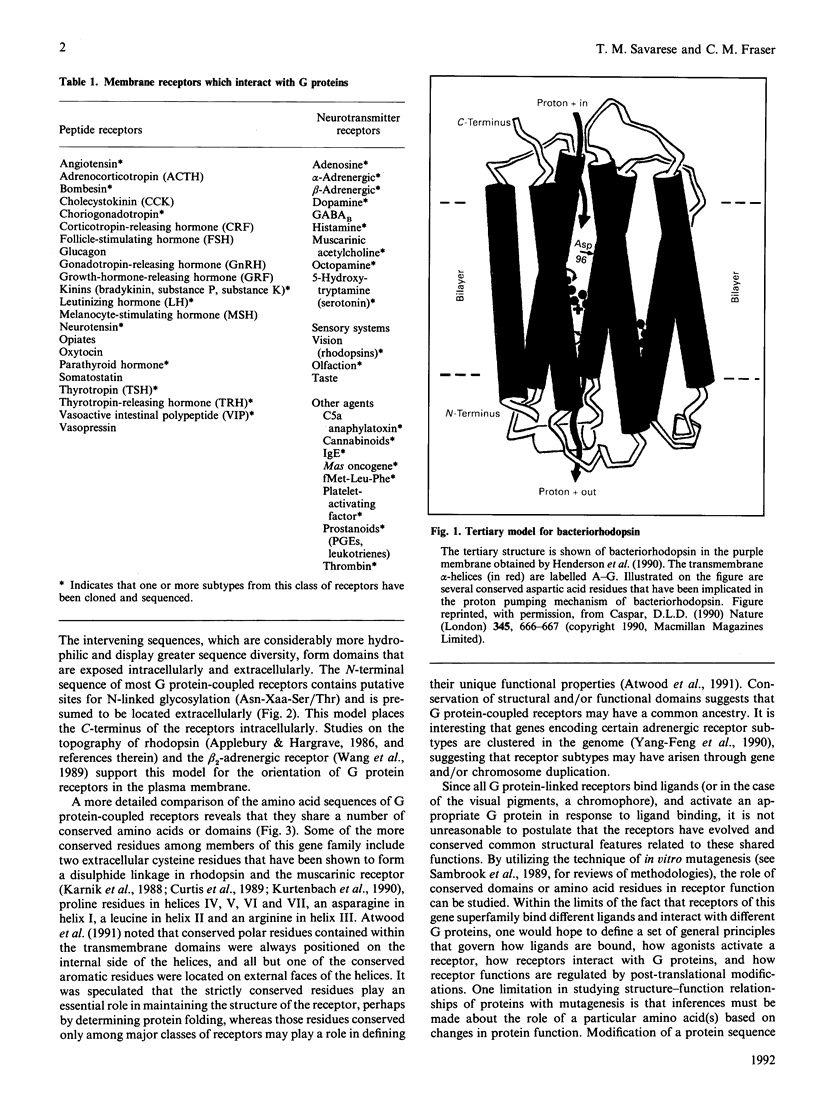
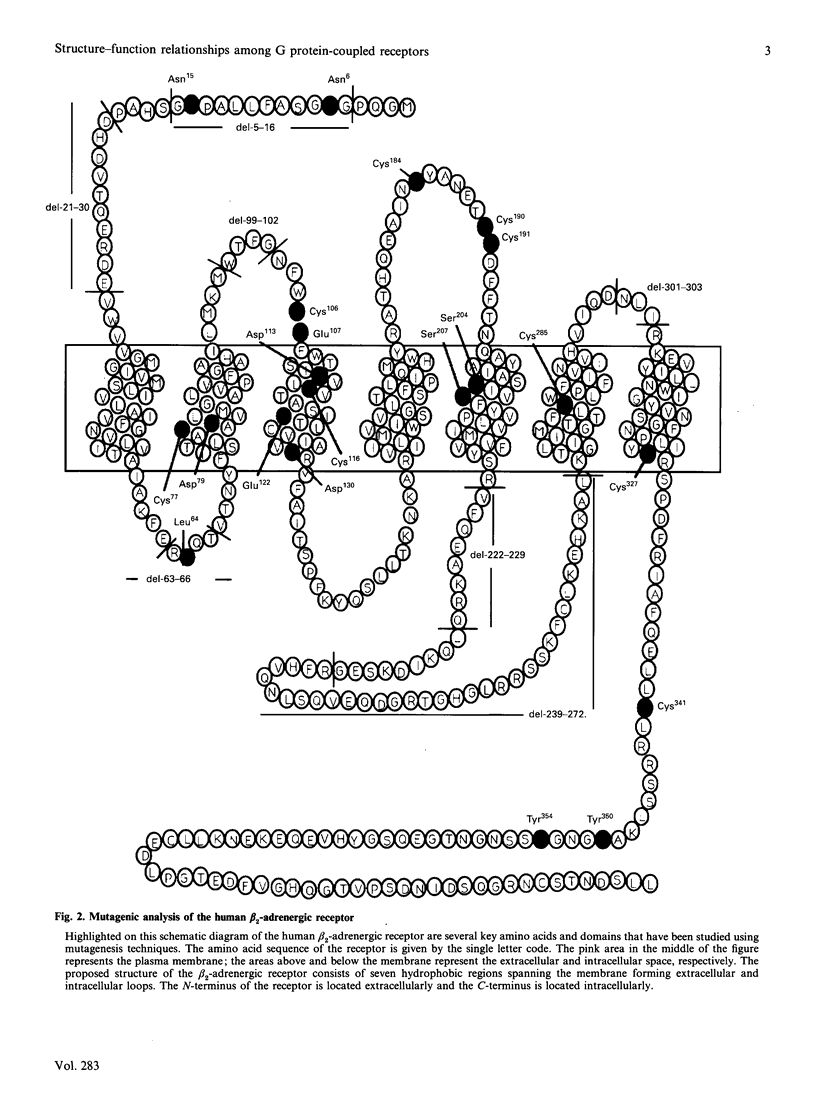
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-Elfattah A. S., Shamoo A. E. Regeneration of a functionally active rat brain muscarinic receptor by D-penicillamine after inhibition with methylmercury and mercuric chloride. Mol Pharmacol. 1981 Nov;20(3):492–497. [PubMed] [Google Scholar]
- Albert P. R., Zhou Q. Y., Van Tol H. H., Bunzow J. R., Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990 Apr 5;265(10):5825–5832. [PubMed] [Google Scholar]
- Applebury M. L., Hargrave P. A. Molecular biology of the visual pigments. Vision Res. 1986;26(12):1881–1895. doi: 10.1016/0042-6989(86)90115-x. [DOI] [PubMed] [Google Scholar]
- Aronstam R. S., Eldefrawi M. E. Reversible conversion between affinity states for agonists of the muscarinic acetylcholine receptor from rat brain. Biochem Pharmacol. 1979 Mar 1;28(5):701–703. doi: 10.1016/0006-2952(79)90162-x. [DOI] [PubMed] [Google Scholar]
- Attwood T. K., Eliopoulos E. E., Findlay J. B. Multiple sequence alignment of protein families showing low sequence homology: a methodological approach using database pattern-matching discriminators for G-protein-linked receptors. Gene. 1991 Feb 15;98(2):153–159. doi: 10.1016/0378-1119(91)90168-b. [DOI] [PubMed] [Google Scholar]
- Barclay P. L., Findlay J. B. Labelling of the cytoplasmic domains of ovine rhodopsin with hydrophilic chemical probes. Biochem J. 1984 May 15;220(1):75–84. doi: 10.1042/bj2200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley H., Huang K. S., Radhakrishnan R., Ross A. H., Takagaki Y., Khorana H. G. Site of attachment of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2225–2229. doi: 10.1073/pnas.78.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J. L., Kühn H., Weyand I., Codina J., Caron M. G., Lefkowitz R. J. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci U S A. 1987 Dec;84(24):8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J. L., Mayor F., Jr, Staniszewski C., Lefkowitz R. J., Caron M. G. Purification and characterization of the beta-adrenergic receptor kinase. J Biol Chem. 1987 Jul 5;262(19):9026–9032. [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstein G., Haga K., Haga T., Ichiyama A. Agonist and antagonist binding of muscarinic acetylcholine receptors purified from porcine brain: interconversion of high- and low-affinity sites by sulfhydryl reagents. J Neurochem. 1988 Jun;50(6):1687–1694. doi: 10.1111/j.1471-4159.1988.tb02464.x. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989 Apr;12(4):148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Bouvier M., Collins S., O'Dowd B. F., Campbell P. T., de Blasi A., Kobilka B. K., MacGregor C., Irons G. P., Caron M. G., Lefkowitz R. J. Two distinct pathways for cAMP-mediated down-regulation of the beta 2-adrenergic receptor. Phosphorylation of the receptor and regulation of its mRNA level. J Biol Chem. 1989 Oct 5;264(28):16786–16792. [PubMed] [Google Scholar]
- Bouvier M., Hausdorff W. P., De Blasi A., O'Dowd B. F., Kobilka B. K., Caron M. G., Lefkowitz R. J. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature. 1988 May 26;333(6171):370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- Bouvier M., Leeb-Lundberg L. M., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of alpha 1- and beta 2-adrenergic receptors by protein kinase C and the cyclic AMP-dependent protein kinase. J Biol Chem. 1987 Mar 5;262(7):3106–3113. [PubMed] [Google Scholar]
- Bownds D., Dawes J., Miller J., Stahlman M. Phosphorylation of frog photoreceptor membranes induced by light. Nat New Biol. 1972 May 24;237(73):125–127. doi: 10.1038/newbio237125a0. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Buczyłko J., Gutmann C., Palczewski K. Regulation of rhodopsin kinase by autophosphorylation. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2568–2572. doi: 10.1073/pnas.88.6.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Caspar D. L. Protein microscopy. Bacteriorhodopsin--at last! Nature. 1990 Jun 21;345(6277):666–667. doi: 10.1038/345666a0. [DOI] [PubMed] [Google Scholar]
