medium to a final volume of 3 ml. Diluted amplicon vector stocks were incubated for 10 min at room temperature with the following antibodies and serum to a final dilution of 1:50: rabbit anti-HSV type 1 polyclonal antibody (B0114; Dako, Glostrup, Denmark), normal rabbit serum (Vector, Burlingame, Calif.), rabbit anti-LacZ antibody (55975; ICN Pharmaceuticals, Costa Mesa, Calif.) as an unrelated rabbit antibody, and PBS as a control. Other than this incubation period the experiment was repeated as described above. Each experimental condition was repeated in triplicate.
(iii) Retrovirus production in glioma cells.
One day prior to infection 5 × 105 cells were plated on 60-mm-diameter plates. The following day, cells were infected at different MOIs, as above, prior to harvesting the supernatant for retrovirus titering. Each MOI was determined in triplicate. Retrovirus titers were determined as described above. For retrovirus production kinetics, on the day of harvesting, the total number of cells per plate was determined by using a cell counter (Coulter, Miami, Fla.), and 5 × 105 cells were replated on 60-mm plates. Two days later, the medium was replaced with 3 ml of fresh medium, and the following day the supernatant was harvested in order to determine the titer. The same process was repeated at each time point.
Stability of transgene expression.
One day prior to infection, J3T and Gli-36 cells were plated in six-well dishes at a density of 105 cells per well. The following day, cells were infected with amplicon vectors at an MOI of 2 in a total volume of 1 ml in growth medium and returned to the 37°C incubator for 24 h, after which the medium was replaced by 1.5 ml of fresh medium. Cells were kept in six-well dishes until day 4, after which they were transferred to 60-mm-diameter plates. Cells were then split 1:5 every 3 days. The experiment was repeated twice in triplicate for each cell line.
To measure the β-galactosidase activity, cells were harvested at different time points in cell lysis buffer (Promega, Madison, Wis.) and stored at −80°C. The total amount of protein for all samples was determined by using the Coomassie Plus protein assay reagent (Pierce, Rockford, Ill.) and a bovine serum albumin (BSA) standard (Bio-Rad, Hercules, Calif.). β-Galactosidase activity in each sample was measured by using a β-galactosidase assay kit (Promega). Samples and standards were incubated for 30 min at 37°C.
Western blot.
Cells were lysed in a buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1% NP-40, and Complete-Mini cocktail of protease inhibitors (Boehringer-Mannheim, Indianapolis, Ind.). Protein concentrations were determined as described above. An equal amount of total cell protein (60 μg) was denatured and separated by electrophoresis on 10% polyacrylamide gels with sodium dodecyl sulfate. Rainbow markers (Amersham Life Sciences, Arlington Heights, Ill.) were used as molecular weight markers. Proteins were transferred to nitrocellulose membrane (Bio-Rad Trans-Blot Transfer Medium Pure, 0.45 μm [pore size]) in transfer buffer (25 mM Tris, 192 mM glycine; pH 8.3) by using a Bio-Rad Transblot Cell for 3 h at 0.5 mA at 4°C. Membranes were stained with 0.2% Ponceau S (Sigma) to ensure equal loading of samples and proper transfer. After the staining, the membranes were blocked overnight in 10% nonfat dry milk powder in TBST (150 mM NaCl; 50 mM Tris, pH 7.9; 0.05% Tween 20). The following day, membranes were washed twice for 15 min and twice for 5 min in TBST and then incubated for 1 h at room temperature with a 1:3,000 dilution of the primary antibodies in 2% nonfat dry milk powder in TBST. Goat anti-p30 and anti-p70 antibodies were developed against Rauscher murine virus p30 (CA protein) and p69/71 (SU protein) proteins, respectively (Quality Biotech, Inc.). The membranes were washed as before and incubated for 30 min with a 1:5,000 dilution of anti-goat immunoglobulin G (IgG) peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) in TBST with 5% milk. After a washing as described above, the blots were developed by using enhanced chemiluminescence (ECL) reagents (Amersham Life Sciences). Membranes were then exposed to film for 30 s to 30 min. Films were scanned, and the bands were analyzed for their integrated densities by using the NIH Image 1.62 software.
Fluorescence-activated cell sorting (FACS) analysis.
Cells were trypsinized and centrifuged for 5 min at 500 × g. After resuspension in PBS, cells were analyzed for the percentage of green fluorescent protein (GFP)-positive cells by using a FacsCalibur analyzer (Becton-Dickinson, Franklin Lakes, N.J.).
Immunofluorescence.
J3T and Gli-36 cells infected with HERAlacZ B7 amplicon vector at MOIs of 2 and 0.1, respectively, were fixed with 4% paraformaldehyde at 37°C for 30 min at 2 and 5 days postinfection. After a thorough washing with PBS, cells were treated with 0.1% NP-40 in PBS for 20 min, followed by treatment with a blocking solution (10% goat serum [Vector Laboratories, Burlingame, Calif.] in PBS) for 45 min. Cells were incubated with an anti-LacZ rabbit primary antibody (ICN Pharmaceuticals) at a 1:250 dilution in PBS plus 1% BSA (Sigma) for 1 h. A rhodamine-conjugated goat anti-rabbit secondary antibody (Biosource International, Camarillo, Calif.) was then used (1 h with a 1:200 dilution in PBS–1% BSA). A coverslip was placed over the cell monolayer in each well, and cells were viewed by using a Bio-Rad MRC1000 confocal microscope to detect GFP and LacZ (rhodamine) expression. All incubations were performed at room temperature; between each step, cells were washed four times with PBS.
RESULTS
Construction of HERE and HERA amplicons.
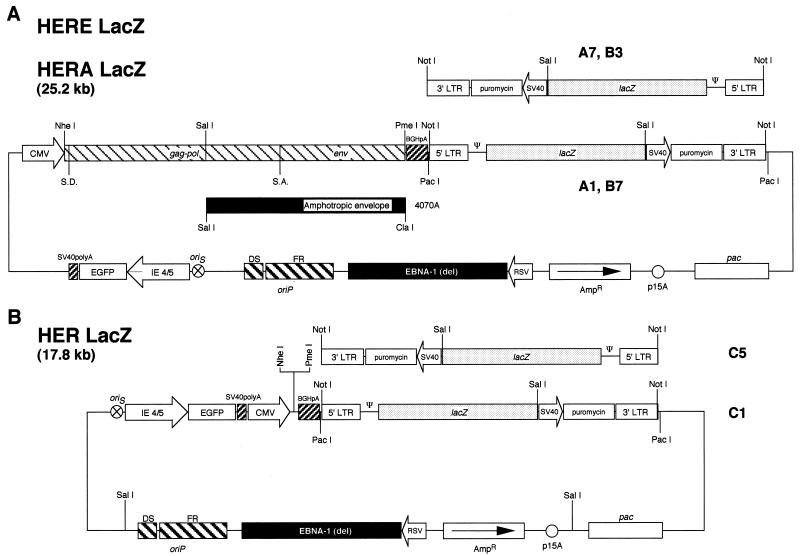
The vector design takes advantage of the host range and retention properties of an HSV-EBV hybrid amplicon to develop a single vector system which enables cells to produce recombinant retrovirus vectors after transduction. Retrovirus genes, gag-pol and env (GPE), and retroviral vector sequences were cloned into an HSV-EBV hybrid amplicon (54a) (Fig. 1). This amplicon has in its backbone the EBV origin of replication (oriP) and a mutant version of the EBNA-1 gene derived from the plasmid p205 (67) under the control of the Rous sarcoma virus promoter. In addition, it carries the cDNA for an enhanced GFP (Clontech, Palo Alto, Calif.), under the control of the HSV immediate-early gene 4/5 promoter (IE4/5), as well as an HSV origin of replication (oriS) and packaging signal (pac). GFP expression can be used to monitor the fate of the amplicon vector backbone. Ecotropic and amphotropic retrovirus GPE expression cassettes were cloned between the CMV immediate-early promoter and the bovine growth hormone polyadenylation signal, generating the HSV-EBV-retrovirus hybrid vectors, HERE and HERA, which code for the ecotropic (E) and the amphotropic (A) envelopes, respectively. Retrovirus vector sequences encoding lacZ were cloned downstream from the GPE expression unit in two different orientations to evaluate potential interference between the CMV and 5′ LTR promoters (Fig. 1A). Vectors HERElacZ A1 and HERAlacZ B7 have the 5′ LTR promoter placed so that transcription from this promoter occurs in the same direction as the CMV-GPE cassette, with vectors HERElacZ A7 and HERAlacZ B3 in the opposite orientation. Two control vectors were also constructed in which the retrovirus vector sequences were cloned in the hybrid amplicon without the gag-pol-env genes, generating the vectors HER lacZ C1 and HER lacZ C5 with the same and opposite orientations relative to the CMV promoter, respectively (Fig. 1B).
FIG. 1.
