Abstract
The prognosis of patients with peritoneal metastases from pancreatic cancer is poor, largely due to massive ascites, which precludes systemic treatment. Two patients with a poor performance status and malignant ascites were treated with cell-free and concentrated ascites reinfusion therapy followed by combined chemotherapy with intraperitoneal paclitaxel, intravenous gemcitabine, and nab-paclitaxel. These patients achieved a survival of 19 and 36 weeks with a relatively good quality of life. Combined intraperitoneal paclitaxel and systemic chemotherapy may provide effective palliative management for some patients with peritoneal metastases from pancreatic cancer.
Keywords: pancreatic cancer, peritoneal metastasis, malignant ascites, intraperitoneal chemotherapy, paclitaxel, cell-free and concentrated ascites reinfusion therapy (CART)
Introduction
Pancreatic cancer is associated with an overall poor prognosis, showing a 5-year survival rate of <10% (1). The prognosis for patients presenting with massive malignant ascites remains extremely poor. The median survival of patients with peritoneal dissemination in population-based studies of malignant ascites has been reported to be only 6-16 weeks (2-5). Treatment of peritoneal carcinomatosis with massive ascites from pancreatic cancer with systemic chemotherapy has little effect on the overall survival (OS). Peritoneal carcinomatosis has long been regarded as the end stage for patients with this disease (6). The quality of life (QOL) of patients with peritoneal carcinomatosis is greatly diminished by symptoms, such as pain, abdominal distension, nausea, loss of appetite, and dyspnea.
Recently, intraperitoneal and systemic combined chemotherapy regimens have been developed with efficacy against peritoneal carcinomatosis in patients with gastric and pancreatic cancer (7-12). This combined treatment approach is a promising option for treating patients with peritoneal dissemination and a good performance status (PS). However, patients with peritoneal carcinomatosis from pancreatic cancer often have a poor PS owing to ascites, which precludes the use of some treatment modalities.
Two patients were treated with combined chemotherapy with intraperitoneal paclitaxel, intravenous nab-paclitaxel, and gemcitabine. These patients presented with a poor PS and massive ascites due to advanced pancreatic cancer at the first visit. The treatment of these two patients was approved by the Institutional Review Board of Jichi Medical University, and the patients' written informed consent was obtained for this treatment.
Case Reports
Patient 1
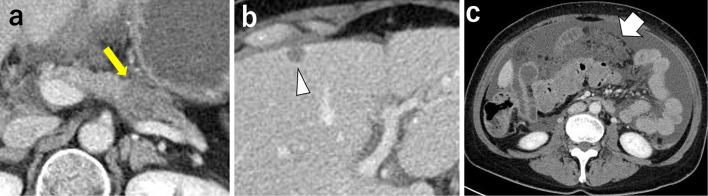
A 57-year-old woman presented with advanced pancreatic cancer. Contrast-enhanced computed tomography (CT) showed a 33×20-mm low-attenuation mass in the pancreatic tail (Fig. 1a) and a 10-mm metastatic mass in S4 of the liver (Fig. 1b). There was a large amount of ascites, and the omentum was 40 mm thick, consistent with peritoneal carcinomatosis (Fig. 1c). An endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) biopsy of the pancreatic tumor revealed adenocarcinoma, and peritoneal fluid cytology was class V. Although she experienced abdominal distension, her Eastern Cooperative Oncology Group Performance Status (ECOG PS) was stable at 2. The patient received cell-free and concentrated ascites reinfusion therapy (CART) followed by combined chemotherapy, including intravenous gemcitabine and nab-paclitaxel, as well as intraperitoneal administration of paclitaxel. The treatment was performed according to jRCTs 031180095, which included nab-paclitaxel at a dose of 125 mg/m2 and gemcitabine at 1,000 mg/m2 administered intravenously; paclitaxel at 30 mg/m2 in 1 L saline at room temperature was administered intraperitoneally on days 1, 8, and 15, followed by 1 week of rest (13).
Figure 1.