- Cheung A. H., Dixon R. A., Hill W. S., Sigal I. S., Strader C. D. Separation of the structural requirements for agonist-promoted activation and sequestration of the beta-adrenergic receptor. Mol Pharmacol. 1990 Jun;37(6):775–779. [PubMed] [Google Scholar]
- Cheung A. H., Sigal I. S., Dixon R. A., Strader C. D. Agonist-promoted sequestration of the beta 2-adrenergic receptor requires regions involved in functional coupling with Gs. Mol Pharmacol. 1989 Jan;35(1):132–138. [PubMed] [Google Scholar]
- Chung F. Z., Lentes K. U., Gocayne J., Fitzgerald M., Robinson D., Kerlavage A. R., Fraser C. M., Venter J. C. Cloning and sequence analysis of the human brain beta-adrenergic receptor. Evolutionary relationship to rodent and avian beta-receptors and porcine muscarinic receptors. FEBS Lett. 1987 Jan 26;211(2):200–206. doi: 10.1016/0014-5793(87)81436-9. [DOI] [PubMed] [Google Scholar]
- Chung F. Z., Wang C. D., Potter P. C., Venter J. C., Fraser C. M. Site-directed mutagenesis and continuous expression of human beta-adrenergic receptors. Identification of a conserved aspartate residue involved in agonist binding and receptor activation. J Biol Chem. 1988 Mar 25;263(9):4052–4055. [PubMed] [Google Scholar]
- Clark R. B., Friedman J., Dixon R. A., Strader C. D. Identification of a specific site required for rapid heterologous desensitization of the beta-adrenergic receptor by cAMP-dependent protein kinase. Mol Pharmacol. 1989 Sep;36(3):343–348. [PubMed] [Google Scholar]
- Cotecchia S., Exum S., Caron M. G., Lefkowitz R. J. Regions of the alpha 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2896–2900. doi: 10.1073/pnas.87.8.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S., Schwinn D. A., Randall R. R., Lefkowitz R. J., Caron M. G., Kobilka B. K. Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. A., Wheatley M., Bansal S., Birdsall N. J., Eveleigh P., Pedder E. K., Poyner D., Hulme E. C. Propylbenzilylcholine mustard labels an acidic residue in transmembrane helix 3 of the muscarinic receptor. J Biol Chem. 1989 Jan 5;264(1):489–495. [PubMed] [Google Scholar]
- Dalman H. M., Neubig R. R. Two peptides from the alpha 2A-adrenergic receptor alter receptor G protein coupling by distinct mechanisms. J Biol Chem. 1991 Jun 15;266(17):11025–11029. [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Rands E., Register R. B., Candelore M. R., Blake A. D., Strader C. D. Ligand binding to the beta-adrenergic receptor involves its rhodopsin-like core. Nature. 1987 Mar 5;326(6108):73–77. doi: 10.1038/326073a0. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Bouvier M., Benovic J. L., Caron M. G., Lefkowitz R. J. The multiple membrane spanning topography of the beta 2-adrenergic receptor. Localization of the sites of binding, glycosylation, and regulatory phosphorylation by limited proteolysis. J Biol Chem. 1987 Oct 15;262(29):14282–14288. [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., DeBlasi A., Frielle T., Lefkowitz R. J. Role of extracellular disulfide-bonded cysteines in the ligand binding function of the beta 2-adrenergic receptor. Biochemistry. 1990 Mar 6;29(9):2335–2342. doi: 10.1021/bi00461a018. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Strader C. D., Amlaiky N., Lefkowitz R. J. Identification and sequence of a binding site peptide of the beta 2-adrenergic receptor. Biochemistry. 1988 Mar 22;27(6):1813–1817. doi: 10.1021/bi00406a002. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Brett M., Pappin D. J. Primary structure of C-terminal functional sites in ovine rhodopsin. Nature. 1981 Sep 24;293(5830):314–317. doi: 10.1038/293314a0. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Pappin D. J. The opsin family of proteins. Biochem J. 1986 Sep 15;238(3):625–642. doi: 10.1042/bj2380625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. N., Buzney S. M. Mechanism and specificity of rhodopsin phosphorylation. Biochemistry. 1975 Nov 18;14(23):5110–5117. doi: 10.1021/bi00694a014. [DOI] [PubMed] [Google Scholar]
