Abstract
Two pairs of Sindbis virus (SV) variants that differ in their neuroinvasive and neurovirulent traits in mice have been isolated. Recently, we mapped the genetic determinants responsible for neuroinvasiveness in weanling mice. Here, we extend this study to newborn and adult rats and to rat neuronal cultures. Remarkably, certain aspects of the pathogenesis of these strains in rats were found to be quite distinct from the mouse model. Suckling rats were susceptible to all four isolates, and replication in the brain was observed after both intraperitoneal and intracranial (i.c.) inoculation. None of the isolates was neuroinvasive in adult rats, although all replicated after i.c. inoculation. For the isolate pair that was highly neurovirulent in mice, SVN and SVNI, only SVNI caused death after i.c. inoculation of adult rats. Similarly, only SVNI was cytotoxic for primary cultures of mature neurons. The genetic determinants responsible for the pathogenic properties of SVNI were mapped to the E2 glycoprotein and the 5′ noncoding region (5′NCR). Substitution of two amino acids in SVN E2 with the corresponding residues of SVNI (Met-190 and Lys-260) led to paralysis in 3- and 5-week-old rats. More dramatically, a single substitution in the 5′NCR of SVN (G at position 8) transformed the virus into a lethal pathogen for 3-week-old rats like SVNI. In 5-week-old rats, however, this recombinant was attenuated relative to SVNI by 2 orders of magnitude. Combination of the E2 and 5′NCR determinants resulted in a recombinant with virulence properties indistinguishable from those of SVNI. These data indicate that the 5′NCR and E2 play an instrumental role in determining the age-dependent pathogenic properties of SV in rats.
Neurovirulence, determined as the ability of a virus to cause a lethal infection in the central nervous system, has captured considerable interest in virological research (13, 15, 48, 49). Infection with Sindbis virus (SV), the prototype member of the Alphavirus genus, has provided useful systems for the study of neurovirulence, including an excellent mouse model used for studying the pathogenesis of alphavirus encephalitis (14, 20).
The SV prototype virion contains a positive-stranded RNA genome of 11,703 nucleotides. The 5′ two-thirds of the genome encodes the nonstructural proteins that function in viral replication. The structural protein coding region is located in the 3′ one-third of the genome. The genome RNA is complexed with multiple (total of 240) copies of the capsid protein to form an icosahedral nucleocapsid. The nucleocapsid is surrounded by a host-derived lipid bilayer from which the viral spikes protrude, which consist of three copies of the viral glycoprotein E2-E1 heterodimer (22, 45).
For several alphaviruses, amino acid changes in E1 and E2 have been shown to affect neurovirulence by mediating virus binding and entry into neuronal cells (18).
The E1 glycoprotein is highly conserved among alphaviruses and is involved in cell attachment, membrane fusion, and entry. The E2 glycoprotein, also involved in cell attachment, contains the majority of epitopes able to elicit potent neutralizing antibodies (16).
Recent evidence from several viral systems indicates that noncoding regions of viral genomes may be important determinants of virulence and pathogenicity, presumably through their effects on genome translation, replication, or transcription. Evidence of the importance of the 5′ noncoding region (5′NCR) in poliovirus resulted from comparison of the nucleotide sequences of virulent and attenuated variants of poliovirus and their revertants and from mapping of the determinants of virulence (23).
The effects of deletions and substitutions in the NCRs have also been studied for the alphatogaviruses, including SV (25, 32, 34) and Venezuelan equine encephalomyelitis (VEE) virus (24). These viruses differ in their capacity to produce disease in mice, suggesting that the noncoding region is an important determinant of virulence. Mouse challenge experiments with VEE viruses of the virulent Trinidad donkey (TD) strain or its attenuated vaccine derivative (TC-83) indicate that attenuation is determined in part by mutations within the 5′NCR, although changes in the E2 envelope glycoprotein also play a role.
We have isolated four variants of SV that differ in their neurovirulence and neuroinvasiveness in weanling mice (30). The SVA and SVB strains are nonvirulent after intracranial (i.c.) inoculation, although both can proliferate in the brain. On the other hand, after intraperitoneal (i.p.) injection these strains show similar patterns of viremia, though only SVB invades the brain. The neurovirulent pair, SVN and SVNI, will kill weanling mice after i.c. injection, but only SVNI is neuroinvasive, invading the brain after i.p. inoculation and causing death (30, 31).
Previously, we mapped the neuroinvasive loci of these strains (7). For SVB a single neuroinvasive determinant was mapped to E2 residue 55 (Gln-55), whereas for SVNI neuroinvasive loci were identified in both the 5′NCR (position 8) and E2 (Met-190).