Imaging findings before combined chemotherapy of Patient 1. (a) Contrast-enhanced CT showing a 33×20-mm low-attenuation mass at the pancreatic tail (arrow). (b) A 10-mm metastatic mass is seen in S4 of the liver (arrowhead). (c) There was a large amount of ascites, and the omentum was 40 mm thick, consistent with peritoneal carcinomatosis (arrow).
Because the patients had massive ascites, we did not insert an intraperitoneal access port, concerned about the risk of ascites leakage and subsequent port-related infection. On day 13 of the first course, she developed Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 Grade III febrile neutropenia but recovered quickly after the administration of granulocyte-colony stimulating factor (G-CSF) and antibiotics. She received intraperitoneal paclitaxel monotherapy on day 15 because systemic chemotherapy was avoided owing to concerns about neutropenia. Subsequently, we reduced the dose of systemic chemotherapy as follows: nab-paclitaxel at a dose of 100 mg/m2 and gemcitabine at 800 mg/m2 respectively, continuing with a 100% dose of intraperitoneal paclitaxel. No adverse events of CTCAE v5.0 Grade 3/4 have occurred since then.
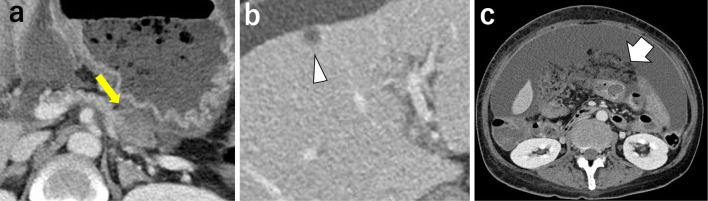
After two courses of combined chemotherapy, repeat CT showed shrinkage of the primary lesion to 25×15 mm (Fig. 2a), that of the metastatic liver tumor in S4 to 6 mm (Fig. 2b), and that of the omental thickness to 30 mm (Fig. 2c). The therapeutic effect, according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 criteria was stable disease (SD). While the amount of ascites seemed to be increasing, ascites assessed on CT at a given point often depends on the drainage timing. The volume of ascites required to relieve her symptoms decreased from 10,000 mL to 6,000 mL from the second to third course. She received a total of 4 courses of combined chemotherapy over 16 weeks. The cytology of ascites was still positive after treatment, and she was unable to tolerate oral intake due to massive ascites and stenosis in the gastrointestinal tract. Chemotherapy was then stopped, and she received supportive care and died three weeks later. The patient ultimately lived for 19 weeks from the start of therapy. CART was performed five times during the treatment.
Figure 2.
Imaging findings after combined chemotherapy treatment of Patient 1. (a) CT showing shrinkage of the primary lesion in the pancreatic tail to 25×15 mm (arrow). (b) The metastatic mass in S4 of the liver decreased to 6 mm (arrowhead). (c) Omental thickening decreased to 30 mm (arrow).
Patient 2
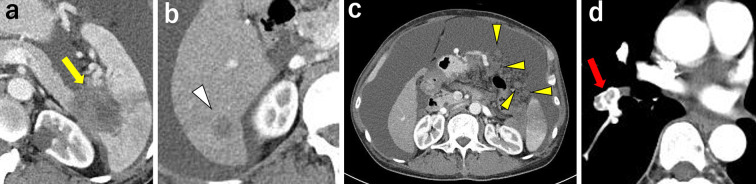
A 58-year-old man presented with advanced pancreatic cancer and malignant ascites. The patient had a history of chronic hepatitis B and was treated with entecavir. Contrast-enhanced CT scan showed a 43×38-mm low-attenuation mass in the pancreatic tail invading the spleen (Fig. 3a). An 18×14-mm metastatic mass was observed in S6 of the liver (Fig. 3b). There was also a large amount of ascites and nodules in the abdominal cavity, which was consistent with peritoneal carcinomatosis (Fig. 3c). Peritoneal fluid cytology was classified as class V. In addition, deep vein thrombosis and pulmonary thromboembolism were also observed (Fig. 3d). Although vital signs, including oxygenation and blood pressure, were unremarkable, the patient was incapable of eating due to massive abdominal distension. The ECOG PS was 3.