- Frank R. N., Cavanagh H. D., Kenyon K. R. Light-stimulated phosphorylation of bovine visual pigments by adenosine triphosphate. J Biol Chem. 1973 Jan 25;248(2):596–609. [PubMed] [Google Scholar]
- Franke R. R., König B., Sakmar T. P., Khorana H. G., Hofmann K. P. Rhodopsin mutants that bind but fail to activate transducin. Science. 1990 Oct 5;250(4977):123–125. doi: 10.1126/science.2218504. [DOI] [PubMed] [Google Scholar]
- Franke R. R., Sakmar T. P., Oprian D. D., Khorana H. G. A single amino acid substitution in rhodopsin (lysine 248----leucine) prevents activation of transducin. J Biol Chem. 1988 Feb 15;263(5):2119–2122. [PubMed] [Google Scholar]
- Fraser C. M., Arakawa S., McCombie W. R., Venter J. C. Cloning, sequence analysis, and permanent expression of a human alpha 2-adrenergic receptor in Chinese hamster ovary cells. Evidence for independent pathways of receptor coupling to adenylate cyclase attenuation and activation. J Biol Chem. 1989 Jul 15;264(20):11754–11761. [PubMed] [Google Scholar]
- Fraser C. M., Chung F. Z., Wang C. D., Venter J. C. Site-directed mutagenesis of human beta-adrenergic receptors: substitution of aspartic acid-130 by asparagine produces a receptor with high-affinity agonist binding that is uncoupled from adenylate cyclase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5478–5482. doi: 10.1073/pnas.85.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C. M. Site-directed mutagenesis of beta-adrenergic receptors. Identification of conserved cysteine residues that independently affect ligand binding and receptor activation. J Biol Chem. 1989 Jun 5;264(16):9266–9270. [PubMed] [Google Scholar]
- Fraser C. M., Wang C. D., Robinson D. A., Gocayne J. D., Venter J. C. Site-directed mutagenesis of m1 muscarinic acetylcholine receptors: conserved aspartic acids play important roles in receptor function. Mol Pharmacol. 1989 Dec;36(6):840–847. [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frielle T., Daniel K. W., Caron M. G., Lefkowitz R. J. Structural basis of beta-adrenergic receptor subtype specificity studied with chimeric beta 1/beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9494–9498. doi: 10.1073/pnas.85.24.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I., Schäffer M., DelValle J., Logsdon C., Campbell V., Uhler M., Yamada T. Molecular cloning of a gene encoding the histamine H2 receptor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):429–433. doi: 10.1073/pnas.88.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard N. P., Eddy R. L., Jr, Shows T. B., Gerard C. The human neurokinin A (substance K) receptor. Molecular cloning of the gene, chromosome localization, and isolation of cDNA from tracheal and gastric tissues. J Biol Chem. 1990 Nov 25;265(33):20455–20462. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gocayne J., Robinson D. A., FitzGerald M. G., Chung F. Z., Kerlavage A. R., Lentes K. U., Lai J., Wang C. D., Fraser C. M., Venter J. C. Primary structure of rat cardiac beta-adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8296–8300. doi: 10.1073/pnas.84.23.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm H. E., Deretic D., Arendt A., Hargrave P. A., Koenig B., Hofmann K. P. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988 Aug 12;241(4867):832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A. The amino-terminal tryptic peptide of bovine rhodopsin. A glycopeptide containing two sites of oligosaccharide attachment. Biochim Biophys Acta. 1977 May 27;492(1):83–94. doi: 10.1016/0005-2795(77)90216-1. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Bouvier M., O'Dowd B. F., Irons G. P., Caron M. G., Lefkowitz R. J. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989 Jul 25;264(21):12657–12665. [PubMed] [Google Scholar]
