Abstract
Immune checkpoint inhibitors (ICIs) can cause immune-related adverse events (irAEs). There are a few case reports of remitting seronegative symmetrical synovitis with pitting edema syndrome (RS3PE) as an irAE. We herein report a 49-year-old Japanese man who developed acute-onset polyarthralgia and edema of the back of both hands and bilateral lower legs after pembrolizumab administration for lung cancer. The patient's lung cancer was in complete remission, leading to the diagnosis of RS3PE induced by pembrolizumab rather than malignancy. When patients show RS3PE during ICI treatment, rheumatologists should consider the possibility of an irAE after excluding malignancy and systemic diseases.
Keywords: immune-related adverse events, remitting seronegative symmetrical synovitis with pitting edema syndrome
Introduction
Immune checkpoint inhibitors (ICIs) activate antitumor immunity and exert therapeutic effects on various malignant tumors by directing against negative regulators in the immune system (1). However, ICIs often induce immune-related adverse events (irAEs) by activating the immune system, causing inflammatory side effects in whole-body systems (2).
Musculoskeletal irAEs account for 7.7% of such side effects, including inflammatory arthritis, myositis, and polymyalgia rheumatica (3). There are a few case reports of remitting seronegative symmetrical synovitis with pitting edema syndrome (RS3PE) as an irAE (4-7). Although pembrolizumab, which inhibits the programmed death protein 1 (PD-1) receptor, rarely causes RS3PE as an irAE (8,9), the clinical picture is unclear.
We herein report a case of irAE of RS3PE caused by pembrolizumab. Rheumatologists should recognize RS3PE as an irAE manifestation and appropriately treat it.
Case Report
A 49-year-old Japanese man with polyarthralgia and immobility was admitted to our hospital. Two years prior, he had experienced chest pain, productive cough, and hemoptysis for 14 days. Chest computed tomography (CT) at the time had revealed an approximately 37-mm tumor in the right lower lobe of the lung. The tumor had merged with the right hilar lymph nodes. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT had revealed an abnormal FDG uptake in the tumor and mediastinal lymph nodes. A transbronchial biopsy confirmed squamous cell carcinoma of the right hilum (cStage T2aN3M1a, Stage IVA). As treatment, the patient received a three-drug chemotherapy regimen consisting of carboplatin, nab-paclitaxel, and pembrolizumab.
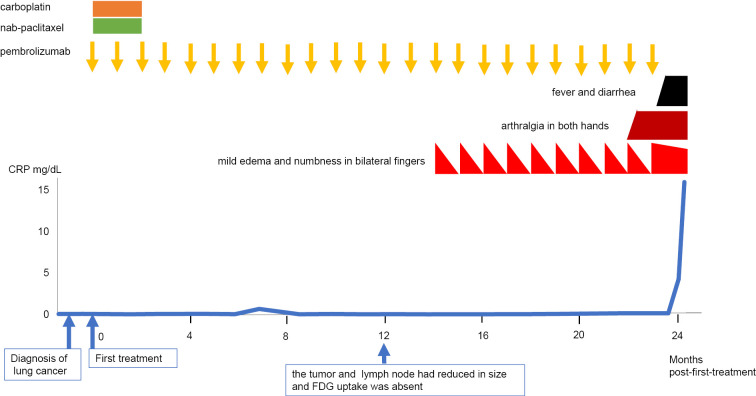
From 3 months post-first-treatment, the patient continued monthly pembrolizumab treatment (Fig. 1). At 12 months post-first-treatment, the tumor and mediastinal lymph node had reduced in size, and FDG uptake was absent in PET-CT, indicating a complete response. However, at 14 months post-first-treatment, the patient experienced mild edema and numbness in both fingers immediately after pembrolizumab infusion. Although the bilateral finger edema improved spontaneously within three weeks, the symptoms recurred immediately after pembrolizumab infusion. At 22 months post-first-treatment, he noticed arthralgia in both hands. At 23 months post-first-treatment he developed a 39°C fever and diarrhea 1 week after the 24th infusion of pembrolizumab. Antibiotics did not improve the symptoms, and he was admitted to the hospital.
Figure 1.
Clinical course before the onset of remitting seronegative symmetrical synovitis with pitting edema syndrome.