Figure 3.
Imaging findings before combined chemotherapy of Patient 2. (a) Contrast-enhanced CT showed a 43×38-mm low-attenuation mass in the pancreatic tail, invading the spleen (arrow). (b) An 18×14-mm metastatic mass is found in S6 of the liver (arrowhead). (c) There was a large amount of ascites and many disseminated nodules (arrowheads) in the abdominal cavity, indicative of peritoneal carcinomatosis. (d) Large thrombus in the pulmonary artery (arrow).
To alleviate abdominal distension, nausea, and anorexia, a diuretic agent was administered and CART was performed through an intraperitoneal catheter. For venous thromboembolism and pulmonary embolus, edoxaban tosylate hydrate was administered. The patient's general condition gradually improved, and oral intake also increased. Two weeks after beginning treatment, the ECOG PS improved to 2, and he then received combined chemotherapy with intravenous administration of gemcitabine and nab-paclitaxel and intraperitoneal administration of paclitaxel. He received initial treatment with systemic chemotherapy using nab-paclitaxel at a dose of 100 mg/m2 and gemcitabine at 800 mg/m2 due to concerns about bone marrow suppression without reduction of intraperitoneal administration of paclitaxel. Before administration of intraperitoneal paclitaxel, 6,500 mL of ascites was drained. After initial treatment, he developed grade 2 neutropenia which required no treatment, therefore, he received intraperitoneal paclitaxel monotherapy at days 8 and 19. Thereafter, the chemotherapy dose was further reduced: nab-paclitaxel at a dose of 75 mg/m2 and gemcitabine at 600 mg/m2 and intraperitoneal paclitaxel at a dose of 20 mg/m2, respectively.
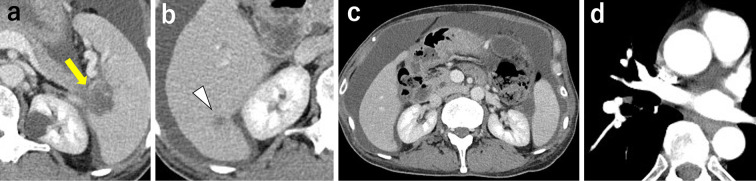
After the first course of treatment, the ascites markedly improved, and the patient was discharged. He then received systemic chemotherapy twice with nab-paclitaxel and gemcitabine without intraperitoneal paclitaxel because the ascites volume had significantly reduced, and obtaining percutaneous abdominal access at this time was believed to incur excessive risk. Two months after starting chemotherapy, the size of the primary lesion had markedly decreased to 29×23 mm (Fig. 4a) and that of the metastatic liver tumor in S6 to 8×7 mm (Fig. 4b), and disseminated nodules in the abdominal cavity were not observed (Fig. 4c). The therapeutic effect according to RECIST ver. 1.1 criteria was partial response (PR). Furthermore, the thrombus in the pulmonary artery resolved (Fig. 4d).
Figure 4.
Imaging findings after combined chemotherapy treatment of Patient 2. (a) The primary lesion was markedly reduced in size to 29×23 mm (yellow arrow). (b) The metastatic mass in S6 of the liver decreased to 8×7 mm (arrowhead). (c) Disseminated nodules in the abdominal cavity disappeared. (d) The thrombus in the pulmonary artery resolved.