- Hausdorff W. P., Hnatowich M., O'Dowd B. F., Caron M. G., Lefkowitz R. J. A mutation of the beta 2-adrenergic receptor impairs agonist activation of adenylyl cyclase without affecting high affinity agonist binding. Distinct molecular determinants of the receptor are involved in physical coupling to and functional activation of Gs. J Biol Chem. 1990 Jan 25;265(3):1388–1393. [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Krause J. E. Molecular characterization of a functional cDNA encoding the rat substance P receptor. Science. 1990 Feb 23;247(4945):958–962. doi: 10.1126/science.2154852. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Brandon S., Wilson A. L., Guyer C. A., Cragoe E. J., Jr, Limbird L. E. An aspartate conserved among G-protein receptors confers allosteric regulation of alpha 2-adrenergic receptors by sodium. J Biol Chem. 1990 Dec 15;265(35):21590–21595. [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Nakamura S., Kaziro Y., Takahashi T., Takahashi K., Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991 Jul;10(7):1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Blair L. A., Marshall J., Goedert M., Hanley M. R. The mas oncogene encodes an angiotensin receptor. Nature. 1988 Sep 29;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Janssen J. J., De Caluwé G. L., De Grip W. J. Asp83, Glu113 and Glu134 are not specifically involved in Schiff base protonation or wavelength regulation in bovine rhodopsin. FEBS Lett. 1990 Jan 15;260(1):113–118. doi: 10.1016/0014-5793(90)80080-3. [DOI] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr, Hock J., Potts J. T., Jr, Kronenberg H. M. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991 Nov 15;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- Karnik S. S., Khorana H. G. Assembly of functional rhodopsin requires a disulfide bond between cysteine residues 110 and 187. J Biol Chem. 1990 Oct 15;265(29):17520–17524. [PubMed] [Google Scholar]
- Karnik S. S., Sakmar T. P., Chen H. B., Khorana H. G. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., MacGregor C., Daniel K., Kobilka T. S., Caron M. G., Lefkowitz R. J. Functional activity and regulation of human beta 2-adrenergic receptors expressed in Xenopus oocytes. J Biol Chem. 1987 Nov 15;262(32):15796–15802. [PubMed] [Google Scholar]
- Kobilka B. K., Matsui H., Kobilka T. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J., Regan J. W. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987 Oct 30;238(4827):650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- Kubo T., Bujo H., Akiba I., Nakai J., Mishina M., Numa S. Location of a region of the muscarinic acetylcholine receptor involved in selective effector coupling. FEBS Lett. 1988 Dec 5;241(1-2):119–125. doi: 10.1016/0014-5793(88)81043-3. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- Kurtenbach E., Curtis C. A., Pedder E. K., Aitken A., Harris A. C., Hulme E. C. Muscarinic acetylcholine receptors. Peptide sequencing identifies residues involved in antagonist binding and disulfide bond formation. J Biol Chem. 1990 Aug 15;265(23):13702–13708. [PubMed] [Google Scholar]
- Kühn H., Dreyer W. J. Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 1972 Jan 15;20(1):1–6. doi: 10.1016/0014-5793(72)80002-4. [DOI] [PubMed] [Google Scholar]
- Kühn H., Hall S. W., Wilden U. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 1984 Oct 29;176(2):473–478. doi: 10.1016/0014-5793(84)81221-1. [DOI] [PubMed] [Google Scholar]