In this study, we examined the outcome of infections caused by these four strains of SV in rats. None of the strains showed induction of viremia and neuroinvasion in adult rats, and only SVNI was found to be neurovirulent, after i.c. inoculation. Surprisingly, we found that the major determinant of the neurovirulence of SVNI is located in the 5′NCR, and changes in the E2 protein may contribute to the age-dependent pathogenesis.
MATERIALS AND METHODS
Viruses and recombinants.
The isolation and phenotype of the SVA/SVB and SVN/SVNI viruses have been described (30). Briefly, the two nonvirulent variants SVA and SVB were isolated by plaque purification from an SV strain. The two virulent strains in mice, SVN and SVNI, were isolated by serial passages of the same original SV strain. All parental viruses and recombinants used for this study were derived from infectious cDNA clones (7). For each virus, a first-passage virus stock was obtained by electroporation (Bio-Rad Gene Pulser) of BHK-21 (baby hamster kidney) cells with RNA transcripts from linearized full-length cDNA templates (7). The supernatant was harvested after incubation at 37°C for 24 h and stored at −70°C in small aliquots. SVA, SVB, SVN, and SVNI are virus stocks derived from the parental full-length cDNA clones.
Virus plaque assay on Vero cell monolayers.
The Vero cell line, derived from kidneys of normal African green monkeys, was grown in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS). For quantitation of SV, the original plaque technique in Vero cells was used (9). A dilution of virus was added to Vero cell monolayers in tissue culture dishes and incubated at 37°C for 1 h to permit viral adsorption. The monolayer was overlaid with minimal essential medium with 2% tragacanth (Gum tragacanth, grade III G-1128; Sigma), 2% FBS, and 2.4% NaHCO3. Cultures were incubated (37°C, 5% CO2) for 48 h, and plaques were counted after being stained with 0.05% neutral red (30, 31).
Animal inoculation and determination of LD50.
All rats used for animal studies were obtained from Charles River Laboratories. Viral phenotypes were assayed in suckling rats (3 days old), weanling rats (3 weeks old), or adult rats (5 weeks old).
Animals were inoculated by either i.p. or i.c. injection of 10-fold serial dilutions of the stock virus, as described earlier (30). For each parental or recombinant plasmid DNA template, two independent transcript preparations were used to derive virus stocks for in vivo assays by using groups of seven rats. The 50% lethal dose (LD50) values were calculated according to the method of Reed and Muench (41). Virus recovered from brains of rats (as a 20% brain homogenate in phosphate-buffered saline) was quantified by the plaque assay, as described above.
Tissue and cell primary cultures of rat cerebral cells.
Dissociated cerebral cells were cultured as monolayers on either glass or plastic-coated surfaces (2). Briefly, hemispheres were obtained from 16-day-old rat fetuses. Cells were mechanically dissociated by using Pasteur pipettes of different pore sizes. The cells were seeded either directly in 35-mm plastic dishes (106 cells/dish) or on round, 13-mm-diameter cover glasses previously coated with 0.1% l-polylysine (Sigma). The nutrient medium consisted of 10% heat-inactivated FBS and Eagle basal medium supplemented with 1% l-glutamine (2 mM), 1% dextrose, and 0.2% gentamicin (Gibco). Cultures were incubated for 2 to 3 weeks at 37°C in a 5% CO2 atmosphere. Single and small groups of neurons attached randomly and sprouted above a layer of dividing nonneuronal cells. The proliferation of the nonneuronal cells was controlled by the addition of cytosine arabinoside (AraC, 10−7 M; Sigma). Neurons could be continuously observed and were highly accessible for morphological, immunocytochemical, and electrophysiological studies.
Light and immunofluorescence microscopy.
Cultures were examined daily by a Zeiss inverted microscope prior to and after virus or mock infections. Analysis of cytological damage was assayed by phase-contrast microscopy.
For immunofluorescent staining, cells seeded on glass coverslips were fixed with cold acetone for 15 min at −20°C. The fixed cells were incubated with polyclonal anti-SV serum for 60 min, washed three times, and treated with goat anti-rabbit antibody conjugated to fluorescein isothiocyanate (Sigma) for 60 min. Observation was done with a Zeiss fluorescence microscope.
RESULTS
To characterize the in vivo and in vitro pathogenic properties of the four SV variants in the rat host, we studied the outcome of infection in newborn and young adult rats and in rat primary neuronal cultures.
Virus growth in suckling and adult rat brains.