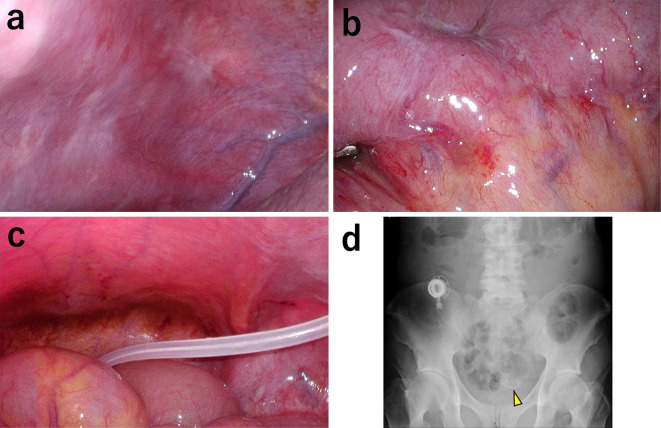
Laparoscopy was performed to place an intraperitoneal access port. There were nodules on the abdominal wall, small intestine, and mesentery, which were thought to have reduced in size after chemotherapy treatment (Fig. 5a, b), with a peritoneal cancer index score of 18 (14). An intraperitoneal access port was implanted in the subcutaneous space to facilitate continuous intraperitoneal chemotherapy. The tip of the catheter was located in the pouch of Douglas (Fig. 5c, d).
Figure 5.
Laparoscopic images and abdominal X-ray findings after combined therapy treatment of Patient 2. (a) Shrinkage of disseminated nodules on the peritoneal surface after chemotherapy. (b) Shrinkage of disseminated nodules on the small intestine after chemotherapy. (c, d) An intraperitoneal access port was placed. The tip of the catheter is in the Douglas pouch (arrowhead).
The patient subsequently received stable combined chemotherapy without any adverse events or trouble with intraperitoneal chemotherapy. The cytology of ascites turned negative for 11 weeks, and he returned to work 15 weeks after the first chemotherapy. By the time his condition deteriorated such that chemotherapy was stopped, the patient had received 8 courses of chemotherapy for 30 weeks. He was then given the best supportive care and died four weeks later. The patient ultimately lived for 36 weeks after starting therapy. He did not require ascites drainage for symptom relief after the first course of combined chemotherapy.
Discussion
Peritoneal carcinomatosis often develops in patients with pancreatic cancer. A population-based study of 2,924 patients with pancreatic cancer showed synchronous peritoneal carcinomatosis in 9% of patients, and an autopsy study reported that 22% of patients who died from pancreatic cancer had peritoneal carcinomatosis (3,15).
In patients with a good PS, modern chemotherapy regimens, such as FOLFIRINOX or nab-paclitaxel plus gemcitabine treatment, have been reported to improve the median survival in cases of metastatic pancreatic ductal adenocarcinoma to 11.1 and 8.5 months, respectively (16,17).
The median survival of patients with peritoneal dissemination in population-based studies of malignant ascites has been reported to be just 6-16 weeks (2-5). Takeda et al. recently reported that the prognosis of patients with peritoneal metastasis from pancreatic cancer has significantly improved over time with the advent of FOLFIRINOX and nab-paclitaxel plus gemcitabine. In this study, the median OS of patients who received FOLFIRINOX or nab-paclitaxel plus gemcitabine treatment was 6.8 months (18). In general, the PS of patients in studies using these strong chemotherapies is 0 or 1. However, data concerning the prognosis of patients with a poor PS due to massive ascites using these regimens are insufficient at present.
The most difficult problem associated with the treatment of patients with peritoneal carcinomatosis from pancreatic cancer is that the presence of peritoneal metastasis is associated with symptoms of abdominal fullness, abdominal pain, appetite loss, obstructive ileus, malnutrition, fatigue, impaired movement, deep venous thrombosis, pulmonary embolus, and dyspnea, all of which affect the QOL and lead to a poor PS, which in turn prevents patients from receiving chemotherapy (11,19). Such patients require treatment using approaches to improve their general condition and QOL. Diuretics are commonly administered to patients with massive ascites (20). Paracentesis is a common practice for relieving symptoms within a short period of time. However, paracentesis often necessitates protein replacement, as it causes a loss of protein contained in the ascitic fluid, leading to severe hypoalbuminemia, which has been reported to be a risk factor for febrile neutropenia, a side effect of cancer chemotherapy (21,22). CART is often a better option for treating refractory ascites than paracentesis and includes several components. After the ascites from the patient was filtered to remove cell components, it was concentrated to reduce the volume. The fluid obtained, including proteins, such as albumin and globulin, was reinfused intravenously. This therapy has been widely used in Japan to reduce the symptoms of massive ascites without causing severe hypoalbuminemia in patients with malignant ascites (22,23).