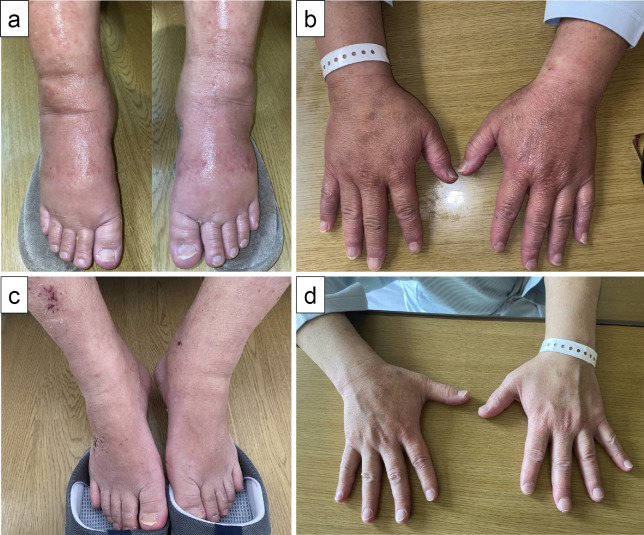
On day 1 of hospitalization, he developed polyarthralgia and edema over the dorsum of both hands and both lower legs, rendering him immobile. His medical history included primary aldosteronism, type 2 diabetes, and asthma-chronic obstructive pulmonary disease overlap syndrome. He used to smoke frequently but had quit at 47 years old. His recent medications included teneligliptin, brotizolam, mecobalamin, pregabalin, loxoprofen, rebamipide, and mirogabalin. On an examination at admission, his temperature was 37.7°C, blood pressure 118/70 mmHg, and heart rate 90 beats/min. He had pitting edema on the backs of both hands and bilateral lower legs (Fig. 2a, b). He had mild tenderness in both shoulder joints but no tenderness in both elbows, knees, hands, or wrist joints; however, he complained of pain in these joints with active movement and no pain with passive movement. Laboratory tests revealed a white blood cell count of 13,770 /μL, C-reactive protein 20.8 mg/dL, and other serological abnormalities, including matrix metalloproteinase-3 (MMP-3) 117 ng/mL, and vascular endothelial growth factor (VEGF) 1,510 pg/mL (Table 1). Rheumatoid factor, anti-cyclic citrullinated peptide antibody, and antinuclear autoantibodies were within the respective normal ranges. Although proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA) was mildly positive, symptoms suggestive of systemic vasculitis, such as polyneuritis multiplex, were not evident.
Figure 2.
Clinical presentation of remitting seronegative symmetrical synovitis with pitting edema syndrome on admission (a, b) and after prednisolone therapy (c, d). (a, b) Pitting edema on the back of both hands and bilateral lower legs. (c, d) Two days after prednisolone therapy. Pitting edema completely disappeared.
Table 1.
Laboratory Data on Admission.
| Value | Normal range | |
|---|---|---|
| Urinalysis | ||
| Protein | (-) | (-) |
| Occult blood | (-) | (-) |
| Urinary cast | (-) | (-) |
| Blood count | ||
| White blood cell (/μL) | 13,770 | 3,300-8,800 |
| Neutrophil (%) | 86.4 | |
| Lymphocyte (%) | 4.6 | |
| Hemoglobin (g/dL) | 10.6 | 13.5-17.0 |
| Platelet (/μL) | 38.4 | 13.0-35.0×10⁴ |
| Serum chemistry | ||
| Blood urea nitrogen (mg/dL) | 19 | 8.0-20.0 |
| Creatinine (mg/dL) | 0.77 | 0.60-1.00 |
| Sodium (mEq/L) | 135 | 135-149 |
| Potassium (mEq/L) | 4.3 | 3.5-4.9 |
| Chloride (mEq/L) | 97 | 96-108 |
| Aspartate aminotransferase (IU/L) | 21 | 13-33 |
| Alanine aminotransferase (IU/L) | 33 | 8-42 |
| Lactate dehydrogenase (IU/L) | 165 | 119-229 |