We examined the brains of 3-day-old rats injected i.c. and i.p. with 103 PFU of the four viral strains. With both routes of inoculation, all four viruses proliferated well in the brains of suckling rats, reaching levels of 1.5 × 108 to 2.5 × 108 PFU/brain with SVA and SVB and 2.0 × 109 to 3.0 × 109 PFU/brain with SVN and SVNI (Table 1). All infections were lethal, and infected rats succumbed within 2 to 3 days.
TABLE 1.
Viral content in brains of suckling and adult rats inoculated with SVsa
| Virus strain | PFU/brainb
|
|||
|---|---|---|---|---|
| Suckling rats
|
Adult rats
|
|||
| i.c. | i.p. | i.c. | i.p. | |
| SVA | (1.5 ± 2.1) × 108 | (2.2 ± 1.6) × 108 | (3.8 ± 2.7) × 104 | <50 |
| SVB | (2.5 ± 1.7) × 108 | (1.4 ± 1.9) × 108 | (4.9 ± 2.0) × 104 | <50 |
| SVN | (3.0 ± 2.4) × 109 | (2.2 ± 2.6) × 109 | (1.8 ± 2.7) × 106 | <50 |
| SVNI | (2.5 ± 2.2) × 109 | (2.7 ± 2.1) × 109 | (2.4 ± 2.2) × 106 | <50 |
103 PFU of each strain was injected i.p. or i.c. as indicated.
Mean ± the standard error from seven animals. PFU were determined on Vero cells as described in Materials and Methods. Mouse brains were harvested on day 2 (suckling rats) or day 4 (adult rats) postinfection and used to prepare a 20% homogenate in phosphate-buffered saline, and titers were determined by plaque assay on Vero cells.
When young adult rats were inoculated i.p. with the four strains, neither viremia nor virus growth in the brain was observed despite the induction of high levels of anti-SV antibodies (data not shown). After i.c. injection, however, the nonvirulent (as defined in mice) viruses SVA and SVB proliferated to a titer of 4 × 104 to 5 × 104 PFU/brain, without any overt symptoms. Both mouse neurovirulent strains, SVN and SVNI, proliferated to a level of 2 × 106 PFU/brain (Table 1), but only SVNI was neurovirulent, causing the death of the infected rats. The LD50 after i.c. injection (ICLD50) of SVNI was found to be 16.4 ± 3.2 PFU per rat, with an average time to death of 5.5 days, whereas doses of 106 PFU of SVN were not lethal (Table 2).
TABLE 2.
Neurovirulence of SV strains in adult rats
| Virus strain | Virus titer
|
PFU/ICLD50 | Avg survival time (days) ± SE | |
|---|---|---|---|---|
| Tissue culture (PFU/ml)a | Rat (ICLD50/ml)b | |||
| SVA | (1.5 ± 1.2) × 108 | <102 | >106 | |
| SVB | (1.9 ± 1.7) × 108 | <102 | >106 | |
| SVN | (1.4 ± 1.0) × 108 | <102 | >106 | |
| SVNI | (2.3 ± 1.9) × 108 | (1.4 ± 1.5) × 107 | 16 | 5.5 ± 0.3 |
Mean ± the standard error (SE) from five titrations on Vero cells.
Mean ± the SE from seven animals (for each dilution) in three independent experiments. The LD50 was calculated by the Reed and Muench method.
Virus growth in primary rat neuronal cultures.
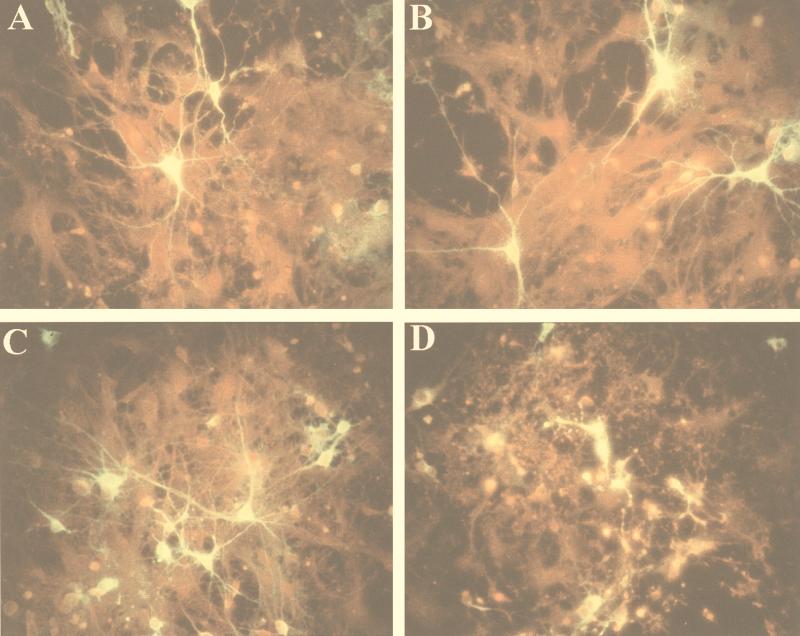
Primary neuronal cultures were prepared from dispersed rat brain cells as described in Materials and Methods. In order to prevent massive proliferation of glial cells, AraC (1-β-d-arabinofuranosylcytosine) was added. Thus, the majority of the cells in these cultures were neurons. Cultures were inoculated with viruses derived from infectious cDNA clones of the four SV strains. All four viruses showed similar growth kinetics in the neuronal cultures, reaching titers of 108 PFU/ml after 48 h. Cultures were monitored daily for signs of cytopathic effect, and the degree of infection was monitored by immunofluorescence. No morphologic changes or signs of cytopathic effect were observed in SVN- and SVNI-infected neurons or neurites at 24 h postinfection (Fig. 1A and C). However, by 48 h the damage caused by SVNI was clearly visible (Fig. 1D). In contrast, SVN (Fig. 1B)- or SVA/SVB (data not shown)-infected cells remained unchanged, and the neuronal disintegration seen in the SVNI-infected cultures was not observed even after 8 days (the longest time tested).