- Kühn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978 Oct 17;17(21):4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Adrenergic receptors. Models for the study of receptors coupled to guanine nucleotide regulatory proteins. J Biol Chem. 1988 Apr 15;263(11):4993–4996. [PubMed] [Google Scholar]
- Liao C. F., Themmen A. P., Joho R., Barberis C., Birnbaumer M., Birnbaumer L. Molecular cloning and expression of a fifth muscarinic acetylcholine receptor. J Biol Chem. 1989 May 5;264(13):7328–7337. [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr ATP mediates rapid reversal of cyclic GMP phosphodiesterase activation in visual receptor membranes. Nature. 1980 Oct 23;287(5784):734–736. doi: 10.1038/287734a0. [DOI] [PubMed] [Google Scholar]
- Limbird L. E. GTP and Na+ modulate receptor-adenyl cyclase coupling and receptor-mediated function. Am J Physiol. 1984 Jul;247(1 Pt 1):E59–E68. doi: 10.1152/ajpendo.1984.247.1.E59. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Lobel P., Fujimoto K., Ye R. D., Griffiths G., Kornfeld S. Mutations in the cytoplasmic domain of the 275 kd mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell. 1989 Jun 2;57(5):787–796. doi: 10.1016/0092-8674(89)90793-9. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lomasney J. W., Lorenz W., Allen L. F., King K., Regan J. W., Yang-Feng T. L., Caron M. G., Lefkowitz R. J. Expansion of the alpha 2-adrenergic receptor family: cloning and characterization of a human alpha 2-adrenergic receptor subtype, the gene for which is located on chromosome 2. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5094–5098. doi: 10.1073/pnas.87.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Hanoune J., Bockaert J. Chemical modification of the beta adrenergic receptors coupled with adenylate cyclase by disulfide bridge-reducing agents. Mol Pharmacol. 1978 Mar;14(2):227–236. [PubMed] [Google Scholar]
- Maenhaut C., Van Sande J., Libert F., Abramowicz M., Parmentier M., Vanderhaegen J. J., Dumont J. E., Vassart G., Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mayor F., Jr, Benovic J. L., Caron M. G., Lefkowitz R. J. Somatostatin induces translocation of the beta-adrenergic receptor kinase and desensitizes somatostatin receptors in S49 lymphoma cells. J Biol Chem. 1987 May 15;262(14):6468–6471. [PubMed] [Google Scholar]
- Miller J. L., Dratz E. A. Phosphorylation at sites near rhodopsin's carboxyl-terminus regulates light initiated cGMP hydrolysis. Vision Res. 1984;24(11):1509–1521. doi: 10.1016/0042-6989(84)90313-4. [DOI] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxham C. P., Ross E. M., George S. T., Malbon C. C. Beta-adrenergic receptors display intramolecular disulfide bridges in situ: analysis by immunoblotting and functional reconstitution. Mol Pharmacol. 1988 May;33(5):486–492. [PubMed] [Google Scholar]
- Mullen E., Johnson A. H., Akhtar M. The identification of Lys216 as the retinal binding residue in bacteriorhodopsin. FEBS Lett. 1981 Aug 3;130(2):187–193. doi: 10.1016/0014-5793(81)81116-7. [DOI] [PubMed] [Google Scholar]
- Nassal M., Mogi T., Karnik S. S., Khorana H. G. Structure-function studies on bacteriorhodopsin. III. Total synthesis of a gene for bacterio-opsin and its expression in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9264–9270. [PubMed] [Google Scholar]
- Nathans J., Davenport C. M., Maumenee I. H., Lewis R. A., Hejtmancik J. F., Litt M., Lovrien E., Weleber R., Bachynski B., Zwas F. Molecular genetics of human blue cone monochromacy. Science. 1989 Aug 25;245(4920):831–838. doi: 10.1126/science.2788922. [DOI] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochemistry. 1990 Jan 30;29(4):937–942. doi: 10.1021/bi00456a013. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Cox B. A., Henningsen R. A., Spanoyannis A., Neve R. L. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol. 1991 Jun;39(6):733–739. [PubMed] [Google Scholar]