| Creatinine kinase (IU/L) | 11 | 59-248 |
| Total protein (g/dL) | 5.3 | 6.7-8.3 |
| Albumin (g/dL) | 2.2 | 4.0-5.0 |
| Immunological findings | ||
| C-reactive protein (mg/dL) | 20.8 | 0-0.3 |
| Immunoglobulin G (mg/dL) | 825 | 870-1,700 |
| Immunoglobulin A (mg/dL) | 248 | 110-410 |
| Immunoglobulin M (mg/dL) | 87 | 46-260 |
| C3 (mg/dL) | 120 | 65-135 |
| C4 (mg/dL) | 8 | 13-35 |
| 50% hemolytic unit of complement (U/mL) | 41 | 25.0-48.0 |
| Soluble interleukin-2 receptor (U/mL) | 4,567 | 157-474 |
| Anti-nuclear antibody | <40 | <40 |
| Rheumatoid factor (IU/mL) | <5 | <15 |
| Anti-cyclic citrullinated peptide antibody (U/mL) | <1.0 | <4.5 |
| Matrix metalloproteinase-3 (ng/mL) | 117.0 | 36.9-121 |
| Ferritin (ng/mL) | 199 | 20-250 |
| Cryoglobulin | (-) | (-) |
| Vascular endothelial growth factor (pg/mL) | 1,510 | 143.1-658.8 |
| MPO-ANCA (U/mL) | <1.0 | <1.0 |
| PR3-ANCA (IU/mL) | 2.5 | <1.0 |
| Thyroid stimulating hormone (μU/mL) | 1.08 | 0.27-4.20 |
| Free triiodothyronine (pg/mL) | 1.98 | 2.3-4.0 |
| Free thyroxine (ng/mL) | 1.05 | 1.0-1.8 |
| Adrenocorticotropic hormone (pg/mL) | 74.8 | <46 |
| Cortisol (μg/dL) | 23.5 | 6.2-19.4 |
| Hepatitis B surface antigen (IU/mL) | <0.001 | <0.05 |
| Hepatitis B core antibody | 0.1 | <1.0 |
| Hepatitis C virus antibody | (-) | (-) |
| Interferon-gamma release assay | (-) | (-) |
MPO-ANCA: myeloperoxidase antineutrophil cytoplasmic antibody, PR3-ANCA: proteinase-3-antineutrophil cytoplasmic antibody
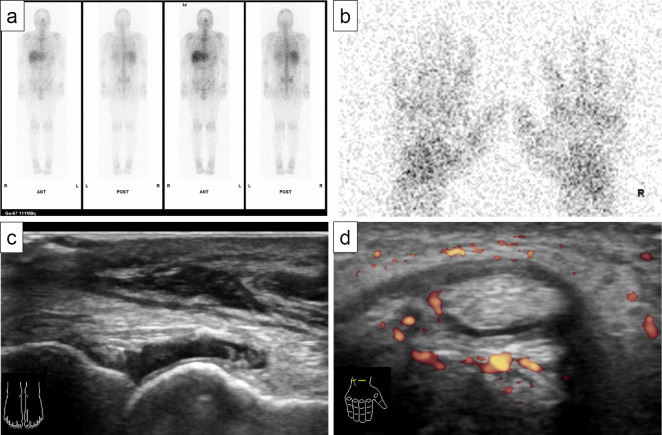
67Ga scintigraphy revealed no accumulation suggestive of malignancy recurrence or metastasis, including in the right lung and mediastinal lymph nodes (Fig. 3a), but mild accumulation in the fingers and both hands was noted (Fig. 3b). Musculoskeletal ultrasonography showed mild synovitis on a gray scale of 1, according to the system proposed by Outcomes Measures in Rheumatology-EULAR (10), in the metacarpophalangeal, proximal interphalangeal, and metatarsophalangeal joints (Fig. 3c), and tenosynovitis was revealed by hypoechoic and power Doppler around the finger extensor tendon (Fig. 3d).
Figure 3.
Image findings of 67Ga scintigraphy (a, b) and musculoskeletal ultrasonography (c, d). (a) No accumulation suggestive of recurrence or metastasis of malignancy was noted in the whole body, including the right lung and mediastinal lymph nodes. (b) Mild accumulation in fingers and both hands. (c) Mild synovitis in the metatarsophalangeal joint. (d) Tenosynovitis was revealed by hypoechoic and power Doppler around the finger extensor tendon.