Amplicon constructs. (A) Schematic representation of the HSV-EBV-retrovirus hybrid amplicons which code for the MMLV gag-pol and env genes. These genes are under the control of the CMV promoter and are followed by the bovine growth hormone polyadenylation signal (BGHpA). The HERElacZ A1 and A7 amplicons code for the ecotropic env gene, while the HERAlacZ B3 and B7 amplicons code for the amphotropic env gene derived from the 4070A genome. (B) Schematic representation of the HERlacZ amplicons C1 and C5 which are missing the MMLV gag-pol-env genes. Amplicon clones A7, B3, and C5 bear the retrovirus lacZ cassette in opposite orientation to the GPE cassette, whereas clones A1, B7, and C1 have it in the same orientation. Abbreviations: S.D., splicing donor; S.A., splicing acceptor; ψ, retrovirus packaging signal; lacZ, E. coli β-galactosidase gene; SV40, simian virus 40 promoter; pac, HSV packaging signal; p15A, E. coli origin of plasmid replication; Ampr, ampicillin resistance gene; RSV, RSV promoter; EBNA-1 (del), EBNA-1 with most of the internal Gly-Ala repetitive sequence deleted (67); oriP, EBV latent origin of replication which contains two elements, the family of repeats (FR) and dyad symmetry (DS) element; oriS, HSV origin of DNA replication; IE4/5; HSV immediate/early 4/5 promoter; EGFP, enhanced green fluorescent protein gene; SV40polyA, SV40 polyadenylation signal.
To minimize the risk of recombination events taking place in this new amplicon packaging system which could generate RCRs, the MMLV gag-pol-env genes and vector elements were modified to reduce overlap between them. The gag-pol-env cassette derived from the plasmid MOVΨ− has a 350-bp deletion that removes the retrovirus packaging signal (41). In addition, the promoter elements were deleted from the 5′ LTR, leaving it intact to avoid altering the splicing mechanism that generates the message encoding the envelope glycoproteins (SU protein, gp70; TM protein, p15E). The 3′ end was modified to remove all noncoding sequences after the stop codon of the env gene. Two different gag-pol-env cassettes were constructed, one conferring an ecotropic (MOV12) and another an amphotropic (MOVAmpho) host range. These cassettes were cloned into pcDNA3.1Neo(−) vector under the control of the CMV promoter, flanked at the 3′ end with the BGH polyadenylation signal (BGHpolyA).
The retrovirus vector component of this amplicon vector was derived from the retrovirus vector, BabePuro (43). To reduce the overlap with the GPE cassettes, the packaging signal was reduced to its minimal sequence, removing the gag gene region. The 3′ end of the vector contains the 3′ LTR and the 35-bp sequence that separates the stop codon of the env gene from the 3′ LTR, and thus it has no sequence overlap with the modified retrovirus genes. With these two sequence alterations, the homology between the vector element and the GPE cassette was reduced to 164 bp at the 5′ end.
Testing the redesigned retrovirus elements for production of recombinant retrovirus vectors.
Human 293T/17 cells were cotransfected with pcDNA3.1MOV12 and pcDNA3.1MOVAmpho plasmids and a lacZ-encoding retroviral vector plasmid, BabePuro LacZ, with transfection efficiencies of 80 to 90%, as evaluated by X-Gal staining at 48 h. Cotransfections of the pcDNA3.1MOV12 plasmid produced titers of 5.9 × 106 ± 1.6 × 106 TU/ml (± the standard deviation), while the plasmid pcDNA3.1MOVAmpho generated retrovirus titers of 1.8 × 106 ± 4.5 × 105 TU/ml. By comparison, cotransfection of the original MOV-Ψ− plasmid (ecotropic) with BabePuro LacZ plasmid yielded titers of 1.3 × 106 ± 2.9 × 105 TU/ml. Phoenix-E cells, derived from 293 cells, are an established packaging cell line for transient production of retrovirus vectors with ecotropic host range. Transfection of these cells with BabePuro LacZ plasmid resulted in retrovirus titers of 1.2 × 106 ± 3.3 × 105 TU/ml. These results indicate that the GPE expression cassettes generated in this study are fully functional and at least as efficient as the original MOV-Ψ− plasmid or an established packaging cell line in generating retrovirus vectors. The effect of deleting the gag gene region from the extended packaging signal on vector titers was also assessed. Cotransfection of the gag-deleted vector, TVBPlacZ, with pcDNA3.1MOV12 into 293T/17 cells resulted in the production of 7.1 × 106 ± 6.0 × 105 TU/ml, while a similar vector, TVBPgaglacZ, in which the gag region was retained, resulted in similar retrovirus titers of 8.2 × 106 ± 3.9 × 105 TU/ml. Although previous studies have reported that inclusion of the gag region in retroviral constructs resulted in a 10-fold increase in retroviral titers (2, 43), this effect could be construct specific since other reports have also shown that deletion of this gag region from some retrovirus vectors has little or no effect on viral titers (31).
Testing the HERE and HERA amplicons and amplicon vectors for retrovirus vector production.
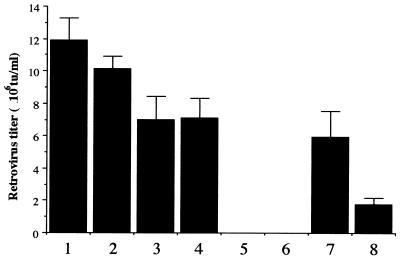
The HERE and HERAlacZ amplicons were transfected into 293T/17 cells (Fig. 2). HERElacZ and HERAlacZ amplicons generated retrovirus stocks with average titers of 1 × 107 and 7 × 106 TU/ml, respectively, values ca. twofold higher than with the GPE cassette and the retrovirus vector in two different plasmids with the same promoters and polyadenylation signals. No significant difference in titers resulted from the two orientations of the retrovirus vector cassette. The presence of both retrovirus elements in one construct means that all transfected cells receive equimolar amounts of both elements and that their rates of loss are the same. No retrovirus vectors were generated when constructs HERlacZ C1 or C5 were transfected into 293T/17 cells, demonstrating that these cells cannot provide retroviral functions necessary for packaging.
FIG. 2.
Retrovirus production in 293T/17 cells after transfection with amplicon constructs. Two million cells were transfected by calcium phosphate coprecipitation with HERElacZ A1 (1), HERElacZ A7 (2), HERAlacZ B3 (3), HERAlacZ B7 (4), HERlacZ C1 (5), and HERlacZ C5 (6) amplicon plasmids. Cells were also cotransfected with the BABE LacZ and pcDNA3.1MOV12 plasmids (7) and the BABE LacZ and pcDNA3.1MOVAmpho plasmids (8). Retrovirus titers assessed 48 h posttransfection represent the average of two experiments repeated in triplicate, and the error bars represent standard deviations.
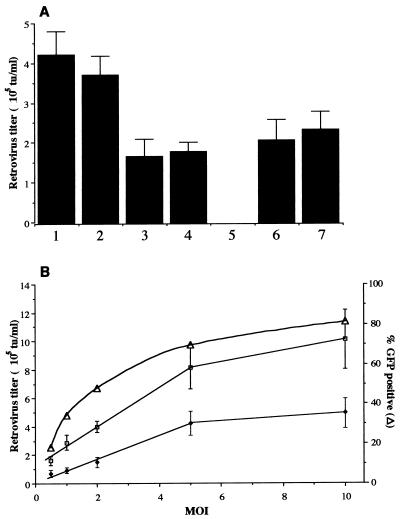
The HERE, HERA, and HERlacZ amplicons were packaged in HSV virion particles in 2-2 cells by using the helper virus-free packaging system developed by Fraefel et al. (18). Typical amplicon vector titers assessed on 293T/17 cells varied between 2 × 106 and 1 × 107 TU/ml. HERE and HERAlacZ amplicon vectors were used to infect naive 293T/17 cells at an MOI of 2. The transduction efficiency was 50%, measured as the percentage of GFP-positive cells at 48 h postinfection by FACS analysis. At this time the supernatants were harvested for titering (Fig. 3A). The HERElacZ amplicon vectors yielded retrovirus titers of 4.2 × 105 ± 6.2 × 104 TU/ml for HERElacZ A1 and 3.7 × 105 ± 4.7 × 104 TU/ml for HERElacZ A7, values ca. twofold higher than those obtained for the HERAlacZ amplicon vectors (HERA lacZ B3, 1.7 × 105 ± 4.3 × 104 TU/ml; HERElacZ B7, 1.8 × 105 ± 2.5 × 104 TU/ml), with no significant difference between the two orientations in either set.
FIG. 3.
Retrovirus vector production in 293T/17 cells 48 h after infection with amplicon vectors. (A) To test whether the orientation of the retrovirus vector cassette in relation to the CMV-GPE cassette had any effect on retrovirus titers, cells were infected at an MOI of 2 with amplicon vectors HERElacZ A1 (1), HERElacZ A7 (2), HERAlacZ B3 (3), and HERAlacZ B7 (4), and 48 h later the supernatants were harvested for titering. A neutralization experiment was performed to demonstrate that retrovirus production is dependent on amplicon transduction. HERAlacZ B7 amplicon vector stocks (MOI of 2) were incubated for 10 min with a rabbit anti-HSV-1 antibody (5), normal rabbit serum (6), and an unrelated rabbit antibody (7) before infection of 293T/17 cells. Media were harvested 48 h later for determination of titers. (B) Relation between MOI and retrovirus titers generated 48 h postinfection. Cells were infected at different MOIs with HERElacZ A1 (□) and HERAlacZ B7 (⧫). The relation between MOI and transduction efficiency was also evaluated (▵). When supernatants were harvested for retrovirus titering, cells were analyzed by FACS to determine the percentage of cells expressing GFP as a measure of the amplicon transduction efficiency.