- Neve K. A., Henningsen R. A., Kinzie J. M., De Paulis T., Schmidt D. E., Kessler R. M., Janowsky A. Sodium-dependent isomerization of dopamine D-2 receptors characterized using [125I]epidepride, a high-affinity substituted benzamide ligand. J Pharmacol Exp Ther. 1990 Mar;252(3):1108–1116. [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989 May 5;264(13):7564–7569. [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Regan J. W., Leader W. M., Caron M. G., Lefkowitz R. J. Site-directed mutagenesis of the cytoplasmic domains of the human beta 2-adrenergic receptor. Localization of regions involved in G protein-receptor coupling. J Biol Chem. 1988 Nov 5;263(31):15985–15992. [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Jakes S., Ingebritsen T. S., Hargrave P. A. Regulation of rhodopsin dephosphorylation by arrestin. J Biol Chem. 1989 Sep 25;264(27):15770–15773. [PubMed] [Google Scholar]
- Pedersen S. E., Ross E. M. Functional activation of beta-adrenergic receptors by thiols in the presence or absence of agonists. J Biol Chem. 1985 Nov 15;260(26):14150–14157. [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Bach A. W., Wozny M., Taleb O., Dal Toso R., Shih J. C., Seeburg P. H. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J. 1988 Dec 20;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E., Candelore M. R., Cheung A. H., Hill W. S., Strader C. D., Dixon R. A. Mutational analysis of beta-adrenergic receptor glycosylation. J Biol Chem. 1990 Jun 25;265(18):10759–10764. [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Towner P., Akhtar M. Functional rhodopsin complex consisting of three noncovalently linked fragments. Biochemistry. 1977 Dec 13;16(25):5641–5649. doi: 10.1021/bi00644a040. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Nakanishi S. Molecular characterization of rat substance K receptor and its mRNAs. Biochem Biophys Res Commun. 1989 Dec 15;165(2):695–702. doi: 10.1016/s0006-291x(89)80022-1. [DOI] [PubMed] [Google Scholar]
- Schwinn D. A., Lomasney J. W., Lorenz W., Szklut P. J., Fremeau R. T., Jr, Yang-Feng T. L., Caron M. G., Lefkowitz R. J., Cotecchia S. Molecular cloning and expression of the cDNA for a novel alpha 1-adrenergic receptor subtype. J Biol Chem. 1990 May 15;265(14):8183–8189. [PubMed] [Google Scholar]
- Shapiro R. A., Nathanson N. M. Deletion analysis of the mouse m1 muscarinic acetylcholine receptor: effects on phosphoinositide metabolism and down-regulation. Biochemistry. 1989 Oct 31;28(22):8946–8950. doi: 10.1021/bi00448a039. [DOI] [PubMed] [Google Scholar]
- Shichi H., Somers R. L. Light-dependent phosphorylation of rhodopsin. Purification and properties of rhodopsin kinase. J Biol Chem. 1978 Oct 10;253(19):7040–7046. [PubMed] [Google Scholar]
- Shichi H., Yamamoto K., Somers R. L. GTP binding protein: properties and lack of activation by phosphorylated rhodopsin. Vision Res. 1984;24(11):1523–1531. doi: 10.1016/s0042-6989(84)80001-2. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Benovic J. L., Daniel K., Lefkowitz R. J. Phosphorylation/dephosphorylation of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9408–9412. doi: 10.1073/pnas.83.24.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Caron M. G., Lefkowitz R. J. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. J Biol Chem. 1985 Apr 10;260(7):3883–3886. [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader C. D., Candelore M. R., Hill W. S., Dixon R. A., Sigal I. S. A single amino acid substitution in the beta-adrenergic receptor promotes partial agonist activity from antagonists. J Biol Chem. 1989 Oct 5;264(28):16470–16477. [PubMed] [Google Scholar]
- Strader C. D., Candelore M. R., Hill W. S., Sigal I. S., Dixon R. A. Identification of two serine residues involved in agonist activation of the beta-adrenergic receptor. J Biol Chem. 1989 Aug 15;264(23):13572–13578. [PubMed] [Google Scholar]