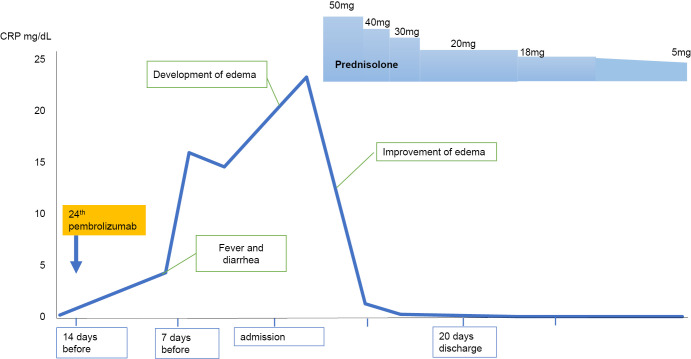
Because there was no evidence of malignancy recurrence, we diagnosed the patient with RS3PE as an irAE induced by pembrolizumab. Because diarrhea during his admission was also likely to have been colitis due to an irAE, and his symptoms were likely to be equivalent to grade 3 inflammatory arthritis induced by an irAE (11), we administered prednisolone at a dose of 50 mg/day (0.7 mg/kg/day) (Fig. 4).
Figure 4.
Clinical course after the onset of remitting seronegative symmetrical synovitis with pitting edema syndrome.
His polyarthralgia improved the day after starting treatment, and edema of the back of both hands and dorsum pedis soon disappeared (Fig. 2c, d). The systemic inflammation subsided, so the prednisolone dose was decreased to 20 mg/day over 2 weeks, and the patient was discharged. As he had been treated with the 24th infusion of pembrolizumab and was in a stage of complete remission, pembrolizumab was discontinued. At 8 months after glucocorticoid initiation, prednisolone was tapered to 5 mg/day, without relapse of lung cancer or RS3PE.
Discussion
We encountered a case of RS3PE that was an irAE induced by pembrolizumab. During ICI treatment for lung cancer, the patient developed polyarthralgia with edema on the back of both hands and dorsum pedis that gradually worsened each month following pembrolizumab infusion. Systemic inflammation was notable, and disease-specific autoantibodies for rheumatic diseases were not detected, leading to a diagnosis of RS3PE. Although he showed slight PR3-ANCA positivity, he did not complain of any symptoms of systemic vasculitis. Paraneoplastic syndrome was unlikely because his lung cancer was in complete response (12), and 67Ga scintigraphy showed no accumulation suggestive of recurrence or metastasis. Because the patient was under 50 years old, the probability of idiopathic RS3PE was low (13). Furthermore, polyarthralgia with edema developed immediately after pembrolizumab infusion, suggesting that pembrolizumab triggered these symptoms. These symptoms were soon resolved by prednisolone and pembrolizumab withdrawal, which supported the diagnosis.
Notably, pembrolizumab-induced irAEs manifested as RS3PE in this case. RS3PE etiology includes idiopathic and secondary comorbidities of other diseases and drugs (14). Malignancy (20-54%) is the most common cause (15,16), while other diseases include systemic lupus erythematosus, gout, Sjögren's syndrome, polyarteritis nodosa, ankylosing spondylitis, sarcoidosis, amyloidosis, relapsing polychondritis, bronchiolitis obliterans, and organizing pneumonia (17). Recurrence of malignancy or other systemic diseases that can cause RS3PE was not observed in the present case. In addition, some drugs can cause RS3PE (14). Indeed, dipeptidyl peptidase-4 (DPP4) inhibitors are risk factors for RS3PE (18), and the effect of DPP4 inhibitors on the development of RS3PE in this case cannot be completely ruled out, as he had been taking teneligliptin; however, RS3PE symptoms occurred after ICI administration and remained improved after prednisolone treatment even with the continuous use of DPP4 inhibitors. There was also a discrepancy between the onset of RS3PE symptoms and the initiation of DPP4 inhibitors, which prompted us to consider the effect of ICI rather than teneligliptin. Finally, the concurrence of diarrhea at the time of RS3PE development may have increased the risk of irAEs. Given these possibilities, we propose that pembrolizumab was the most likely factor inducing RS3PE in the present case.
To date, nine cases of RS3PE as an irAE have been reported (4-6,8,9,19-22). (Table 2). The clinical features of RS3PE as an irAE and idiopathic RS3PE are similar, including negative serologies for rheumatoid factor and anti-cyclic citrullinated peptide antibody. The duration between the initial ICI administration and RS3PE diagnosis usually varies from 1 to 18 months. Our patient was diagnosed 23 months after ICI initiation, which is longer than usual. Thus, when treated with an ICI, patients may develop RS3PE as an irAE at any time. There appears to be no trend in the type of ICI agent causing such side effects in the literature; five of nine patients were treated with nivolumab, three with ipilimumab, one with durvalumab, and three with pembrolizumab (Table 2), suggesting that RS3PE as an irAE is unpredictable by the type of ICI alone.
