Abstract
A 72-year-old man presented with bilateral ground-glass opacities in the lower lung fields on chest radiography. Computed chest tomography showed ground-glass opacities and micronodules in both lower lungs. A video-assisted thoracoscopic biopsy of the right lower lung showed homogeneous thickening of the alveolar septa with fibrosis and inflammatory cell infiltration consistent with fibrotic non-specific interstitial pneumonia (fNSIP). Cicatricial organizing pneumonia and intraluminal pulmonary ossification containing bone marrow that was considered to represent dendriform pulmonary ossification. Idiopathic fNSIP was diagnosed. The patient remains stable under antifibrotic treatment.
Keywords: cicatricial organizing pneumonia, dendriform pulmonary ossification, fibrotic interstitial pneumonia, antifibrotic treatment
Introduction
Cicatricial organizing pneumonia (CiOP) is a cryptogenic organizing pneumonia (COP) in which the loose fibromyxoid connective tissue of the organizing pneumonia exhibits progressive fibrosis with the formation of dense intraluminal eosinophilic scar tissue without destruction of the underlying lung architecture (1). CiOP is seen not only in cases of COP cases, but also as a component of histopathological findings in fibrosing interstitial pneumonia (2). In addition, CiOP is often associated with intraluminal ossification with dendriform features (3,4).
However, information on the clinical features and significance of CiOP with dendriform pulmonary ossification (DPO) is still limited. In this communication, we introduce a case of idiopathic fibrotic nonspecific interstitial pneumonia (fNSIP) associated with CiOP with intraluminal pulmonary ossification containing bone marrow that was considered to represent DPO. We also summarize recent knowledge regarding CiOP and DPO to incresese the awareness of physicians regarding these relatively rare conditions and discuss the mechanisms underlying the development of CiOP and intraluminal pulmonary ossification that contains bone marrow in this patient with fNSIP.
Case Report
A 72-year-old man was referred to our hospital for a detailed examination of an abnormality identified on chest radiography. Ground-glass opacities had been detected incidentally in bilateral lower lungs in a medical check-up. He had smoked 27 pack years. He denied any histories of environmental or occupational exposure that might cause interstitial pneumonia. His history included acute myocardial infarction at 60 years old, and he had been taking aspirin since then. Fine crackles were audible in the bilateral lower lungs. No findings suggestive of connective tissue disease were evident. Other physical findings were unremarkable.
Laboratory data showed elevated serum levels of Krebs von den Lungen-6 (956 U/mL) and surfactant protein-D (249 ng/mL). Serum autoantibodies were negative. Hypoxemia was not evident on an arterial blood gas analysis. The results of respiratory function testing were a vital capacity of 2.25 L (77.7% predicted); forced vital capacity, 2.59 L (79.0% predicted); forced expiratory volume in 1 second, 1.93 L (80.8% predicted); and lung carbon monoxide diffusion capacity, 7.90 mL/min/mmHg (44.5% predicted).
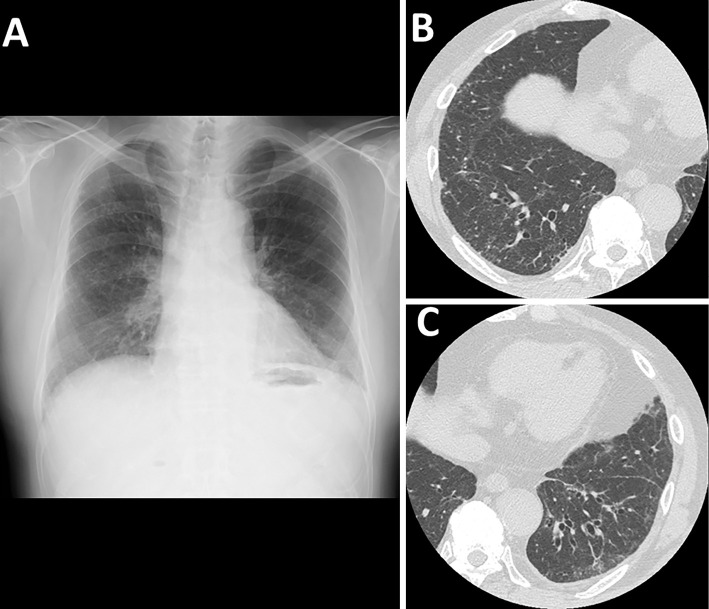
Chest radiography showed bilateral ground-glass opacities in the lower lung fields (Fig. 1A). Computed tomography (CT) of the chest showed bilateral ground-glass opacities and micronodules in the lower lobes (Fig. 1B, C). Using a mediastinal window setting for chest CT, no dense calcified lesions were observed. The analysis of the bronchoalveolar lavage fluid (BALF) showed a cell count of 2.16×105/mL, with a cell differential of 86.5% macrophages and 6.5% lymphocytes and a CD4/CD8 ratio of 1.8. No microorganisms were detected in the BALF culture. A transbronchial lung biopsy specimen obtained from the right upper lobe showed no significant findings.