- Strader C. D., Dixon R. A., Cheung A. H., Candelore M. R., Blake A. D., Sigal I. S. Mutations that uncouple the beta-adrenergic receptor from Gs and increase agonist affinity. J Biol Chem. 1987 Dec 5;262(34):16439–16443. [PubMed] [Google Scholar]
- Strader C. D., Gaffney T., Sugg E. E., Candelore M. R., Keys R., Patchett A. A., Dixon R. A. Allele-specific activation of genetically engineered receptors. J Biol Chem. 1991 Jan 5;266(1):5–8. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Blake A. D., Cheung A. H., Register R. B., Rands E., Zemcik B. A., Candelore M. R., Dixon R. A. The carboxyl terminus of the hamster beta-adrenergic receptor expressed in mouse L cells is not required for receptor sequestration. Cell. 1987 Jun 19;49(6):855–863. doi: 10.1016/0092-8674(87)90623-4. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Register R. B., Candelore M. R., Rands E., Dixon R. A. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R. H., Benovic J. L., Caron M. G., Lefkowitz R. J. Beta-agonist- and prostaglandin E1-induced translocation of the beta-adrenergic receptor kinase: evidence that the kinase may act on multiple adenylate cyclase-coupled receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6362–6366. doi: 10.1073/pnas.83.17.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Takemoto D. J., Takemoto L. J., Hansen J., Morrison D. Regulation of retinal transducin by C-terminal peptides of rhodopsin. Biochem J. 1985 Dec 15;232(3):669–672. doi: 10.1042/bj2320669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P., Findlay J. B. Phosphorylation of ovine rhodopsin. Identification of the phosphorylated sites. Biochem J. 1984 Jun 15;220(3):773–780. doi: 10.1042/bj2200773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tota M. R., Strader C. D. Characterization of the binding domain of the beta-adrenergic receptor with the fluorescent antagonist carazolol. Evidence for a buried ligand binding site. J Biol Chem. 1990 Oct 5;265(28):16891–16897. [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Kanaho Y., Halpern J. L., Moss J. Immunological and biochemical differentiation of guanyl nucleotide binding proteins: interaction of Go alpha with rhodopsin, anti-Go alpha polyclonal antibodies, and a monoclonal antibody against transducin alpha subunit and Gi alpha. Biochemistry. 1987 Jul 28;26(15):4728–4733. doi: 10.1021/bi00389a020. [DOI] [PubMed] [Google Scholar]
- Valiquette M., Bonin H., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Involvement of tyrosine residues located in the carboxyl tail of the human beta 2-adrenergic receptor in agonist-induced down-regulation of the receptor. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5089–5093. doi: 10.1073/pnas.87.13.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauquelin G., Bottari S., Kanarek L., Strosberg A. D. Evidence for essential disulfide bonds in beta1-adrenergic receptors of turkey erythrocyte membranes. Inactivation by dithiothreitol. J Biol Chem. 1979 Jun 10;254(11):4462–4469. [PubMed] [Google Scholar]
- Venter J. C., Fraser C. M., Kerlavage A. R., Buck M. A. Molecular biology of adrenergic and muscarinic cholinergic receptors. A perspective. Biochem Pharmacol. 1989 Apr 15;38(8):1197–1208. doi: 10.1016/0006-2952(89)90325-0. [DOI] [PubMed] [Google Scholar]
- Venter J. C., di Porzio U., Robinson D. A., Shreeve S. M., Lai J., Kerlavage A. R., Fracek S. P., Jr, Lentes K. U., Fraser C. M. Evolution of neurotransmitter receptor systems. Prog Neurobiol. 1988;30(2-3):105–169. doi: 10.1016/0301-0082(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Wheaton V. I., Hung D. T., Charo I., Coughlin S. R. Domains specifying thrombin-receptor interaction. Nature. 1991 Oct 17;353(6345):674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- Wang C. D., Buck M. A., Fraser C. M. Site-directed mutagenesis of alpha 2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol Pharmacol. 1991 Aug;40(2):168–179. [PubMed] [Google Scholar]