Pharmacokinetic studies of antitumor therapy for peritoneal carcinomatosis revealed that systemically administered anticancer drugs do not retain a sufficient drug concentration in the peritoneal cavity (24). This may partially explain why patients with peritoneal dissemination have a worse prognosis than those with metastases to other organs. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) have been used in patients with pseudomyxoma, mesothelioma, ovarian, and colorectal cancers, suggesting considerable efficacy for the treatment of peritoneal metastasis at specialized centers, especially in Western countries (7). However, no clinical trials have been conducted to evaluate the effects of cytoreductive surgery or HIPEC in patients with pancreatic cancer with peritoneal dissemination; only case reports and case accumulation studies have been published (25-27). In addition, the perioperative complication rate and treatment-related mortality rate for cytoreductive surgery are reportedly as high as 55.6% and 5.6-25%, respectively (25,26,5). Therefore, the Japanese clinical practice guideline for peritoneal malignancy 2021 weakly recommend that cytoreductive surgery and HIPEC for patients with pancreatic cancer not be performed (5).
Given this situation, attention has recently been paid to the repeated administration of intraperitoneal chemotherapy using paclitaxel, especially in Japan. Paclitaxel is insoluble in water and solubilized with Cremophor ElⓇ and ethanol. The size of the paclitaxel particles in solution is relatively large (10-12 nm). With such features, intra-abdominally administered paclitaxel is not absorbed into blood vessels; however, it is absorbed slowly through the lymphatic system. This results in prolonged retention in the peritoneal cavity compared to other hydrophilic drugs. Because of its pharmacological characteristics, there is little adhesion formation; therefore, it is suitable for repeated administration and, when necessary, subsequent surgery (7,10,23,28-30).
Satoi et al. reported the results of a multicenter phase II study of intravenous and intraperitoneal paclitaxel with S-1 in patients with pancreatic ductal adenocarcinoma with peritoneal metastasis and no other distant metastases. In 33 patients, the response rate was 33%, with a median survival of 16.3 months, and 8 patients (24.2%) underwent conversion surgery with a median survival of 27.8 months (11). Yamada et al. reported the results of a Phase I/II study of combined chemotherapy using intraperitoneal paclitaxel, intravenous gemcitabine, and nab-paclitaxel in patients with pancreatic ductal adenocarcinoma with peritoneal metastasis and no other distant metastases. In 46 patients, the median survival was 14.5 months, and 8 patients (17.4%) underwent conversion surgery, with a significantly longer median survival than those who did not (median survival not reached versus 12.4 months) (12).
The patients in these clinical trials had a favorable ECOG PS of 0 or 1 and no other severe comorbidities. Thus, combined chemotherapy using intraperitoneal paclitaxel and intravenous systemic chemotherapy, if used for patients with a good PS and relatively limited metastatic disease with a small amount of ascites, is a promising option for the treatment of patients with peritoneal dissemination from pancreatic cancer. Therefore, the Japanese clinical practice guideline for peritoneal malignancy 2021 weakly recommend intraperitoneal chemotherapy for patients with peritoneal dissemination from pancreatic cancer who do not have a large volume of ascites (5). Another phase II multicenter randomized clinical trial using intraperitoneal paclitaxel, intravenous gemcitabine, and nab-paclitaxel (jRCTs031180095) and a phase III multicenter randomized clinical trial using intraperitoneal and intravenous paclitaxel and oral S-1 (jRCTs051180199) are ongoing.