Table 2.
Cases of Remitting Seronegative Symmetrical Synovitis with Pitting Edema (RS3PE) Syndrome Induced by irAEs Reported in the Literature.
| Reference | Age (y.o.) | Sex (M/F) | Target disease | ICI | Time from initial ICI therapy to RS3PE diagnosis | First treatment of RS3PE | Treatment outcome | Retreated by ICI | Course after retreatment with ICI |
|---|---|---|---|---|---|---|---|---|---|
| (19) | 80 | M | Malignant melanoma | Nivolumab | 4 weeks | Corticosteroid 0.5 mg/kg/day | Remission | Yes | No relapse, but ICI stopped due to malignancy progression |
| (5) | 70 | M | Malignant melanoma | Nivolumab and ipilimumab | 11 months | PSL 0.5 mg/kg/day | Remission | Yes | Flared after each ICI administration. Continued PSL 7.5 mg/day |
| (20) | 57 | F | Lung adenocarcinoma | Nivolumab | 18 months | PSL 1 mg/kg/day | Remission | Yes | No relapse |
| (4) | 70 | M | Malignant melanoma | Nivolumab | 1 month | Corticosteroid 0.5 mg/kg/day | Remission | Yes | Flared after each ICI administation. Low dose glucocorticoids |
| (21) | 70 | M | Malignant melanoma | Nivolumab and ipilimumab | 6 weeks | PSL 1,000 mg/day | Remission | No | |
| (22) | 70 | M | Prostate cancer | Durvalumab | 1 week | PSL 15 mg/day | Remission | No | |
| (6) | 59 | M | Prostate cancer | Ipilimumab | 9 weeks | PSL 10 mg/day as part of hormone treatment regimen MTX 15 mg/week | Remission | No | |
| (8) | 79 | M | Malignant melanoma | Pembrolizumab | 11 months | PSL 60 mg/day | Remission | No | |
| (2) | 84 | M | Urothelial carcinoma | Pembrolizumab | 6 weeks | PSL 30 mg/day | Remission | Yes | No relapse with continued PSL 3 mg/day |
| Our case | 49 | M | Lung squamous cell carcinoma | Pembrolizumab | 23 months | PSL 50 mg/day | Remission | No |
PSL: predonisolone, MTX: methotrexate, ICI: immune checkpoint inhibitor
Multiple risk factors for the irAE incidence might have contributed to the clinical course of the present case. Numerous risk factors for irAEs, classified as patient-, tumor-, and agent-specific, have been reported (23). The present case had many patient-specific risk factors for irAE development, including being under 60 years old, having a history of smoking, and having a relatively high body mass index, asthma, chronic obstructive pulmonary disease, and hypertension. The use of anti-PD-1 antibodies can increase the risk of arthralgia (23). Patients with these risk factors for irAEs should be carefully monitored during ICI treatment.
Serum VEGF and MMP-3 levels might be helpful in diagnosing RS3PE, even as an irAE. Serum VEGF levels are prone to be high in cases of RS3PE (24,25). VEGF mediates angiogenesis and increases vascular permeability, which can cause synovitis and edema. Malignancy, toxic shock syndrome, and organizing pneumonia elevate the serum VEGF titer (25). These diseases have been reported to cause RS3PE, suggesting an association between serum VEGF and the pathogenesis of RS3PE (25). One case report described a patient with RS3PE as an irAE elevating serum VEGF levels (4), which decreased after treatment for RS3PE. Therefore, serum VEGF elevation might suggest the presence of RS3PE as an irAE as well as idiopathic RS3PE. The present patient also showed elevated serum VEGF levels, which supports this hypothesis. The relationship between ICIs and serum VEGF levels is unclear. Gauci et al. suggested an association between RS3PE and PD-1/programmed death ligand 1 (PDL-1) liaison (19). Inhibition of PD-1 or PDL-1 through ICIs might contribute to the onset of RS3PE, potentially leading to an increase in VEGF production. Serum MMP-3 levels also increase to various degrees in cases of RS3PE, depending on the cause (26,27). MMP-3 is produced by various malignant tumors, in addition to synovial cells in joints, as well as RS3PE (25). Origuchi et al. reported that serum MMP-3 levels in malignancy-induced RS3PE were significantly higher than in idiopathic RS3PE (median: paraneoplastic 437.3 vs. idiopathic 114.7 ng/mL) (27), suggesting a link between a high serum MMP-3 level and malignancy-induced RS3PE rather than idiopathic RS3PE. The present case showed mildly increased MMP-3 levels, similar to those seen with idiopathic RS3PE. This was consistent with our exclusion of active malignancy based on radiological tests to detect lesions that might cause malignancy-induced RS3PE. The serum VEGF and MMP-3 levels might be useful for supporting the diagnosis of not only idiopathic RS3PE but also RS3PE as an irAE. Further research is necessary, as the trends of titers of these biomarkers in RS3PE as an irAE have yet to be clarified, although some biomarkers for irAEs have been proposed (22). Until then, we should eliminate secondary causes of RS3PE with great care when considering diagnosing RS3PE as an irAE.