A neutralization experiment was performed to determine whether production of retrovirus vectors was exclusively dependent on transduction of 293T/17 by the amplicon vector. For this purpose, HERAlacZ B7 amplicon vector stocks were treated with a rabbit antibody directed against HSV-1 envelope glycoproteins, normal rabbit serum, or an unrelated rabbit antibody. Treatment of the amplicon stocks with rabbit anti-HSV-1 antibody reduced the transduction efficiency of the amplicon vector (by 200-fold from 50% to 0.2% at an MOI of 2) as determined both by GFP and LacZ expression, and consequent retrovirus vector production decreased by more than 10,000-fold to less than 10 TU/ml. Treatment of amplicon vector stocks with normal rabbit serum or an unrelated rabbit antibody did not have any effect on transduction efficiency or retrovirus titers (Fig. 3A). The fact that the change in transduction efficiency after antibody neutralization was the same by both methods of determination (GFP and LacZ expression) indicates that most LacZ expression detected at 48 h postinfection was amplicon derived and thus demonstrates that the amplicon stocks are not contaminated to an appreciable extent with functional LacZ retrovirus vectors produced during amplicon packaging.
HERElacZ A1 and HERAlacZ B7 amplicon vectors were used to analyze the relationship between MOI and resulting retrovirus titers 48 h postinfection of 293T/17 cells (Fig. 3B). There was a linear relationship between MOI and retrovirus titers, up to an MOI of 5 (HERElacZ A1 amplicon, y = 1.2 × 105 + 1.4 × 105 x, R2 = 0.99; HERAlacZ B7 amplicon, y = 1.4 × 104 + 8.0 × 104 x, R2 = 0.99). Maximal retrovirus titers of 1.0 × 106 ± 2 × 105 TU/ml and 4.9 × 105 ± 1.0 × 105 TU/ml were achieved at an MOI of 10 for HERE and HERAlacZ amplicon vectors, respectively. For both types of amplicon vectors, the transduction efficiency was 18% at an MOI of 0.5 and reached a maximum of 81% at an MOI of 10 (Fig. 3B).
Retrovirus production in gliomas.
A number of glioma lines derived from human (T98, U87.ΔEFGR, SNB-19, and Gli-36), rat (9L and CNS-1), and dog (J3T) tumors were tested for their ability to produce retroviruses upon infection with the HERAlacZ B7 amplicon vector at an MOI of 2 (Table 1). For three human glioma lines, Gli-36, SNB-19, and U87.ΔEGFR, retrovirus titers were roughly proportional to transduction efficiencies. T98 was an exception to this trend, with less than 10 TU/ml detectable in the supernatant, while the percentage of transduced cells was 13%. The rodent glioma models, 9L and CNS-1, showed low infectability with the amplicon vector and very low production of retrovirus vectors. The dog glioma line J3T generated retroviral titers of 6 × 104 to 8 × 104 TU/ml with 20 to 25% transduced cells.
TABLE 1.
Transduction efficiency and production of retrovirus vectors by glioma lines after infection with HERAlacZ B7 amplicon vector
| Cell line | Species | % GFP-positive cellsa | Retrovirus titer (TU/ml)b |
|---|---|---|---|
| Gli-36 | Human | 80–90 | (1.0–1.2) × 105 |
| SNB-19 | Human | 15–21 | 400–1,000 |
| T98 | Human | 8–13 | <10 |
| U87.ΔEGFR | Human | 5–8 | 200–300 |
| 9L | Rat | ND | <10 |
| CNS-1 | Rat | 7–9 | <30 |
| J3T | Dog | 20–25 | (6–8) × 104 |
Cells were infected with HERAlacZ B7 amplicon vector at an MOI of 2, and 2 days postinfection the percentage of GFP positive cells was determined by FACS analysis. ND, not determined.
Media were harvested to determine retrovirus titers at 2 days postinfection. Titers are an average of two experiments repeated in triplicate.
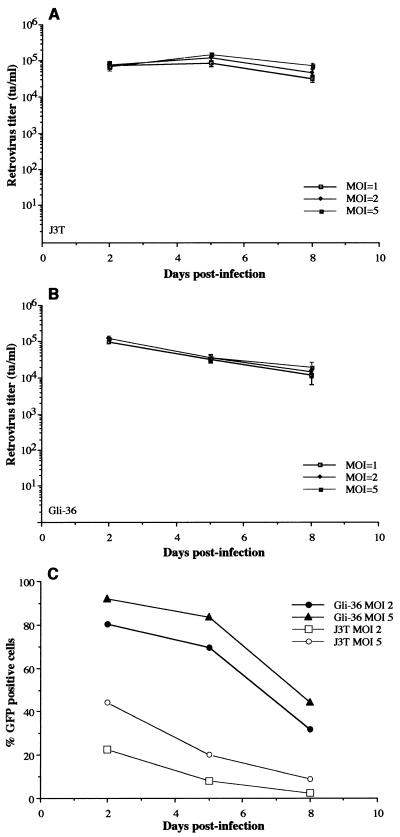
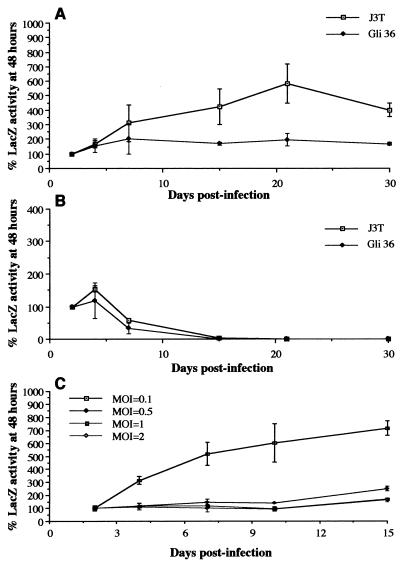
Gli-36 and J3T glioma cells, which yielded the highest retroviral titers, were chosen to analyze the kinetics of retrovirus production. One day after plating, cells were infected at different MOIs with HERAlacZ B7 amplicon vector and 2 days later the supernatants were harvested for to determine the titers of the retrovirus vectors. Cells were then passaged at the same density as on day 0, and this procedure was repeated on days 5 and 8 postinfection. The production of retrovirus vectors over time was different between these two cell lines. J3T cells showed a slight increase in retroviral titer between day 2 and day 5, followed by a small decay between day 5 and day 8 (Fig. 4A). Maximum retroviral titers of 1.6 × 105 TU/ml were obtained for an MOI of 5 at day 5 postinfection. Titers decreased to 8.0 × 104 TU/ml by day 8 for the same MOI. In contrast, Gli-36 cells showed maximal retroviral production of 1.0 × 105 to 1.2 × 105 TU/ml at 48 h postinfection and a decrease thereafter to titers of 2.0 × 104 to 3.0 × 104 TU/ml at 8 days. No significant differences were observed with increasing MOIs for Gli-36 (Fig. 4B).
FIG. 4.
Retrovirus production over time for J3T and Gli-36 cells infected with HERAlacZ B7 amplicon vector at different MOIs. One day prior to infection 5 × 105 cells were seeded on 60-mm plates. (A) J3T cells were infected at MOIs of 1 (□), 2 (⧫), and 5 (■). (B) Gli-36 cells were infected at MOIs of 1 (□), 2 (⧫), and 5 (■). Media were harvested at 2, 5, and 8 days postinfection and used for retrovirus titering. At 2 and 5 days postinfection cells were counted and replated at the same density as on day 0. (C) The percentage of GFP-positive cells was determined at 2, 5, and 8 days postinfection for J3T (MOI = 2, □; MOI = 5, ○) and Gli-36 cells (MOI = 2, ●; MOI = 5, ▴).
The retention of amplicon sequences over time in infected J3T and Gli-36 cells was determined for MOIs of 2 and 5 by evaluating the percentage of GFP-positive cells by FACS analysis. For J3T cells infected at MOIs of 2 and 5, the percentage of GFP-positive cells decreased over time by about 50% from day 2 to day 5 and again from day 5 to day 8 (Fig. 4C). For Gli-36 cells infected at MOIs of 2 and 5 the percentage of amplicon transduced cells decreased about 10% from day 2 to day 5 and about 35% from day 5 to day 8 (Fig. 4C). The apparent slower rate of loss in Gli-36 cells could be due to higher infectivity, with perhaps some cells being infected initially with multiple amplicon vectors.
The ratio of the retrovirus particles produced per producer cell can be used as a measure to estimate the efficiency of a packaging cell line. To calculate this ratio, the assumption was made that all GFP-positive cells produced retrovirus vectors. If we take into account the total number of GFP-positive cells and retrovirus titers for each time point, for Gli-36 cells infected at an MOI of 2 or 5, this packaging ratio was between 0.13 and 0.15 retroviral particles/producer cell at 48 h, decreasing at later time points to about 0.04 to 0.05. For J3T cells infected at an MOI of 2 this ratio was 0.5 at day 2, 0.94 at day 5, and 1.2 at day 8 postinfection, while for an MOI of 5 the ratio remained the same at 0.5 from day 2 to day 8, indicating that J3T cells are inherently more efficient than Gli-36 cells at generating retrovirus vectors with the HERA vector system.
J3T and Gli-36 cells also grew at different rates during the course of the experiment. Gli-36 cells increased in average sixfold for each 3-day time interval and MOI analyzed, corresponding to a doubling time of 29 h, a finding similar to the situation with uninfected cells under the same culture conditions. For the last two time intervals J3T cells showed an average generation time of 24 h for all three MOIs tested, similar to the uninfected cells. However, for the first time interval, the generation times appeared to be longer and dependent on MOI. For MOIs of 1 and 2 the doubling time was 36 h, while for an MOI of 5 it was 48 h, possibly indicating some initial vector toxicity. The rate of loss of GFP expression as a function of cell division was similar for Gli-36 and J3T cells infected at MOIs of 2 and 5 between day 2 and day 8 postinfection: for Gli-36 it was 12 and 10% per generation, respectively, and for J3T it was 14 and 12% per generation, respectively.