FIG. 1.
Immunofluorescent staining of SV envelope proteins in primary rat neuronal cultures infected with SVN (A and B) or SVNI (C and D) examined 24 h (A and C) or 48 h (B and D) postinfection. Neuronal monolayers were seeded on polylysine-coated glass coverslips and infected at a multiplicity of infection of 1 and then subjected to immunofluorescence analysis as described in Materials and Methods.
Localization of SVNI neurovirulence determinants.
To define the genetic determinants contributing to the neurovirulent properties of SVNI, we studied the outcome of infection in adult rats infected with several SVN-SVNI recombinants. Recombinants were constructed by exchanging homologous genomic fragments between SVN and SVNI and then by nucleotide substitution for fine mapping. The contribution of the genetic determinants to neurovirulence was assessed by determining the PFU/LD50 after i.c. injection (PFU/ICLD50).
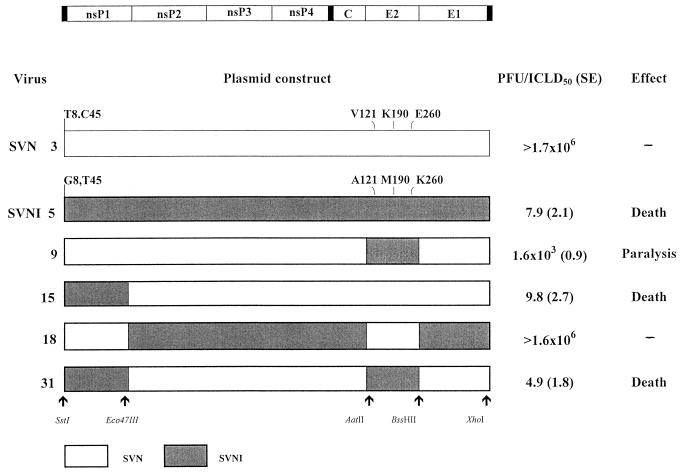
The data for the first set of recombinants are summarized in Fig. 2. The results indicate that a region of 1,909 bases of SVNI, encompassing most of the E2-coding region (recombinant 9), induced paralysis in the rats. However, high viral doses of recombinant 9 were required compared to SVNI. An additional recombinant (recombinant 15), including the 5′NCR and part of nsP1 of SVNI (1,406 bases), was shown to cause the death of the infected rats. However, both the 5′ end and the E2 SVNI neurovirulence determinants (recombinant 31) were required to produce a virus with neurovirulence properties indistinguishable from those of SVNI. Reciprocal recombinants in the SVNI background (recombinant 18) corroborated these results: the 5′ region from SVN combined with the SVN E2 determinant dramatically reduced the neurovirulence of the recombinant virus, behaving similarly to SVN.
FIG. 2.
Mapping of the loci responsible for SVNI neurovirulence in adult rats. A diagram of the SV genome is shown at the top, and the restriction sites used to generate the recombinants are indicated at the bottom. The names of the analyzed viruses, either viruses recovered from cDNA (SVN and SVNI) or various SVN/SVNI recombinants (numbered), are given at the left. cDNA constructs used for recovery of these viruses are diagrammed to show the sequences derived from SVN (open box) and SVNI (shaded box). Viral titers, given as PFU/ICLD50 (± the standard error [SE]), were determined after i.c. inoculation of rats. Rats were injected with 0.03 ml of serial 10-fold dilutions of the indicated virus stock (titers of 1 × 108 to 2 × 108 PFU/ml).