Figure 1.
Chest radiography shows bilateral ground-glass opacities in the lower lung fields (A). Computed tomography of the chest shows bilateral ground-glass opacities and micronodules in the lower lobes (B, C).
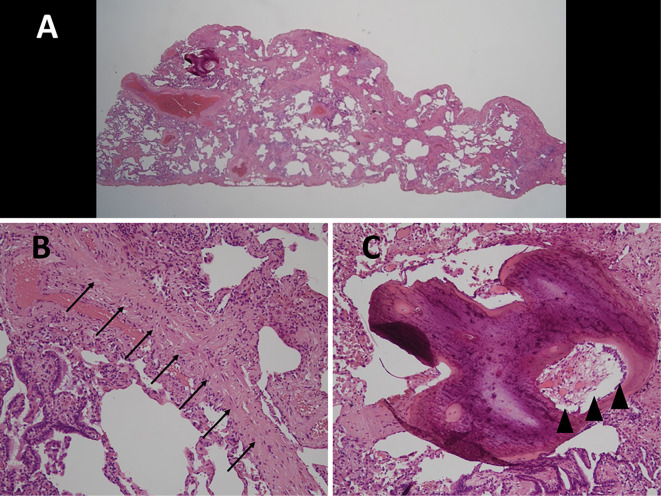
At this time, differential diagnoses included idiopathic NSIP and granulomatous pulmonary diseases, such as sarcoidosis, since interstitial opacities and micronodules were evident on chest CT. To confirm the diagnosis, a video-assisted thoracoscopic biopsy was performed. Specimens obtained from the right upper lobe showed relatively homogeneous thickening of the alveolar septa with fibrosis and infiltration of inflammatory cells consistent with fNSIP (Fig. 2A). CiOP findings, such as dense fibrosis with formation of intraluminal eosinophilic scar tissue without destruction of the underlying lung architecture (Fig. 2B), and intraluminal pulmonary ossification containing bone marrow components, such as fat and haematopoietic cells (Fig. 2C), were also observed. Therefore, idiopathic fNSIP with CiOP and pulmonary ossification was diagnosed.
Figure 2.
Biopsy specimens obtained from the right lower lobe show relatively homogeneous thickening of the alveolar septa with fibrosis and infiltration of inflammatory cells consistent with non-specific interstitial pneumonia [A, Hematoxylin and Eosin (H&E) staining, ×1]. Findings of cicatricial organizing pneumonia such as dense fibrosis with formation of intraluminal eosinophilic scar tissue without destruction of the underlying lung architecture (B, arrow; H&E staining, ×400) and intraluminal pulmonary ossification containing bone marrow such as fat and hematopoietic cells (C, arrowhead, H&E staining, ×400) are seen.
Because the patient was asymptomatic and afraid of the side effects of systemic corticosteroids, nintedanib was prescribed at 300 mg/day. A year later, the forced vital capacity had increased to 2.75 L (84.9% predicted), and serum Krebs von den Lungen-6 levels were normalised without radiological improvement.
Discussion
CiOP was first described by Yousem as a cicatricial variant of COP that may be a more recalcitrant form of COP, requiring morphological separation from classical COP (1). All patients in that report remained alive during the mean observation period of 90 months despite persistent radiological findings under systemic corticosteroid treatment. However, Zaizen et al. reported CiOP as a common histological finding in fibrotic interstitial pneumonia, such as usual interstitial pneumonia (UIP), and the fNSIP pattern as specific histological findings rather than a phenotype of a distinct disease entity. They also stated that CiOP-induced fibrotic interstitial pneumonia had a low risk of acute exacerbation and showed small improvements in restrictive ventilatory impairment during 12 months of follow-up (2). Later, CiOP was reported to be often accompanied by DPO, which was thought to represent a sequel of ordinary organizing pneumonia as well as CiOP (3).