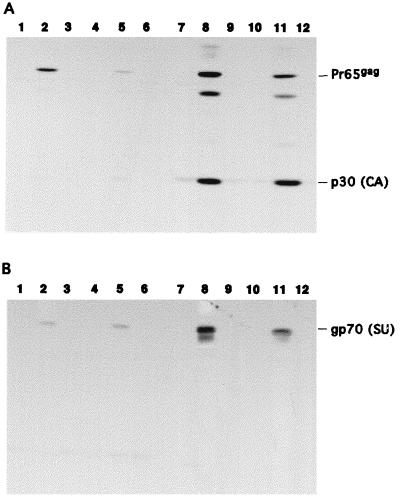
Although the amplicon infection efficiency of Gli-36 cells was about fourfold higher than for J3T, the maximal retrovirus titers produced were similar, and thus the ratio of number of retroviral vectors/producer cell for Gli-36 cells was lower and decreased over the 8-day period, though it remained relatively constant for J3T cells. To determine whether relative production of retrovirus virion proteins was responsible for the difference between lines, expression of Gag-Pol and gp70 (surface protein, SU) retrovirus proteins was analyzed in infected cells over time. Total proteins were extracted at day 2 and day 8 postinfection from cells infected at an MOI of 2 with HERAlacZ B7 and HERlacZ C1 amplicon vectors and naive cells and then analyzed by Western blot by using antibodies capable of recognizing the MMLV Gag-derived capsid protein p30(CA) (Fig. 5A) and gp70 (Fig. 5B). For J3T (Fig. 5A, lanes 2 and 5) and Gli-36 (Fig. 5A, lanes 8 and 11) cells infected with the HERAlacZ B7 amplicon vector, it was possible to detect the Pr65gag precursor protein, as well as the mature p30(CA) capsid protein with the anti-p30 antibody. Other immunoreactive polypeptides were also detected which could be partially cleaved forms of the Pr65gag precursor. The densities of bands corresponding to the precursor Pr65gag and p30(CA) were compared for each cell line at different time points. For J3T cells, there was a 12-fold decrease in the amount of Pr65gag precursor protein between day 2 and day 8 postinfection, while levels of p30(CA) remained the same over this time period. For Gli-36 cells, the Pr65gag precursor decreased 1.8-fold, while p30(CA) remained at the same level over this time interval. Comparison between the two cell lines showed that Pr65gag was 2.2-fold more abundant in Gli-36 cells than in J3T cells at 48 h postinfection, while this difference increased to 16-fold at 8 days. Expression of gp70 (SU) (Fig. 5B) remained the same for J3T cells over time (Fig. 5B, lanes 2 and 5), while for Gli-36 cells it decreased by 1.6-fold (Fig. 5B, lanes 8 and 11). Expression of gp70 in Gli-36 cells was 16-fold higher than in J3T cells at 48 h postinfection. These results indicate that there is no correlation between retrovirus titers and the expression levels of these virion proteins.
FIG. 5.
Western blot analysis of Pr65gag and gp70 expression in J3T and Gli-36 cells at 2 and 8 days postinfection with HERAlacZ B7 and HERlacZ C1 amplicon vectors at an MOI of 2. (A) Pr65gag expression. (B) gp70 expression. Lanes 1 and 4, lysates of naive J3T cells at 2 and 8 days; lanes 2 and 5, lysates of J3T cells at 2 and 8 days postinfection with HERAlacZ B7; lanes 3 and 6, lysates of J3T cells at 2 and 8 days postinfection with HERlacZ C1; lanes 7 and 10, lysates of naive Gli-36 cells at 2 and 8 days; lanes 8 and 11, lysates of Gli-36 cells at 2 and 8 days postinfection with HERAlacZ B7; lanes 9 and 12, lysates of Gli-36 cells at 2 and 8 days postinfection with HERlacZ C1. Goat anti-p30 and anti-gp70 antibodies were used at a 1:3,000 dilution. Anti-goat peroxidase-conjugated IgG was used as secondary antibody at a 1:5,000 dilution. Blots were developed with ECL reagents and exposed to film for 1 min.
Stability of transgene expression.
Stability of amplicon-mediated gene expression in cells transduced by these HERA hybrid amplicon vectors was analyzed above by the percentage of GFP-positive cells, retrovirus vector titers, and expression of retrovirus proteins over time. The question remaining was whether the presence of the retroviral elements, the gag-pol-env cassette and retrovirus vector, have any effect on retention of the transgene (lacZ) carried by the retrovirus vector, i.e., whether retrovirus vectors produced by amplicon transduced cells were able to infect and confer stable transgene expression on other cells in the population. To address this issue, J3T and Gli-36 cells were infected with the HERAlacZ B7 amplicon vectors at an MOI of 2 and LacZ activity was measured in the total population at different times postinfection up to 1 month (Fig. 6A). Since these amplicons have all the functions necessary for packaging retroviruses, it is likely that the amplicon vector stocks themselves contain some retrovirus vectors carrying the lacZ gene, which are produced during packaging of the amplicons in HSV virions, that could stably infect cells, thus contributing to total LacZ activity in the population. To compensate for this factor, LacZ activities in cultures were expressed as a percentage of the activity at 48 h postinfection. The relative LacZ activity for J3T cells, where 22% of cells were initially transduced, increased with time, reaching a maximum increase of fivefold at 3 weeks and maintaining a constant level out to 4 weeks. Gli-36 cells, with 80% transduction with amplicon vector at 48 h, reached a maximum of twofold at 7 days and then remained stable (Fig. 6A). In addition to this difference in relative transgene expression over time, LacZ activity in J3T cells was three- to fourfold higher than in Gli-36 cells at 48 h postinfection. To assess the contribution of the amplicon vector itself to total LacZ expression over time, cells were infected at an MOI of 2 with the HERlacZ C1 amplicon vector, which does not code for any retrovirus proteins. For both cell types, LacZ activity reached a maximum at 4 days postinfection and then decreased rapidly with time, becoming undetectable by day 15 (Fig. 6B).
FIG. 6.
β-Galactosidase activity in amplicon-infected J3T and Gli-36 cells over time. (A) J3T (□) and Gli-36 cells (⧫) were infected with HERAlacZ B7 amplicon vector at an MOI of 2, and the LacZ activity in the population was measured at different time points for up to 1 month. LacZ activities at 48 h for J3T and Gli-36 cells were 317.2 and 79.7 mU/mg of protein, respectively. (B) J3T (□) and Gli-36 cells (⧫) were infected with HERlacZ C1 amplicon vector at an MOI of 2, and the LacZ activity in the population was assayed at different time points of up to 1 month. (C) Gli-36 cells were infected with HERAlacZ B7 amplicon vector at MOIs of 2 (◊), 1 (■), 0.5 (⧫), and 0.1 (□), and LacZ activity in the population was measured at different time points for up to 15 days. LacZ activities at 48 h postinfection for MOIs of 2, 1, 0.5, and 0.1 were 147.5, 94.6, 62.3, and 9.6 mU/mg of protein, respectively. LacZ activities for all experiments were represented as the percentage of LacZ activity at 48 h. Results represent the average of two experiments repeated in triplicate for each cell line.
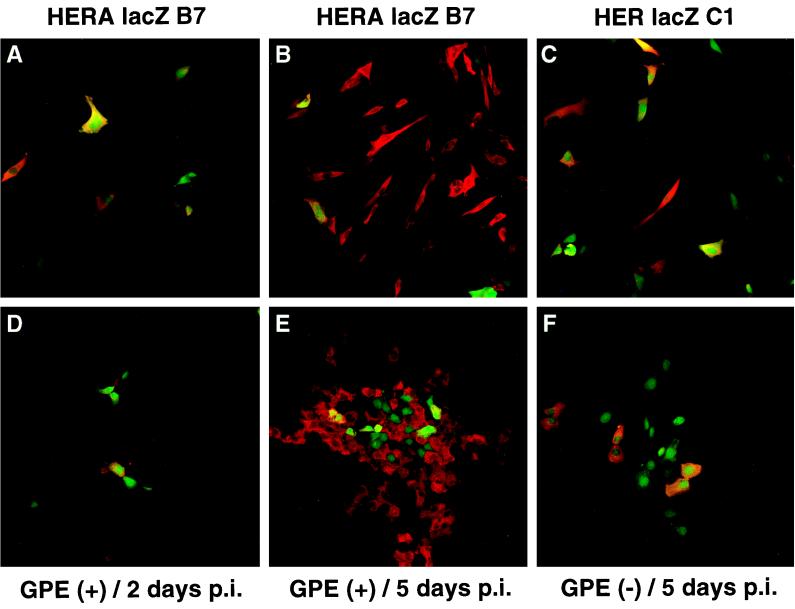
Since there was a greater increase in LacZ activity over time in J3T cells, which had a lower amplicon transduction efficiency than Gli-36 cells, experiments were designed to evaluate whether the relative increase in LacZ activity was dependent on the initial percentage of amplicon infected cells. Gli-36 cells were infected with HERAlacZ B7 amplicon vector at MOIs of 0.1, 0.5, 1, and 2, achieving a range of transduction efficiencies (percent GFP-positive cells) at 2 days postinfection of 17, 49, 66, and 80%, respectively. An inverse correlation between increase in LacZ activity and MOI was observed over a period of 2 weeks (Fig. 6C). For the population infected at an MOI of 0.1, LacZ activity increased by sevenfold, while for an MOI of 2, LacZ activity increased only by about twofold. Absolute LacZ activity levels were 15-fold higher for an MOI of 2 than for an MOI of 0.1 at 48 h postinfection, while at 2 weeks this difference decreased to 3.5-fold. Thus, the final increase in LacZ activity approximately reflected the initial percentage of infected cells, being sevenfold for Gli-36 at 17% transduced and fivefold for J3T at 22%. An additional experiment was performed to visualize the spread of LacZ expression over time in J3T and Gli-36 cell populations (Fig. 7). Cells were infected with HERAlacZ B7 or HERlacZ C1 amplicon vectors at an MOI of 2 for J3T and 0.1 for Gli-36 cells to achieve comparable initial transduction efficiencies of approximately 20%. At 2 days post-amplicon infection the number of LacZ-expressing cells was the same or lower than those expressing GFP for both cell lines (Fig. 7A and D). However, by day 5 post-amplicon infection the number of LacZ-expressing cells was much higher than the number of cells expressing GFP for both cell lines infected with HERAlacZ B7 (Fig. 7B and E). In contrast, for cells infected with HERlacZ C1, the number of cells expressing LacZ remained the same or lower than those expressing GFP at day 5 (Fig. 7C and F).