In the mapped regions SVN and SVNI differ by three amino acids in the E2 region (position 121 in SVN [Val] and SVNI [Ala], position 190 in SVN [Lys] and SVNI [Met], and position 260 in SVN [Glu] and SVNI [Lys]). Three differences are also present in the 5′ region: two in the 5′NCR (position 8 in SVN [U] and SVNI [G] and position 45 in SVN [C] and SVNI [U]) and one in nsP1 (position 425 in SVN [Cys] and SVNI [Arg]).
Modulation of neurovirulence by single nucleotide changes in the viral genome.
To identify the specific residue(s) in the E2 and 5′ determinants responsible for neurovirulence, additional recombinants were assayed by i.c. inoculation of 3-week-old rats (Fig. 3). Recombinant 40, with two amino acids of SVNI in the E2 region (Met-190 and Lys-260) in the SVN background, led to paralysis (but doses 2 orders of magnitude greater were required). Recombinants bearing single mutations (either Met-190 or Lys-260) on the SVN background caused no overt symptoms in the infected rats (data not shown). The 5′ neurovirulence determinant was identified by analysis of additional recombinants. Recombinant 43, with a G at position 8 in the SVN background, produced a neurovirulent virus similar to SVNI. The reciprocal construct, substitution of T for G at position 8 in the SVNI background with the SVN E2 determinant, abolished neurovirulence. As expected, recombinant 47, containing both the G at position 8 of the NCR and E2 Met-190 and E2 Lys-260 in the SVN background, was found to be as neurovirulent in rats as was the SVNI parent. Recombinants 43 and 47, containing G8, were also shown to induce apoptosis in the primary mature neuronal cultures.
FIG. 3.
Identification of specific mutations involved in SVNI neurovirulence toward 3- and 5-week-old rats. Recombinants were used to estimate the contribution of the E2 (recombinant 40) and the 5′ end determinants (recombinants 43 and 45) or the combined effect (recombinant 47) of SVNI determinants. For SVN and SVNI, the differing nucleotides (positions 8 and 45 in the 5′NCR) and amino acids (positions 121, 190, and 266 in E2) in the neurovirulent loci are indicated. For the recombinants, the indicated nucleotides or amino acids are those normally present in the other strain. PFU/ICLD50 titer ± the standard error (SE) values were determined as described in the legend to Fig. 2.
Age-dependent susceptibility of the host.
Comparison of the effects of the different viruses and recombinants on 3- and 5-week-old rats uncovered an age-dependent contribution of the E2 determinant to neurovirulence (Fig. 3). Recombinant 40, bearing the E2 Met-190 and E2 Lys-260, induced paralysis in 3- and 5-week-old rats, requiring larger inocula in the older animals. The outcome of the infections with recombinant 43, SVN with G8, showed a higher dependence on the age of the host, with a higher (30-fold) PFU/ICLD50 in the 5-week-old rats. This dependence is negligible when the E2 determinant of SVNI is added to the 5′NCR determinant. Recombinant 47, containing G8 and E2 Met-190 and E2 Lys-260 on the SVN background, showed infectivity and neurovirulence similar to those of SVNI in 3- and 5-week-old rats. These results indicate that the E2 determinants, in the context of G8 of the 5′NCR, can modulate the neurovirulence in adult rats (5 weeks old). Therefore, we conclude that although 5′NCR G8 is the main player in SVNI neurovirulence, the E2 determinants make an additional contribution to age-dependent neurovirulence.
DISCUSSION
Extensive studies on the pathogenicity of SV have been done with mice (4, 14, 22, 26, 28, 29, 43, 47). We have previously isolated and characterized four variants of SV which differ in their neurovirulence and neuroinvasiveness in weanling mice (30, 31). In the present study we expanded our studies to characterize the genetic determinants of neurovirulence of the SV strains in a less-susceptible host: the rat. Although this rodent is not a commonly used host for SV studies, our results indicate that this model may shed new light on mechanisms mediating the viral pathogenesis.
Suckling rats were found to be susceptible targets for the four SV variants. The viruses proliferated well in the brain after either i.c. or i.p. inoculation, causing the death of the infected rats within 2 to 3 days. The lack of defense mechanisms in suckling rats, due to both an immature immune system and a nonfunctional blood-brain barrier, masked potential differences between the strains. A similar outcome was seen in suckling mice, in which the four viral strains caused death by either inoculation route (30). In adult rats, however, marked differences in pathogenesis were apparent. In contrast to mouse infections, no viremia was detected in adult rats after i.p. inoculation, nor was neuroinvasion observed. Nonetheless, all of the viruses elicited a humoral immune response, which is indicative of proliferation in some organ or tissue. After i.c. inoculation, all four viruses proliferated well in the brains of the infected rats, with SVN and SVNI reaching similar titers, but only the latter caused death. A dose of ca. 20 PFU of SVNI per rat was sufficient to cause death within 5 to 6 days, whereas with SVN a dose of more than 106 PFU per rat was not lethal.