The prognosis of patients with peritoneal dissemination from pancreatic cancer has been reported to vary greatly, depending on the volume of ascites present and the PS. Takahara et al. reported that the median OS of patients with a PS of 0 to 2 with chemotherapy, 0 to 2 with best supportive care, and 3 to 4 with best supportive care were 124, 50, and 15 days, respectively (2). Thus, to improve the prognosis of patients with peritoneal dissemination from pancreatic cancer, it is important to treat the patient appropriately in order to improve their overall status so that they can be treated with chemotherapy, such as reducing the volume of ascites. In the two patients presented here, the therapeutic strategy involved multimodal treatment, including the administration of diuretic agents, paracentesis, and CART to reduce the volume of ascites and improve the PS of the patient, thus enabling the induction of chemotherapy. During chemotherapy, hypoalbuminemia and myelosuppression were minimized using CART and intraperitoneal paclitaxel monotherapy. We previously reported the efficacy of CART combined with intraperitoneal paclitaxel administration in patients with massive ascites due to peritoneal carcinomatosis from gastric cancer (31). In this study, the average volume of processed ascites was 3.1 L, which was concentrated to 0.33 L containing 85.5 g protein on average. Significant increases in urine volume, serum total protein, and albumin levels were found after CART, with no clinically significant adverse events noted. Since peritoneal dissemination from pancreatic cancer is usually aggressive and often causes a large volume of ascites, CART for patients with malignant ascites from pancreatic cancer may also be effective in maintaining PS and allowing further chemotherapy administration for patients with pancreatic cancer.
Owing to its pharmacological properties, intraperitoneal administration of paclitaxel is a local therapy inside the peritoneal cavity that rarely causes myelosuppression. Instead, concomitant systemic chemotherapy is usually required. If myelosuppression occurs with the administration of combined systemic and intraperitoneal chemotherapy, it is usually necessary to stop only systemic chemotherapy, and intraperitoneal chemotherapy can continue, thus continuing an effective treatment for peritoneal carcinomatosis while avoiding the development of severe systemic side effects. This therapeutic strategy may be effective in administering chemotherapy to patients with rapidly progressing pancreatic cancer and massive ascites. These palliative procedures enable the improvement or at least maintenance of a good PS and therefore facilitate the initiation or continuation of aggressive therapy, even in patients with massive ascites.
Takahara et al. reported the results of a phase I study of intraperitoneal paclitaxel combined with gemcitabine plus nab-paclitaxel in patients with pancreatic cancer with peritoneal metastasis. Major grade 3/4 adverse events included neutropenia (58%), anemia (33%), intraperitoneal access port-related complications (obstruction, infection, and transient ascites leakage from the skin wound) (33%), hypoalbuminemia (17%), febrile neutropenia (8%), and thrombocytopenia (8%). There were no adverse events related to intraperitoneal administration of paclitaxel or treatment-related deaths (13). These results suggest that intraperitoneal administration of paclitaxel is relatively safe. In the present cases, both patients developed neutropenia during the first course of treatment and required dose reduction. Therefore, when administering this regimen to patients with a poor PS, supportive care to improve the PS and maintain serum albumin levels, appropriate dose reduction, and careful observation during chemotherapy are needed. In the future, it will be necessary to verify the safety and efficacy of this combined chemotherapy regimen when administered to patients with a relatively poor PS.
In summary, a palliative combined chemotherapy regimen with intraperitoneal paclitaxel and systemic chemotherapy was administered to two patients with a poor PS at presentation due to massive ascites from advanced pancreatic cancer. Multimodal supportive treatment, including CART followed by combined chemotherapy, may be a promising option for the palliative management of patients with peritoneal metastasis from advanced pancreatic cancer.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Tonini V, Zanni M. Pancreatic cancer in 2021: what you need to know to win. World J Gastroenterol 27: 5851-5889, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahara N, Isayama H, Nakai Y, et al. Pancreatic cancer with malignant ascites. Pancreas 44: 380-385, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Thomassen I, Lemmens VE, Nienhuijs SW, Luyer MD, Klaver YL, de Hingh IHJT. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: a population-based study. Pancreas 42: 72-75, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Mackay TM, van Erning FN, van der Geest LGM, et al. Association between primary origin (head, body and tail) of metastasised pancreatic ductal adenocarcinoma and oncologic outcome: a population-based analysis. Eur J Cancer 106: 99-105, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Satoi S, Takahara N, Fujii T, et al. Synopsis of a clinical practice guideline for pancreatic ductal adenocarcinoma with peritoneal dissemination in Japan; Japan Peritoneal Malignancy Study Group. J Hepatobiliary Pancreat Sci 29: 600-608, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. World J Gastroenterol 19: 6979-6994, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitayama J, Ishigami H, Yamaguchi H, et al. Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg 2: 116-123, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol 21: 539-546, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Ishigami H, Yamaguchi H, Yamashita H, et al. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer 20: 128-134, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Meguro Y, Yamaguchi H, Kitayama J, et al. Pathological complete response after intraperitoneal paclitaxel and systemic combined chemotherapy in a patient with peritoneal metastases from gastric cancer: a case report. Surg Case Rep 6: 63, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoi S, Fujii T, Yanagimoto H, et al. Multicenter phase II study of intravenous and intraperitoneal paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients with peritoneal metastasis. Ann Surg 265: 397-401, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Yamada S, Fujii T, Yamamoto T, et al. Phase I/II study of adding intraperitoneal paclitaxel in patients with pancreatic cancer and peritoneal metastasis. Br J Surg 107: 1811-1817, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahara N, Nakai Y, Ishigami H, et al. A phase I study of intraperitoneal paclitaxel combined with gemcitabine plus nab-paclitaxel for pancreatic cancer with peritoneal metastasis. Invest New Drugs 39: 175-181, 2021 Feb. [DOI] [PubMed] [Google Scholar]
- 14.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82: 359-374, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Blastik M, Plavecz E, Zalatnai A. Pancreatic carcinomas in a 60-year, institute-based autopsy material with special emphasis of metastatic pattern. Pancreas 40: 478-480, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817-1825, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369: 1691-1703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda T, Sasaki T, Mie T, et al. Improved prognosis of pancreatic cancer patients with peritoneal metastasis. Pancreatology 21: 903-911, 2021. [DOI] [PubMed] [Google Scholar]
- 19.Meyer HJ, Wienke A, Surov A. Incidental pulmonary embolism in oncologic patients - a systematic review and meta-analysis. Support Care Cancer 29: 1293-1302, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleicher J, Lambert LA. A palliative approach to management of peritoneal carcinomatosis and malignant ascites. Surg Oncol Clin N Am 30: 475-490, 2021 Jul. [DOI] [PubMed] [Google Scholar]
- 21.Kloft C, Wallin J, Henningsson A, Chatelut E, Karlsson MO. Population pharmacokinetic-pharmacodynamic model for neutropenia with patient subgroup identification: comparison across anticancer drugs. Clin Cancer Res 12: 5481-5490, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Ishihara M, Horita N, et al. Effectiveness of cell-free and concentrated ascites reinfusion therapy in the treatment of malignancy-related ascites: a systematic review and meta-analysis. Cancers (Basel) 13: 4873, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito T, Hanafusa N. CART: Cell-free and Concentrated Ascites Reinfusion Therapy against malignancy-related ascites. Transfus Apher Sci 56: 703-707, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol 4: 277-283, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Farma JM, Pingpank JF, Libutti SK, et al. Limited survival in patients with carcinomatosis from foregut malignancies after cytoreduction and continuous hyperthermic peritoneal perfusion. J Gastrointest Surg 9: 1346-1353, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Tentes AA, Pallas N, Karamveri C, Kyziridis D, Hristakis C. Cytoreduction and HIPEC for peritoneal carcinomatosis of pancreatic cancer. J BUON 23: 482-487, 2018. [PubMed] [Google Scholar]
- 27.Lin SD, Soucisse ML, Lansom J, Morris DL. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a patient with peritoneal carcinomatosis from a pancreatic cystadenocarcinoma: a case report. Int J Surg Case Rep 63: 48-52, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med 332: 1004-1014, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm 235: 179-192, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Ishigami H, Kitayama J, Otani K, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology 76: 311-314, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi H, Kitayama J, Emoto S, et al. Cell-free and concentrated ascites reinfusion therapy (CART) for management of massive malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S-1. Eur J Surg Oncol 41: 875-880, 2015. [DOI] [PubMed] [Google Scholar]