FIG. 7.
Spreading of transgene expression in glioma cells. J3T (A to C) and Gli-36 (D to F) glioma cells were infected at MOIs of 2 and 0.1, respectively, with HERAlacZ B7 (A, D, B, and E) and HERlacZ C1 (C and F) amplicon vectors achieving initial percentages of transduction (GFP positive) of 20%. Cells were fixed at 2 (A and B) and 5 (B, C, E, and F) days postinfection. LacZ expression (red) was detected by immunofluorescence by using a rhodamine-conjugated secondary antibody, and GFP was detected by its intrinsic fluorescence.
DISCUSSION
We report here on the development and characterization of a hybrid amplicon vector system capable of single-step conversion of cells to retrovirus vector producer cells. Transduction of several cell types with this vector system was shown to result in the efficient production of retrovirus vectors for extended periods. For the same cell line, retrovirus titers were dependent on the extent of amplicon transduction, but different cell lines displayed different capacities for retrovirus production. Infection of a subpopulation of dividing cells with this system results in a continuing increase in transgene activity until a stable, steady-state level is reached.
Several vector systems have been developed which can induce cells to transiently produce replication-deficient retrovirus vectors after transduction. Some systems use transfection to introduce components of the vector system into cells (39, 45, 47). This process is efficient in culture but relatively inefficient in vivo. Others have used combinations of viral vectors to achieve the same result. In a two-step approach, cells are first infected with a retrovirus vector and then with an HSV amplicon vector coding for the gag-pol and env genes (55) or with an adenovirus vector carrying these genes in the same (40) or separate vectors (40, 68). Retrovirus genes and vector element can both be delivered by adenoviral vectors (13, 15). Some of these adenovirus-based systems can generate relatively high retrovirus titers (105 to 106 CFU/ml) in cultured cells (13, 68). Although systems that employ different vectors to deliver the elements necessary for retrovirus production have a low potential to generate replication-competent retroviruses, output titers depend on the efficiency of cotransduction. The higher the number of different vectors needed, the lower the probability that all will infect the same cell to allow retrovirus vector production. Adenovirus vectors can be produced at very high titers (>1010 PFU/ml) and can achieve remarkable gene transfer efficiencies in some circumstances in vivo. However, in some applications, such as gene transfer to malignant human gliomas, their transduction efficiency has been reported to be only as high as 30 to 35% in certain areas, with an overall efficiency below 11% (52). At this level of transduction the probability that two or three vectors will infect the same cell is low, and therefore the ability to convert cells in situ into the retrovirus packaging cells would be compromised. Nonetheless, Feng et al. demonstrated that in a tumor model in vivo a retrovirus production system based on two adenoviral vectors was capable of increasing the length of transgene expression as compared to a conventional adenovirus vector (15). This study demonstrated the principle, but conceptually the process should be more efficient if a single adenovirus vector contained all the necessary elements to generate retrovirus vectors. Although the size limitation of conventional adenovirus vectors precludes the construction of such a combined vector, “gutless” adenovirus vectors could easily accommodate all of the components (16, 32, 34).
The hybrid amplicon vector system described here has several advantages over existing vector systems for generating retrovirus vectors. (i) All elements necessary for production of replication-deficient retrovirus vectors are present in the same construct. In theory the conversion efficiency per transduced cell is 100%, independent of whether amplicon DNA is introduced into cells by transfection or infection. (ii) Not only do HSV virions have a wide host range, high stability, and high infectability but, due to their large DNA capacity and the mode of viral DNA replication, each amplicon vector carries multiple copies of the amplicon plasmid. This means that for each transduction event multiple copies of the vector producing components and transgenes are delivered to the cell nucleus. This may account for the high retroviral titers obtained with this system. (iii) EBV elements, oriP and the EBNA-1 gene, which mediate episomal replication and retention of amplicon DNA in dividing cells, increase the duration of retrovirus production. (iv) Lastly, HSV amplicons have a large transgene capacity (theoretically up to 150 kb) which will allow the inclusion of other elements to improve the efficiency of retrovirus vector production.
The efficiency of gene delivery mediated by this hybrid amplicon vector system is dependent on a number of cellular factors, including the infectability with HSV and retrovirus virions, the capacity to produce retrovirus vectors, and the retention of HSV-EBV amplicon elements. Infectability of target cells by HSV virions is a primary determinant of the levels of retrovirus vector production by a cell population; the transduction efficiency by retrovirus vectors is ultimately responsible for the capacity for long-term transgene retention. In this study, infection of a variety of cell lines with the same number of hybrid amplicon virions resulted in a wide spectrum of transduction efficiencies (Table 1). Entry of both types of virus is dependent on the expression levels of certain cell surface receptors (35, 42). Since manipulation of these levels in vivo is not an option, optimization can only be accomplished by modifying the virions to increase the variety of cell surface receptors which can be used to enter cells. For HSV virions this could be achieved by using helper functions from different strains of HSV and/or modifying the virion envelope to enhance binding of the vector to receptors abundant on the target cells (37). For retrovirus vectors, a broader host range can be achieved by pseudotyping the virions with vesicular stomatitis virus envelope glycoprotein (5). However, expression of this protein can be toxic and thus limit the length of time that transduced cells can produce vectors. An alternative would be to use other envelope genes that have different host ranges and no toxicity associated with their expression, such as the gibbon ape leukemia virus envelope glycoprotein or chimeric envelope genes which are modified to target specific cell types (29).
Another factor that affects the gene delivery efficiency of this system is the ability of the amplicon-infected cells to produce retrovirus. In the present study, infection of different cell lines with the HERAlacZ amplicon resulted in a wide range of retrovirus titers that did not always correlate with cell infectability, since different cell lines with comparable transduction efficiencies produced very different retrovirus vector titers (Table 1) and the number of retrovirus vector particles produced/amplicon-transduced cell was 10-fold higher for J3T than for Gli-36 cells. Other studies also suggest that cells have an intrinsic maximal capacity to generate retrovirus, which is dependent on cellular factors that are not yet understood (9, 13). Several hypothesis can be advanced to explain the differences in retrovirus vector production among cell lines: limiting amounts of packageable RNA, low expression of retroviral protein(s), or premature processing of retrovirus precursor proteins. The amount of packageable retrovirus vector RNA can be estimated from the level of LacZ activity per GFP-positive cell early in the course of infection (48 h), when most LacZ activity is attributable to amplicon-infected cells and not to subsequently retrovirus-infected cells. At this early time point the LacZ activity per GFP-positive cell is 15-fold higher in J3T cells than in Gli-36 cells, indicating that the level of vector RNA generated from the 5′ LTR promoter is higher, corresponding to higher retrovirus production in J3T cells. Possible explanations are that the MMLV LTR promoter may be weaker in Gli-36 cells than in J3T cells, a result due either to low or absent levels of some transcription factors necessary for efficient promoter activation or to inhibition by the upstream CMV promoter via a deficient transcription termination mechanism mediated by the BGHpolyA sequence.
Although low expression levels of retrovirus proteins could also be a limiting factor in some cells, this does not appear to be a factor in Gli-36 and J3T cells since Pr65gag and gp70 levels are higher in Gli-36 than J3T cells by about the same magnitude as the percentage of transduced cells, which is the inverse of their retrovirus vector production efficiencies. It has been shown that overexpression of the human immunodeficiency virus type 1 (HIV-1) Gag-Pol polyprotein can lead to intracellular activation of the viral protease, mainly resulting in the intracellular production of capsid and matrix proteins, with inhibition of assembly and budding of virus-like particles from the cell membrane (28). The presence of intracellular processed MMLV virion proteins, e.g., p30(CA), has also been reported after infection with adenovirus vectors (13, 40) or SFV-derived expression vectors (39). The presence of mature virion proteins, such as p30(CA), suggests that the viral protease is being prematurely activated and may result, by comparison with HIV, in inhibition of virion formation. Thus, the cellular ratio of Pr65gag and p30(CA) may be a useful indicator for the efficiency of virion assembly and retrovirus production. Based on the density of the bands on the Western blot in this study, between day 2 and day 8 the Pr65gag/p30(CA) ratio for Gli-36 cells decreased by about 2-fold (from 0.9 to 0.5), while that for J3T cells decreased by about 20-fold (from 35 to 1.6). Although these values could serve as a basis to explain the difference in retrovirus production between Gli-36 and J3T cells, the fact that for J3T cells the retrovirus production efficiency ratio (vectors/GFP+ cell) increased by about twofold between day 2 and day 8 argues against this connection. However, since it has not been determined which premature virion proteins are responsible for the inhibitory effect on HIV virion assembly, the presence of this same type of effect for MMLV assembly cannot be ruled out. If premature processing has an important role in determining maximum retrovirus output in different cell types, it will be necessary to understand the cellular factors that regulate this processing before any improvements can be made to increase retroviral production from certain cell types. Alternatively, if the main cause of different retrovirus production capacities among cell types is related to promoter activity, several changes in vector design can be made to increase the efficiency in a broad spectrum of cells: (i) a substitution of the MMLV 5′ LTR by chimeric LTR promoters which can yield higher retrovirus vector titers (31, 45); (ii) an increase in the distance between the GPE expression cassette and the retrovirus vector sequences to reduce the chance of transcriptional interference between the two promoters; (iii) the introduction of additional transcription termination or insulator sequences to reduce promoter interference; and (iv) the construction of a large library of HERA amplicon vectors with different promoter combinations to identify the most efficient configuration for particular cell types.