Since neuronal cultures represent a reasonable model for studying and characterizing the morphological and neurochemical damage of either acute or chronic infection, we infected primary neuronal cultures derived from rat brains with the four viruses. Similar to in vivo virulence in adult rats, only SVNI was cytotoxic for neurons in culture. At 48 h postinfection SVNI cultures showed signs of neuronal degeneration, including disintegration of neuronal fibers. These results indicate that the unique neurovirulent effects of SVNI may be due to the genetic traits of the virus, which are independent of host defense mechanisms. The effect of SVNI on the neuronal cells was shown to be mediated by apoptosis (data not shown), and the molecular mechanisms involved in the process are currently being studied in neuronal cell lines.
Using the recombinants that we prepared for studying neuroinvasion in mice (7), we localized the genetic determinants of SVNI neurovirulence in rats. Substitution of two SVNI E2 amino acids in the SVN genetic background, Met-190 and Lys-260, led to induction of paralysis in 3- and 5-week-old rats. Remarkably, a single substitution in the 5′NCR (G8) transformed SVN into a lethal pathogen for 3-week-old rats, one similar to SVNI. However, in 5-week-old rats, this recombinant was attenuated by 2 orders of magnitude relative to SVNI. Only substitution of both the 5′NCR and E2 SVNI determinants produced a recombinant with virulence properties in 5-week-old rats indistinguishable from those of the SVNI parent.
It is known that amino acid changes in the surface glycoproteins are important determinants of SV neurovirulence in mice (4, 6, 14, 26, 28, 32, 36, 37, 43, 46). In an earlier study with independent SV isolates, a major neurovirulence determinant derived from a neuroadapted strain (NSV) was mapped to the E2 glycoprotein (28). Residues at positions 55 and 172 modulate neurovirulence for mice of different ages (28). His-55 was particularly important for virulence, since five other amino acids tested in this position led to attenuation in 2-week-old mice. However, in attenuated SV, substitution of amino acids into His-55 led to partial virulence in weanling mice (40% mortality compared to the virulent wild-type NSV). The results described in the present study demonstrate that His-55 is not sufficient to induce virulence in rats, although it still may be an obligatory determinant (32).
It has also been reported that mice demonstrate age-related resistance to most alphaviruses (11, 19, 21, 42, 47). The molecular and cellular basis for this resistance is not understood. Resistance seems to correlate not with maturity of the immune system but rather with restriction of viral replication in neurons. This decreased replication may involve changes in neuronal receptors or increased resistance to the virus-induced apoptosis through elevated expression of Bcl-2 or related homologs. If we assume that E2 is involved in receptor binding and penetration, as previously stated, our results can be seen as supporting the hypothesis that age-dependent resistance is influenced by changes in viral receptors during neuronal maturation (17).
Recent evidence from many viral systems suggests that NCRs of viral genomes harbor important determinants of virulence. The effects of deletion mutations in the 3′ and 5′ NCRs have been studied for alphaviruses, including SV (25, 34) and VEE virus (24). SV 5′NCR mutants typically show defects in RNA accumulation and altered growth rates in cultured cells. These viruses also differ in their capacity to produce disease in mice, suggesting that the NCR is an important determinant of virulence in vivo as well as in vitro. Site-directed mutations in the 5′NCR of SV S.A.AR86 significantly reduced mortality and extended survival in adult mice inoculated i.c. (22). Mouse challenge experiments with VEE viruses containing sequences corresponding to those of the virulent TD strain or its attenuated vaccine derivative also indicate that attenuation is determined in part by mutations within the 5′NCR, although changes in the E2 envelope glycoprotein also play a role (24). The importance of the 5′NCR in the determination of virulence has also been shown for poliovirus (1, 10, 23, 35, 40, 44), Theiler’s murine encephalomyelitis virus (3, 27, 39, 50), influenza virus (33), mengovirus (8), hepatitis A virus (5, 12), and retroviruses (38).
This study thus demonstrates that the 5′NCR and E2 SVNI determinants play an instrumental role in neurovirulence in the rat model. Given the correlation between neurovirulence in vivo and the induction of apoptosis in primary neuronal cultures, it may be possible to extend these studies to define relevant pathogenic mechanisms at a molecular level.