The optimal treatment strategy for managing RS3PE as an irAE has not been established. The American Society of Clinical Oncology guidelines recommend the treatment of irAEs, including musculoskeletal manifestations, according to a grading system (11). Although the guidelines do not include RS3PE, treatment strategies for inflammatory arthritis include prednisolone 10-20 mg/day for grade 2 (mild) and 0.5-1.0 mg/kg/day for grade 3 and 4 (severe). As such, cases of RS3PE as an irAE have been treated with glucocorticoids with a wide range of initial doses, from intravenous pulse therapy to prednisolone 10 mg/day (4-6,8,9,19-22) (Table 2), which tend to be higher doses than treatment for idiopathic RS3PE (28). Although we prescribed high-dose prednisolone in the present patient, considering RS3PE as an irAE, it might have been possible to treat him with a lower-dose glucocorticoid.
With regard to the prognosis, all patients quickly responded well to initial glucocorticoid therapy (Table 2). Six of the nine patients, including those who continued glucocorticoid therapy, resumed ICI treatment, with three experiencing relapse and three maintaining remission, supporting the hypothesis that ICIs can trigger RS3PE development. There is some concern that prednisolone >10 mg/day, along with ICI discontinuation, can worsen the overall prognosis (29). ICI-treated patients who develop irAEs have a longer overall survival than those who do not (30). In addition, one study suggested that glucocorticoid administration for irAEs did not hamper ICI efficacy or worsen the overall survival (31), which encouraged us to continue low-dose glucocorticoid therapy as maintenance therapy. A cohort study is needed to clarify the appropriate management strategy and prognosis for RS3PE as an irAE.
In conclusion, we reported a case of RS3PE as an irAE caused by pembrolizumab. When patients develop RS3PE during ICI treatment, rheumatologists should promptly treat them with glucocorticoids, considering the possibility of an irAE after carefully excluding recurrence of malignancy and other systemic autoimmune diseases. Because RS3PE as an irAE is a rare entity, similar cases should be collected to elucidate appropriate management strategies and the prognosis.
Written informed consent was obtained from the patient to publish his case.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Mr. David Price for critically reading the manuscript.