In this study it was evident that transgenes carried in the amplicon backbone decreased in expression with time after infection in dividing cell populations. This was true for both GFP and retrovirus proteins, as well as retrovirus vector production. Since expression of those transgenes was mediated by different promoters, the most likely explanation for this decline is the loss of amplicon DNA, as has been observed by others for HSV-EBV amplicon vectors (63). In the absence of drug selection for amplicon retention, both Gli-36 and J3T cells lost 10 to 12% of GFP-positive cells per generation. Other studies have shown that without drug selection, plasmids carrying the EBV oriP element and the EBNA-1 gene are lost from human cells at rates of between 1 and 5% per generation (67). The apparently faster rate of loss in our study could be due to a number of factors: (i) the rate was measured as loss of transgene expression and could include promoter inactivation, as well as the loss of amplicon DNA; (ii) the mutant EBNA-1 gene used, which has most of the Gly-Ala repeats deleted, has been shown to be less effective than full-length versions in episome replication and maintenance (65); (iii) the presence in the amplicon vector of multiple origins of DNA replication (both EBV oriP and HSV oriS) might interfere with DNA replication; (iv) the concatemeric nature of the amplicon episome and its size (150 kb) may be conducive to a high frequency of deletional recombination events disrupting transgene expression; and (v) expression of some of the genes present in this triple hybrid amplicon (tribrid) might increase the doubling time of transduced cells and thus select against their frequency in the population over time.
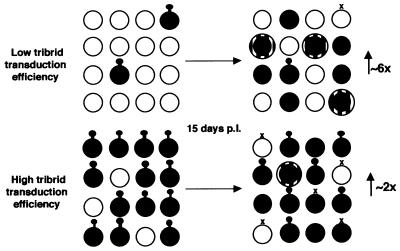
This tribrid vector system provides a means to stably deliver a transgene to a large percentage of cells in a dividing population starting with transduction of a small percentage of cells. Gli-36 and J3T populations infected at an MOI of 2 with the HERAlacZ amplicon vector displayed different profiles of LacZ activity over time, with large (sixfold) and small (twofold) increases, respectively. The main reason for this difference appears to be the percentage of cells initially transduced, since when this was normalized to about 20% initially transduced (MOI = 2 for J3T and MOI = 0.1 for Gli-36), both populations increased LacZ activity over successive generations by about sixfold over 2 weeks post-amplicon infection (Fig. 6). These dynamics can be explained as follows. Shortly after infection of a dividing cell population with the amplicon vector, two different cell genotypes are present while a third one arises later. The first genotype, amplicon-transduced cells, express GFP and LacZ encoded by the episomal amplicon and produce retrovirus vectors carrying the lacZ gene but are themselves resistant to infection by those same retrovirus vectors due to the presence of the gp70 envelope proteins on the cell surface (8). The second initial genotype represents uninfected cells which do not express any transgenes but are susceptible to infection by retrovirus vectors produced by amplicon-transduced cells. With time, a third cell type appears in the population which expresses LacZ from retrovirus vector sequences stably integrated in their genome and is receptive to multiple retroviral infections. Due to the episomal nature of the amplicon and the absence of any selection pressure for retention, the first population decreases in representation over time, and therefore overall retrovirus vector production also declines. Cells which lose the amplicon also lose expression of LacZ and retroviral proteins but become susceptible to retroviral infection, depending on the half-life of gp70 on the cell surface. The third cell type becomes more prominent with time, until it is the sole contributor to LacZ activity in the population and essentially no more retrovirus vectors are produced. Total LacZ activity in the population at any time is the sum of LacZ expressed from the amplicon episomes (six copies/episome × number of episomes per cell) and from the retrovirus vectors stably integrated in the cell genome (one copy/infection × number of infections). Absolute levels of LacZ activity will depend on the relative activity of the LTR promoter in the context of the episome or at different sites of integration in different cell types. The behavior of cell populations in accumulating transgene activity can be described by the following hypothetical model (Fig. 8). At low initial levels of amplicon transduction, the number of transduced cells and levels of LacZ activity are low in the early postinfection period. At the same time there is a large pool of cells which are susceptible to retrovirus infection and hence able to achieve stable LacZ activity. In contrast, at high initial levels of transduction, LacZ activity is high in the early period postinfection, but the percentage of retrovirus infectable cells is small and increases only as retrovirus titers are declining, thus achieving only a modest increase in LacZ activity. Interestingly, for both Gli-36 and J3T cells, expression levels of gp70 declined more slowly (Fig. 5) than retrovirus titers (Fig. 4), suggesting that cells which have lost their ability to produce retrovirus might still not be susceptible to retrovirus infection due to the continuing presence of envelope protein on the cell surface. This model would explain why lowering the percentage of amplicon-transduced cells (lower MOIs) results in a higher increase in LacZ activity in the population over time (Fig. 6C). Immunofluorescence analysis of LacZ expression over time in J3T and Gli-36 glioma cells showed a dramatic increase in the ratio of LacZ-expressing cells to GFP-expressing cells only in populations infected with the HERAlacZ B7 amplicon (Fig. 7A, B, D, and E). This demonstrates that transgene retention and spreading in dividing cell populations infected with the HERA system is mediated by retrovirus vectors produced in situ.
FIG. 8.
Model of cumulative increase in transgene expression in cell populations infected with the tribrid vector. The hypothesized dynamics of transgene expression are shown for two different transduction efficiencies immediately after infection with the tribrid amplicon vector and 2 weeks later, assuming a dividing cell population. Right after infection, uninfected cells (indicated as open circles) do not express the LacZ transgene, and cells infected with the amplicon vector (solid circles with “lollipop”) express LacZ and retroviral proteins, which interfere with their ability to be infected with retrovirus vectors. After 2 weeks a number of parallel phenomena have occurred. Some initially uninfected cells are now infected once by a retrovirus vector (LacZ+, solid circles) or multiple times with retrovirus vectors (higher LacZ expression, solid circles with halo). Over the same period, some cells initially infected with the amplicon vector have lost the episomal amplicon and hence their ability to produce LacZ or retroviral proteins (open circle with “×”), while some of these have subsequently become infected by retrovirus vectors produced by amplicon-infected cells that retain the episomal tribrid (LacZ+, solid circles with “×”). Therefore, at low transduction efficiency, when only a small fraction of cells are initially infected with the amplicon vector, there is a larger increase in the total LacZ activity of the population than in populations with high levels of initial transduction. Eventually the episome tribrid is lost from all cells, and then the population should reach a steady-state level of LacZ expression, reflecting the number of successful infections with retrovirus vectors.
The HERA tribrid gene delivery system could be used in vivo to directly modify specific cell populations, which by their intrinsic location, migratory properties, or tissue- and/or organ-targeting properties, could secondarily deliver retrovirus vectors to progenitor or stem cells or to a larger population of dividing cells, resulting in expanded numerical and spatial distribution of transgene-expressing cells. Several applications for this gene delivery amplification mechanism can be anticipated, including tumor therapy and genetic modification of the hematopoietic system, lung epithelium, developing CNS, and other proliferating cell populations. Use of this gene delivery-amplification mechanism in the adult is limited to some extent by the fact that the retrovirus elements in this tribrid system are derived from MMLV, which can only infect dividing cells. However, this limitation could be overcome by replacing these elements with lentivirus elements, thus allowing production of lentivirus vectors that can infect both dividing and nondividing cells.