ACKNOWLEDGMENTS
We thank P. Schneider and Y. Shemesh for excellent technical assistance.
J.D. and C.M.R. were supported by Public Health Service grant AI24134, and C.B. was supported by Israel Science Foundation grant 72696-16.6.
REFERENCES
- 1.Almond J W. The attenuation of poliovirus neurovirulence. Annu Rev Microbiol. 1987;41:153–180. doi: 10.1146/annurev.mi.41.100187.001101. [DOI] [PubMed] [Google Scholar]
- 2.Amir A, Pittel Z, Shahar A, Fisher A, Heldman E. Cholinotoxicity of the ethylcholine aziridinium ion in primary cultures from rat central nervous system. Brain Res. 1988;454:298–307. doi: 10.1016/0006-8993(88)90830-x. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay P K, Pritchard A E, Jensen K, Lipton H L. A three-nucleotide insertion in the H stem-loop of the 5′ untranslated region of Theiler’s virus attenuates neurovirulence. J Virol. 1993;67:3691–3695. doi: 10.1128/jvi.67.6.3691-3695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies N L, Fuller F J, Dougherty W G, Olmsted R A, Johnston R E. A single nucleotide change in the E2 glycoprotein gene of Sindbis virus affects penetration rate in cell culture and virulence in neonatal mice. Proc Natl Acad Sci USA. 1986;83:6771–6775. doi: 10.1073/pnas.83.18.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day S P, Murphy P, Brown E A, Lemon S M. Mutations within the 5′ non-translated region of hepatitis A virus RNA which enhance replication in BS-C-1 cells. J Virol. 1992;66:6533–6540. doi: 10.1128/jvi.66.11.6533-6540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dropulic L K, Hardwick J M, Griffin D E. A single amino acid change in the E2 glycoprotein of Sindbis virus confers neurovirulence by altering an early step of virus replication. J Virol. 1997;71:6100–6105. doi: 10.1128/jvi.71.8.6100-6105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubuisson J, Lustig S, Ruggli N, Akov Y, Rice C M. Genetic determinants of Sindbis virus neuroinvasiveness. J Virol. 1997;71:2636–2646. doi: 10.1128/jvi.71.4.2636-2646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke G M, Osorio J E, Palmenberg A C. Attenuation of Mengo virus through genetic engineering of the 5′ noncoding poly(c) tract. Nature. 1990;343:474–476. doi: 10.1038/343474a0. [DOI] [PubMed] [Google Scholar]
- 9.Dulbecco R, Vogt M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1956;99:167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans D, Dunn G, Minor P. Increased neurovirulence associated with a single nucleotide change in a non-coding region of the Sabin type 3 poliovaccine genome. Nature. 1985;314:548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- 11.Fazakerley J K, Pathak S, Scallan M, Amor S, Dyson H. Replication of the A7(74) strain of Semliki Forest virus is restricted in neurons. Virology. 1993;195:627–637. doi: 10.1006/viro.1993.1414. [DOI] [PubMed] [Google Scholar]
- 12.Funkhouser A W, Purcell R H, D’Hondt E, Emerson S U. Attenuated hepatitis A virus: genetic determinants of adaptation to growth in MRC-5 cells. J Virol. 1994;68:148–157. doi: 10.1128/jvi.68.1.148-157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Scarano F, Tyler K L. Molecular pathogenesis of neurotropic viral infections. Ann Neurol. 1987;22:565–574. doi: 10.1002/ana.410220502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin D E. Molecular pathogenesis of Sindbis virus encephalitis in experimental animals. Adv Virus Res. 1989;36:255–271. doi: 10.1016/s0065-3527(08)60587-4. [DOI] [PubMed] [Google Scholar]
- 15.Griffin D E. Viral infections of the central nervous system. In: Galasso G J, Whitley R J, Merigan T C, editors. Antiviral agents and viral diseases of man. 3rd ed. New York, N.Y: Raven Press, Ltd.; 1990. pp. 461–495. [Google Scholar]
- 16.Griffin D E. Roles and reactivities of antibodies to alphaviruses. Semin Virol. 1995;6:249–255. [Google Scholar]
- 17.Griffin D E, Hardwick J M. Apoptosis in alphavirus encephalitis. Semin Virol. 1998;8:481–489. [Google Scholar]
- 18.Griffin D E, Levine B, Ubol S, Hardwick J M. The effects of alphavirus infection on neurons. Ann Neurol. 1994;35:S23–S27. doi: 10.1002/ana.410350709. [DOI] [PubMed] [Google Scholar]
- 19.Griffin D E, Levine B, Tyor W R, Tucker P C, Hardwick J M. Age-dependent susceptibility to fatal encephalitis: alphavirus infection of neurons. Arch Virol. 1994;9:31–39. doi: 10.1007/978-3-7091-9326-6_4. [DOI] [PubMed] [Google Scholar]