- Wang H., Lipfert L., Malbon C. C., Bahouth S. Site-directed anti-peptide antibodies define the topography of the beta-adrenergic receptor. J Biol Chem. 1989 Aug 25;264(24):14424–14431. [PubMed] [Google Scholar]
- Wang J. K., McDowell J. H., Hargrave P. A. Site of attachment of 11-cis-retinal in bovine rhodopsin. Biochemistry. 1980 Oct 28;19(22):5111–5117. doi: 10.1021/bi00563a027. [DOI] [PubMed] [Google Scholar]
- Weinshank R. L., Zgombick J. M., Macchi M., Adham N., Lichtblau H., Branchek T. A., Hartig P. R. Cloning, expression, and pharmacological characterization of a human alpha 2B-adrenergic receptor. Mol Pharmacol. 1990 Nov;38(5):681–688. [PubMed] [Google Scholar]
- Weiss E. R., Kelleher D. J., Woon C. W., Soparkar S., Osawa S., Heasley L. E., Johnson G. L. Receptor activation of G proteins. FASEB J. 1988 Oct;2(13):2841–2848. doi: 10.1096/fasebj.2.13.3139484. [DOI] [PubMed] [Google Scholar]
- Wess J., Bonner T. I., Dörje F., Brann M. R. Delineation of muscarinic receptor domains conferring selectivity of coupling to guanine nucleotide-binding proteins and second messengers. Mol Pharmacol. 1990 Oct;38(4):517–523. [PubMed] [Google Scholar]
- Wess J., Brann M. R., Bonner T. I. Identification of a small intracellular region of the muscarinic m3 receptor as a determinant of selective coupling to PI turnover. FEBS Lett. 1989 Nov 20;258(1):133–136. doi: 10.1016/0014-5793(89)81633-3. [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., Kühn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982 Jun 8;21(12):3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]
- Wong S. K., Parker E. M., Ross E. M. Chimeric muscarinic cholinergic: beta-adrenergic receptors that activate Gs in response to muscarinic agonists. J Biol Chem. 1990 Apr 15;265(11):6219–6224. [PubMed] [Google Scholar]
- Wong S. K., Slaughter C., Ruoho A. E., Ross E. M. The catecholamine binding site of the beta-adrenergic receptor is formed by juxtaposed membrane-spanning domains. J Biol Chem. 1988 Jun 15;263(17):7925–7928. [PubMed] [Google Scholar]
- Yang-Feng T. L., Xue F. Y., Zhong W. W., Cotecchia S., Frielle T., Caron M. G., Lefkowitz R. J., Francke U. Chromosomal organization of adrenergic receptor genes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1516–1520. doi: 10.1073/pnas.87.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Rodriguez H., Wong S. K., Brandt D. R., May D. C., Burnier J., Harkins R. N., Chen E. Y., Ramachandran J., Ullrich A. The avian beta-adrenergic receptor: primary structure and membrane topology. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6795–6799. doi: 10.1073/pnas.83.18.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]
- Young D., Waitches G., Birchmeier C., Fasano O., Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986 Jun 6;45(5):711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]
- Zeng D. W., Harrison J. K., D'Angelo D. D., Barber C. M., Tucker A. L., Lu Z. H., Lynch K. R. Molecular characterization of a rat alpha 2B-adrenergic receptor. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3102–3106. doi: 10.1073/pnas.87.8.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. Y., Grandy D. K., Thambi L., Kushner J. A., Van Tol H. H., Cone R., Pribnow D., Salon J., Bunzow J. R., Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990 Sep 6;347(6288):76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]
- Zhukovsky E. A., Oprian D. D. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989 Nov 17;246(4932):928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- van Koppen C. J., Nathanson N. M. Site-directed mutagenesis of the m2 muscarinic acetylcholine receptor. Analysis of the role of N-glycosylation in receptor expression and function. J Biol Chem. 1990 Dec 5;265(34):20887–20892. [PubMed] [Google Scholar]