Two modalities of therapeutic gene delivery to tumors are considered here by way of example. In one, the retrovirus producer cells would be derived from the tumor cells themselves and thus presumably share the same growth and migratory patterns; in the other, normal cells with migratory or tumor-targeting properties could be used as gene delivery vehicles, e.g., neuronal progenitor cells (1), endothelial cells (4, 36), and tumor-infiltrating lymphocytes (30). Several studies have shown that packaging cell lines currently in use, which are derived from mouse fibroblasts, do not display migratory properties in the context of brain tumors (53, 61, 62). This characteristic presents several restrictions to effective gene delivery. The packaging cells tend to form clusters around the injection site and, given the limited diffusion of retroviruses through cell layers, only retrovirus vectors released close to the cluster surface can infect tumor cells. As a result, the number of vector particles that reach tumor cells is much lower than what would be expected from the number of packaging cells implanted. Furthermore, retrovirus vectors have a relatively short half-life and can only infect dividing cells. In experimental brain tumor models, which have high mitotic indexes, these small “clusters” of gene delivery can be effective in therapeutic strategies. However, in human glioblastomas, where it is estimated that only 1 to 8% of the cells are actively dividing (69), the probability of a packaging cell being close to a dividing cell is extremely low. Even more problematic for gene therapy of human glioblastomas is the invasive nature of these cells, which migrate extensively throughout the CNS (51, 66), whereas injection of vector-producing cells is focal. Tamura et al. showed that if the same type of glioma cells is implanted in a tumor mass, these cells can migrate along the same routes as the initial tumor cells and eventually reach them (62). Thus, the use of tumor cells themselves as retrovirus producers could serve as a strategy for spreading vector production over larger areas. To achieve this, however, producer cells would need to survive for extended periods in the brain. Although the CNS is relatively immune privileged, an immune response to expressed retroviral proteins or transgene products can limit the survival of amplicon-derived packaging cells. Possible ways to reduce the immune response would be to incorporate in the amplicon certain genes that code for immune-modulatory molecules, such as those that some tumors use to evade the immune system, e.g., CD95 ligand, transforming growth factor-β, and interleukin-10 (22) or viral genes, such as HSV ICP47, which interfere with antigen presentation (19, 24). The main advantage of this tribrid amplicon gene delivery system for tumor therapy is that it should mediate retrovirus vector production in situ over an extended range after direct injection of amplicon vector stocks into the tumor mass.
This transgene delivery amplification mechanism could also be used to expand the range of gene delivery in the developing CNS. Several studies performed with replication-competent retrovirus vectors during development have shown that transgenes can be delivered to a large number of cells throughout the entire CNS (14). However, RCRs carry with them not only the transgene of interest but also a high risk of insertional oncogenesis. The HERE-HERA system could be used in a similar, but safer, manner to achieve widespread gene delivery in the CNS. Genetic modification of cells in the subventricular zone would create two amplifying effects. (i) Production of retrovirus vector in that area could result in genetic modification of neuronal progenitors that would later divide, migrate into the brain, and differentiate into neurons or glia. (ii) Amplicon-transduced cells could themselves migrate to other regions of the brain where they could come into close contact with populations of dividing cells, e.g., during gliogenesis. This spatial expansion of transgene expression would be useful for disease states where the entire CNS is involved and can be corrected by diffusible factors, as is the case for some lysosomal storage disorders.
Another example of the usefulness of this gene delivery approach is genetic modification of the hematopoietic system. This could be achieved by transducing CD34-positive cells in culture, followed by reimplantation and migration of these cells to the bone marrow, where they would come in close contact with dividing hematopoietic stem cells. Alternatively, similar to what was attempted with fibroblast-derived packaging cells (46), the tribrid amplicon vector could be directly injected into the bone marrow. These strategies could be used for gene delivery to normal bone marrow but possibly also in the context of leukemia. Since bone marrow stem cells divide very slowly, it is possible that retrovirus vectors produced in situ would preferentially infect leukemic cells. As a safety mechanism, cells could be transduced with prodrug activating enzymes that result in the production of metabolites whose cytotoxic effects are cell cycle dependent, such as the HSV-tk–ganciclovir system, so that any tumor cells could be selectively eliminated by drug treatment.
The principle of the primary producer cells being in close proximity to secondary target cells might also prove useful to attain stable gene delivery to the lung. Adenovirus vectors and liposomes are capable of transducing the lung epithelium with various degrees of efficiency. Due to their nonintegrative nature and the turnover of cells in the lung epithelium, these strategies only achieve transient expression of transgenes. Furthermore, in some disease states, such as cystic fibrosis, the rate of replacement seems to be accelerated (38). Repeated administration of some of these agents results in strong inflammatory immune responses. Even with integrating vectors such as lentivirus vectors (20) repeated administration would likely be required. Since 95% of the epithelial surface is occupied by type I pneumocytes and only 3% by type II pneumocytes (stem cells which give rise to type I cells), direct vector administration will most likely transduce terminally differentiated type I cells, which are eventually replaced. Since type I cells are derived from type II cells, the latter dividing cells are the appropriate targets to achieve stable genetic modification of the lung epithelium. In theory, single infection of the lung epithelium with HERA amplicons should convert type I cells into retrovirus producer cells, which can, in turn, deliver vectors to type II stem cells in the vicinity when they divide and they, in turn, would continuously give rise thereafter to genetically modified type I cells.
This new tribrid amplicon delivery system provides a means to extend retrovirus gene delivery to large numbers of cells over a wide distribution in the body by direct injection of a HSV-derived amplicon vector. Retroviral delivery to dividing cell populations results in cumulative increases in transgene activity reaching high, stable levels. This new gene delivery system should have wide application in methods of gene therapy.
ACKNOWLEDGMENTS
This work was supported by National Institute of Neurologic Disorders and Stroke grant NS-24279 (X.O.B.), National Cancer Institute grant CA-69246 (X.O.B. and E.A.C.), and Junta Nacional de Investigação Científica e Tecnológica (Portugal)-Programa Praxis grant XXI/BD/3248/94 (M.S.-E.).
We thank Richard Mulligan for providing numerous reagents and helpful suggestions and discussion. We thank Deborah E. Schuback for artwork and critical reading of the manuscript and Jürgen Hampl for critical reading of the manuscript and helpful discussions.
REFERENCES
- 1.Aboody, K. S., N. Rainov, J. E. Small, S. Liu, K. A. Bower, U. Herrlinger, P. Black, X. O. Breakefield, and E. Y. Snyder. Neural stem cells migrate throughout and express foreign genes within experimental gliomas: a potential gene therapy approach to brain tumors. Submitted for publication.
- 2.Bender M A, Palmer T D, Gelinas R E, Miller A D. Evidence that the packaging signal of Moloney leukemia virus extends into the gag region. J Virol. 1987;61:1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berens M E, Bjotvedt G, Levesque D C, Rief M D, Shapiro J R, Coons S W. Tumorigenic, invasive, karyotypic, and immunocytochemical characteristics of clonal cell lines derived from a spontaneous canine anaplastic astrocytoma. In Vitro Cell Dev Biol Anim. 1993;29A:310–318. doi: 10.1007/BF02633959. [DOI] [PubMed] [Google Scholar]
- 4.Brown, A. B., E. Marecos, R. Weissleder, and X. O. Breakefield. Vascular targeting of therapeutic cells to tumors. Submitted for publication.
- 5.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: Concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty A K, Zink M A, Hodgson C P. Transmission of endogenous VL30 retrotransposons by helper cells used in gene therapy. Cancer Gene Ther. 1994;1:113–118. [PubMed] [Google Scholar]
- 7.Chattopadhyay S K, Oliff A I, Linemeyer D L, Lander M R, Lowy D R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981;39:777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin J M. Retroviridae: the virus and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Raven Publishers; 1996. pp. 1767–1848. [Google Scholar]
- 9.Cosset F-L, Takeuchi Y, Battini J-L, Weiss R A, Collins M K L. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culver K W, Ram Z, Wallbridge S, Ishii H, Oldfield E H, Blaese R M. In vivo gene transfer with retroviral vector-producing cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham C, Davison A J. A cosmid-based system for constructing mutants of herpes simplex virus type I. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 12.Dranoff G, Jaffe E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duisit G, Salvetti A, Moullier P, Cosset F L. Functional characterization of adenoviral/retroviral chimeric vectors and their use for efficient screening of retroviral producer cell lines. Hum Gene Ther. 1999;10:189–200. doi: 10.1089/10430349950018986. [DOI] [PubMed] [Google Scholar]
- 14.Fekete D M, Cepko C L. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng M, Jackson J W H, Goldman C K, Rancourt C, Wang M, Dusing S K, Siegal G, Curiel D T. Stable in vivo gene transduction via a novel adenoviral/retroviral chimeric vector. Nat Biotechnol. 1997;15:866–870. doi: 10.1038/nbt0997-866. [DOI] [PubMed] [Google Scholar]