Diffuse pulmonary ossification is divided into nodular-type ossification and DPO (5). Pulmonary nodular ossification can be associated with passive congestion due to heart failure, mitral stenosis, and hypertrophic subaortic stenosis. DPO is a rare condition characterised by metaplastic bone formation in the lung parenchyma and can be idiopathic or occur in association with underlying lung diseases, such as idiopathic pulmonary fibrosis (IPF) (5-8). Egashira et al. reported that DPO is common among patients with fibrosing interstitial lung disease and is significantly more prevalent in patients with IPF than in those with other fibrosing interstitial lung diseases. However, DPO is also observed in patients with NSIP and chronic hypersensitivity pneumonitis, although its prevalence in these patients is significantly lower than in patients with IPF (6). In addition, Kim et al. reported that DPO was seen only in UIP patients, not in NSIP patients, although the number of patients involved in their study was small (7). In the present case, the intraluminal pulmonary ossification differed from the morphology typical of DPO but did contain bone marrow, such as fat and haematopoietic cells, representing characteristic findings of DPO (3). Therefore, we considered the intraluminal pulmonary ossification in our case to represent DPO. Our case demonstrated improvement in restrictive ventilatory impairment without acute exacerbation under antifibrotic treatment, although follow-up for only one year is insufficient.
The association of fNSIP, CiOP, and DPO may have been coincidental in the present patient. However, CiOP has been observed in fNSIP as well as in UIP (2), and DPO has also been observed in NSIP, although its prevalence in NSIP is significantly lower than that in IPF (6). In addition, CiOP and DPO are believed to represent sequelae of normal organizing pneumonia, which is induced by lung injury (3), and DPO is confined to areas of more pronounced fibrosis, suggesting that DPO may reflect the chronicity and/or severity of interstitial pneumonia (7). Based on these observations, we speculated that relatively robust lung injury, similar to that involved in the pathogenesis of IPF, may have resulted in the development of intraluminal pulmonary ossification containing bone marrow in this patient with fNSIP, although the cause of the lung injury is unknown. This case demonstrated that DPO can develop in instances of fibrotic interstitial pneumonias other than IPF. To clarify the pathogenesis underlying the associations between fibrotic interstitial pneumonia, CiOP, and DPO, further research is needed. In addition, physicians should be aware of these relatively rare conditions.
In conclusion, we reported a rare case of idiopathic fNSIP with CiOP and intraluminal pulmonary ossification containing bone marrow. Relatively robust lung injury similar to that of IPF might have resulted in the development of CiOP and intraluminal pulmonary ossification.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Dr. Kazuhiro Tabata from the Department of Pathology at the Kagoshima University Graduate School of Medical and Dental Science for the pathological diagnosis.
References
- 1.Yousem SA. Cicatricial variant of cryptogenic organizing pneumonia. Hum Pathol 64: 76-82, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Zaizen Y, Tabata K, Yamano Y, et al. Cicatricial organizing pneumonia associated with fibrosing interstitial pneumonia - a clinicopathological study. Histopathol 80: 279-290, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Bin Saeedan M, Farver C, Mehta AC, Yadav R. Cicatricial organizing pneumonia with dendriform pulmonary ossification: an unusual cause for a recurrent pneumothorax. Case Rep Pulmonol 2019: 237915, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woge MJ, Ryu JH, Bartholmai BJ, Yi ES. Cicatricial organizing pneumonia: a clinicopathologic and radiologic study on a cohort diagnosed by surgical lung biopsy at a single institution. Hum Pathol 101: 58-63, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Enomoto T, Takimoto T, Kagawa T, et al. Histology proven dendriform pulmonary ossification: a five-case series. Intern Med 60: 2261-2268, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egashira R, Jacob J, Kokosi MA, et al. Diffuse pulmonary ossification in fibrosing interstitial lung diseases: prevalence and associations. Radiology 284: 255-263, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Kim TS, Han J, Chung MP, et al. Disseminated dendriform pulmonary ossification associated with usual interstitial pneumonia: incidence and thin-section CT-pathologic correlation. Eur Radiol 15: 1581-1585, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Nioshioka Y, Toyoda Y, Egashira R, et al. Nationwide retrospective observational study of idiopathic dendriform pulmonary ossification: clinical features with a progressive phenotype. BMJ Open Respir Res 9: e001337, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]