References
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 33: 1974-1982, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158-168, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Angelopoulou F, Bogdanos D, Dimitroulas T, Sakkas L, Daoussis D. Immune checkpoint inhibitor-induced musculoskeletal manifestations. Rheumatol Int 41: 33-42, 2021. [DOI] [PubMed] [Google Scholar]
- 4.Wada N, Uchi H, Furue M. Case of remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome induced by nivolumab in a patient with advanced malignant melanoma. J Dermatol 44: e196-e197, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Ngo L, Miller E, Valen P, Gertner E. Nivolumab induced remitting seronegative symmetrical synovitis with pitting edema in a patient with melanoma: a case report. J Med Case Rep 12: 48, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim ST, Murphy WA Jr, Aparicio A, Subudhi SK. RS3PE following treatment with combination of hormonal therapies plus ipilimumab in a patient with metastatic prostate cancer. J Immunother Precis Oncol 3: 128-132, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakhmalla M, Dahiya DS, Kichloo A, Fatima T, Edigin E, Wani F. Remitting seronegative symmetrical synovitis with pitting edema: a review. J Investig Med 69: 86-90, 2021. [DOI] [PubMed] [Google Scholar]
- 8.Hansmaennel A, Verhoeven F, Chouk M, Prati C, Aubin F, Wendling D. RS3PE syndrome induced by Pembrolizumab. Joint Bone Spine 88: 105216, 2021. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura A, Yamanaka K, Tadokoro R, et al. Remitting seronegative symmetrical synovitis with pitting edema syndrome induced by pembrolizumab in patient with urothelial carcinoma. IJU Case Rep 5: 219-222, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Agostino MA, Terslev L, Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce - part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 3: e000428, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Lacchetti C, Schneider BJ, et al.; the National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36: 1714-1768, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 85: 838-854, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivé A, del Blanco J, Pons M, Vaquero M, Tana X. The clinical spectrum of remitting seronegative symmetrical synovitis with pitting edema. The Catalán Group for the Study of RS3PE. J Rheumatol 24: 333-336, 1997. [PubMed] [Google Scholar]
- 14.Borges T, Silva S. RS3PE syndrome: autoinflammatory features of a rare disorder. Mod Rheumatol 33: 640-646, 2023. [DOI] [PubMed] [Google Scholar]
- 15.Yao Q, Su X, Altman RD. Is remitting seronegative symmetrical synovitis with pitting edema (RS3PE) a subset of rheumatoid arthritis? Semin Arthritis Rheum 40: 89-94, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Altman RD, Yao Q. RS3PE: clinical and research development. Curr Rheumatol Rep 17: 49, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Varshney AN, Singh NK. Syndrome of remitting seronegative symmetrical synovitis with pitting edema: a case series. J Postgrad Med 61: 38-41, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horai Y, Shimizu T, Okada A, et al. Dipeptidyl peptidase-4 inhibitor use is associated with a lower erythrocyte sedimentation rate in patients with remitting seronegative symmetrical synovitis with pitting oedema and pre-existing diabetes mellitus. Mod Rheumatol 32: 830-833, 2022. [DOI] [PubMed] [Google Scholar]
- 19.Gauci ML, Baroudjian B, Laly P, et al. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome induced by nivolumab. Semin Arthritis Rheum 47: 281-287, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Filetti M, Anselmi E, Macrini S, et al. Resolution of remitting seronegative symmetrical synovitis with pitting edema (RS3PE) during Nivolumab therapy for non-small cell lung cancer: a case report. Semin Arthritis Rheum 48: e17-e20, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Amini-Adle M, Piperno M, Tordo J, et al. Remitting seronegative symmetric synovitis with pitting edema associated with partial melanoma response under anti-CTLA-4 and anti-programmed death 1 combination treatment. Arthritis Rheumatol 70: 1358, 2018. [DOI] [PubMed] [Google Scholar]
- 22.Redman JM, Rhea LP, Cordes L, et al. A case of anti-PD-L1-associated remitting seronegative symmetric synovitis with pitting edema. Clin Genitourin Cancer 17: e549-e552, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chennamadhavuni A, Abushahin L, Jin N, Presley JP, Manne A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol 13: 779691, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arima K, Origuchi T, Tamai M, et al. RS3PE syndrome presenting as vascular endothelial growth factor associated disorder. Ann Rheum Dis 64: 1653-1655, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenzaka T. The relationship between remitting seronegative symmetrical synovitis with pitting edema and vascular endothelial growth factor and matrix metalloproteinase 3. Intern Med 59: 1021-1022, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawashiri SY, Nakano M, Kawakami A, Eguchi K. Monitoring of therapeutic efficacy in a patient with RS3PE syndrome by serologic variables and radiographic methods. Rheumatol Int 30: 1677-1680, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Origuchi T, Arima K, Kawashiri SY, et al. High serum matrix metalloproteinase 3 is characteristic of patients with paraneoplastic remitting seronegative symmetrical synovitis with pitting edema syndrome. Mod Rheumatol 22: 584-588, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Karmacharya P, Donato AA, Aryal MR, et al. RS3PE revisited: a systematic review and meta-analysis of 331 cases. Clin Exp Rheumatol 34: 404-415, 2016. [PubMed] [Google Scholar]
- 29.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36: 2872-2878, 2018. [DOI] [PubMed] [Google Scholar]
- 30.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 7: 306, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skribek M, Rounis K, Afshar S, et al. Effect of corticosteroids on the outcome of patients with advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Eur J Cancer 145: 245-254, 2021. [DOI] [PubMed] [Google Scholar]