- 16.Fisher K J, Choi H, Burda J, Chen S-J, Wilson J M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 17.Fraefel C, Breakefield X O, Jacoby D R. HSV-1 amplicon. In: Chiocca E A, Breakefield X O, editors. Gene therapy for neurological disorders and brain tumors. Totowa, N.J: Humana Press, Inc.; 1998. pp. 63–82. [Google Scholar]
- 18.Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller A I. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 20.Goldman M J, Lee P-S, Yang J-S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 21.Gross J L, Behrens D L, Mullins D E, Kornblith P L, Dexter D L. Plasminogen activator and inhibitor activity in human glioma cells and modulation by sodium butyrate. Cancer Res. 1988;48:291–296. [PubMed] [Google Scholar]
- 22.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 23.Hatzoglou M, Hodgson C P, Mularo F, Hanson R W. Efficient packaging of a specific VL30 retroelement by psi 2 cells which produce MoMLV recombinant retroviruses. Hum Gene Ther. 1990;1:385–397. doi: 10.1089/hum.1990.1.4-385. [DOI] [PubMed] [Google Scholar]
- 24.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 25.Isacson O, Breakefield X O. Benefits and risks of hosting animal cells in the human brain. Nat Med. 1997;3:964–969. doi: 10.1038/nm0997-964. [DOI] [PubMed] [Google Scholar]
- 26.Johnston K M, Jacoby D R, Pechan P A, Fraefel C, Borghesani P, Schuback D, Dunn R J, Smith F I, Breakefield X O. HSV/AAV hybrid amplicon vectors extend transgene expression in human glioma cells. Hum Gene Ther. 1997;8:359–370. doi: 10.1089/hum.1997.8.3-359. [DOI] [PubMed] [Google Scholar]
- 27.Jolly D. Viral vector systems for gene therapy. In: Sobol R E, Scanlon K J, editors. The internet book of gene therapy. Cancer therapeutics. Stamford, Conn: Appleton and Lange; 1995. pp. 51–64. [Google Scholar]
- 28.Karacostas V, Wolffe E J, Nagashima K, Gonda M A, Moss B. Overexpression of the HIV-1 Gag-Pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993;193:661–671. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- 29.Kasahara N, Dozy A M, Kan Y W. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 30.Kasid A, Morecki S, Abersold P, Cornetta K, Culver K, Freeman S, Director E, Lotze M T, Blaese R M, Anderson W F, et al. Human gene transfer: characterization of human tumor-infiltrating lymphocytes as vehicles for retroviral-mediated gene transfer in man. Proc Natl Acad Sci USA. 1990;87:473–477. doi: 10.1073/pnas.87.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S H, Yu S S, Park J S, Robbins P D, An C S, Kim S. Construction of retroviral vectors with improved safety, gene expression, and versatility. J Virol. 1998;72:994–1004. doi: 10.1128/jvi.72.2.994-1004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochanek S, Clemens P R, Mitani K, Chen H-H, Chan S, Caskey C T. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and β-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruse C A, Molleston M C, Parks E P, Schiltz P M, Kleinschmidt-DeMasters B K, Hickey W F. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neurooncol. 1994;22:191–200. doi: 10.1007/BF01052919. [DOI] [PubMed] [Google Scholar]
- 34.Kumar-Singh R, Chamberlain J S. Encapsidated adenovirus minichromosomes allow delivery and expression of a 14 kb dystrophin cDNA to muscle cells. Hum Mol Genet. 1996;5:913–921. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- 35.Kurre P, Kiem H-P, Morris J, Heyward S, Battini J-L, Miller A D. Efficient transduction by an amphotropic retrovirus vector is dependent on high-level expression of the cell surface virus receptor. J Virol. 1999;73:495–500. doi: 10.1128/jvi.73.1.495-500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lal B, Indurti R R, Couraud P O, Goldstein G W, Laterra J. Endothelial cell implantation and survival within experimental gliomas. Proc Natl Acad Sci USA. 1994;91:9695–9699. doi: 10.1073/pnas.91.21.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laquerre S, Anderson D B, Stolz D B, Glorioso J C. Recombinant herpes simplex virus type 1 engineered for targeted binding to erythropoietin receptor-bearing cells. J Virol. 1998;72:9683–9697. doi: 10.1128/jvi.72.12.9683-9697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leigh M W, Kylander J E, Yankaskas J R, Boucher R C. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- 39.Li K-J, Garoff H. Production of infectious recombinant Moloney murine leukemia virus particles in BHK cells using Semliki Forest virus-derived RNA expression vectors. Proc Natl Acad Sci USA. 1996;93:11658–11663. doi: 10.1073/pnas.93.21.11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin X. Construction of new retroviral producer cells from adenoviral and retroviral vectors. Gene Ther. 1998;5:1251–1258. doi: 10.1038/sj.gt.3300720. [DOI] [PubMed] [Google Scholar]
- 41.Mann R, Mulligan R C, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33:153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 42.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 43.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagane M, Coufal F, Lin H, Bogler O, Cavenee W K, Huang H-J S. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 45.Naviaux R K, Costanzi E, Haas M, Verma I M. The pCL system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson D M, Metzger M E, Donahue R E, Morgan R A. In vivo retrovirus-mediated gene transfer into multiple hematopoietic lineages in rabbits without preconditioning. Hum Gene Ther. 1997;8:747–754. doi: 10.1089/hum.1997.8.6-747. [DOI] [PubMed] [Google Scholar]
- 47.Noguiez-Hellin P, Robert-LeMeur M, Salzmann J-L, Klatzmann D. Plasmoviruses: nonviral/viral vectors for gene therapy. Proc Natl Acad Sci USA. 1996;93:4175–4180. doi: 10.1073/pnas.93.9.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palombo F, Monciotti A, Recchia A, Cortese R, Ciliberto G, LaMonica N. Site-specific integration in mammalian cells mediated by a new hybrid baculovirus-adeno-associated virus vector. J Virol. 1998;72:5025–5034. doi: 10.1128/jvi.72.6.5025-5034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patience C, Takeuchi Y, Cosset F-L, Weiss R A. Packaging of endogenous retroviral sequences in retroviral vectors produced by murine and human packaging cells. J Virol. 1998;72:2671–2676. doi: 10.1128/jvi.72.4.2671-2676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pederson P-H, Marienhagen K, Mork S, Bjerkvig R. Migratory pattern of fetal rat brain cells and human glioma cells in the adult rat brain. Cancer Res. 1993;53:5158–5165. [PubMed] [Google Scholar]
- 52.Puumalainen A-M, Vapalahti M, Agrawal R S, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S. β-Galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 53.Ram Z, Culver K W, Oshiro E M, Viola J J, DeVroom H L, Otto E, Long Z, Chiang Y, McGarrity G J, Muul L M, Katz D, Blaese R M, Oldfield E H. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 54.Rother R P, Fodor W L, Springhorn J P, Birks C W, Setter E, Sandrin M S, Squinto S P, Rollins S A. A novel mechanism of retrovirus inactivation in human serum mediated by anti-alpha-galactosyl natural antibody. J Exp Med. 1995;182:1345–1355. doi: 10.1084/jem.182.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54a.Saeki, Y. Unpublished data.
- 55.Savard N, Cosset F L, Epstein A L. Defective herpes simplex virus type 1 vectors harboring gag, pol, and env genes can be used to rescue defective retrovirus vectors. J Virol. 1997;71:4111–4117. doi: 10.1128/jvi.71.5.4111-4117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scadden D T, Fuller B, Cunningham J M. Human cells infected with retrovirus vectors acquire an endogenous murine provirus. J Virol. 1990;64:424–427. doi: 10.1128/jvi.64.1.424-427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sena-Esteves M, Aghi M, Pechan P A, Kaye E M, Breakefield X O. Gene delivery to the nervous system using retroviral vectors. In: Latchman D S, editor. Genetic manipulation of the nervous system. London, England: Academic Press; 1996. pp. 149–180. [Google Scholar]
- 58.Smith I L, Hardwicke M A, Sandri-Goldin R M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 59.Spaete R R, Frenkel N. The herpes simplex virus amplicon: analysis of cis-acting replication functions. Cell. 1982;30:295–304. doi: 10.1073/pnas.82.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeuchi Y, Porter C D, Strahan K M, Preece A F, Gustafsson K, Cosset F L, Weiss R A, Collins M K L. Sensitization of cells and retroviruses to human serum by alpha(1-3)galactosyltransferase. Nature. 1996;379:85–88. doi: 10.1038/379085a0. [DOI] [PubMed] [Google Scholar]
- 61.Tamiya T, Wei M X, Chase M, Ono Y, Lee F, Breakefield X O, Chiocca E A. Transgene inheritance and retroviral infection contribute to the efficiency of gene expression in solid tumors inoculated with retroviral vector producing cells. Gene Ther. 1995;2:531–538. [PubMed] [Google Scholar]
- 62.Tamura M, Shimizu K, Yamada M, Miyao Y, Hayakawa T, Ikenaka K. Targeted killing of migrating glioma cells by injection of HTK-modified glioma cells. Hum Gene Ther. 1997;8:381–391. doi: 10.1089/hum.1997.8.4-381. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Vos J M. A hybrid herpesvirus infectious vector based on Epstein-Barr virus and herpes simplex virus type 1 for gene transfer into human cells in vitro and in vivo. J Virol. 1996;70:8422–8430. doi: 10.1128/jvi.70.12.8422-8430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weizsaecker M, Deen D F, Rosenblum M L, Hoshino T, Gutin P H, Barker M. The 9L rat brain tumor: description and application of an animal model. J Neurol. 1981;224:183–192. doi: 10.1007/BF00313280. [DOI] [PubMed] [Google Scholar]
- 65.Wendelburg B J, Vos J-M H. An enhanced EBNA-1 variant with reduced IR3 domain for long-term episomal maintenance and transgene expression of oriP-based plasmid in human cells. Gene Ther. 1998;5:1389–1399. doi: 10.1038/sj.gt.3300736. [DOI] [PubMed] [Google Scholar]
- 66.Yamada M, Shimizu K, Miyao Y, Hayakawa T, Nakajima K, Nakahira K, Nakagawa H, Mikoshiba K, Ikenaka K. Migration of genetically labeled glioma cells after implantation into murine brain. J Neurosci Res. 1994;38:415–423. doi: 10.1002/jnr.490380407. [DOI] [PubMed] [Google Scholar]
- 67.Yates J L, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida Y, Emi N, Hamada H. VSV-G-pseudotyped retroviral packaging through adenovirus-mediated inducible gene expression. Biochem Biophys Res Commun. 1997;232:379–382. doi: 10.1006/bbrc.1996.5976. [DOI] [PubMed] [Google Scholar]
- 69.Yoshii Y, Maki Y, Tsuboi K, Tomono Y, Nakagawa K, Hoshino T. Estimation of growth fraction with bromodeoxyuridine in human central nervous system tumors. J Neurosurg. 1986;65:659–663. doi: 10.3171/jns.1986.65.5.0659. [DOI] [PubMed] [Google Scholar]