- 20.Jackson A C, Moench T R, Trapp B D, Griffin D E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Investig. 1988;58:503–509. [PubMed] [Google Scholar]
- 21.Johnson R I, McFarland H F, Levy S E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972;125:257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- 22.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. New York, N.Y: Raven Press, Ltd.; 1996. pp. 843–898. [Google Scholar]
- 23.Kawamura N, Kohara M, Abe S, Komatsu T, Tago K, Arita M, Nomoto A. Determinants in the 5′ non-coding region of poliovirus Sabin 1 RNA that influence the attenuation phenotype. J Virol. 1989;63:1302–1309. doi: 10.1128/jvi.63.3.1302-1309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney R M, Chang G-J, Tsuchiya K R, Sneider J M, Roehrig J T, Woodward T M, Trent D W. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5′-noncoding region and the E2 envelope glycoprotein. J Virol. 1993;67:1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn R J, Griffin D E, Zhang H, Niesters H G M, Strauss J H. Attenuation of Sindbis virus neurovirulence by using defined mutations in nontranslated regions of the genome RNA. J Virol. 1992;66:7121–7127. doi: 10.1128/jvi.66.12.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine B, Griffin D E. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol. 1993;67:6872–6875. doi: 10.1128/jvi.67.11.6872-6875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipton H L, Calenoff M, Bandyopadhyay P K, Miller S D, Dal Canto M C, Gerety S, Jensen K. The 5′ noncoding sequences from a less virulent Theiler’s virus dramatically attenuate GDVII neurovirulence. J Virol. 1991;65:4370–4377. doi: 10.1128/jvi.65.8.4370-4377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lustig S, Jackson A, Hahn C S, Griffin D E, Strauss E G, Strauss J H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988;62:2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lustig S, Danenberg H D, Kafri Y, Kobiler D, Ben-Nathan D. Viral neuroinvasion and encephalitis induced by lipopolysaccharide and its mediators. J Exp Med. 1992;176:707–712. doi: 10.1084/jem.176.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lustig S, Halevy M, Ben-Nathan D, Akov Y. A novel variant of Sindbis virus is both neurovirulent and neuroinvasive in adult mice. Arch Virol. 1992;122:237–248. doi: 10.1007/BF01317186. [DOI] [PubMed] [Google Scholar]
- 31.Lustig S, Halevy M, Ben-Nathan D, Rice C M, Kobiler D. The role of host immunocompetence in neuroinvasion of Sindbis virus. Arch Virol. 1999;144:1159–1171. doi: 10.1007/s007050050576. [DOI] [PubMed] [Google Scholar]
- 32.McKnigt K L, Simpson D A, Lin S-C, Knott T A, Polo G M, Pence D F, Johannsen D B, Heidner H W, Davis N L, Johnston R E. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol. 1996;70:1981–1989. doi: 10.1128/jvi.70.3.1981-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muster T, Subbarao E K, Enami M, Murphy B R, Palese P. An influenza virus containing influenza B virus 5′- and 3′-noncoding regions on the neuraminidase gene is attenuated in mice. Proc Natl Acad Sci USA. 1991;88:5177–5181. doi: 10.1073/pnas.88.12.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niesters H G M, Strauss J H. Defined mutations in the 5′ nontranslated sequence of Sindbis virus RNA. J Virol. 1990;64:4162–4168. doi: 10.1128/jvi.64.9.4162-4168.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomoto A, Omata T, Toyoda H, Kuge S, Horie H, Kataoka Y, Genba Y, Nakano Y, Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci USA. 1982;79:5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polo J M, Johnston R E. Attenuating mutations in glycoproteins E1 and E2 of Sindbis virus produce a highly attenuated strain when combined in vitro. J Virol. 1990;64:4438–4444. doi: 10.1128/jvi.64.9.4438-4444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polo J M, Davis N L, Rice C M, Huang H V, Johnston R E. Molecular analysis of Sindbis virus pathogenesis in neonatal mice using recombinants constructed in vitro. J Virol. 1988;62:2124–2133. doi: 10.1128/jvi.62.6.2124-2133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portis J L, Perryman S, McAtee F J. The R-U5-5′ leader sequence of neurovirulent wild mouse retrovirus contains an element controlling the incubation period of neurodegenerative disease. J Virol. 1991;65:1877–1883. doi: 10.1128/jvi.65.4.1877-1883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pritchard A E, Calenoff M A, Simpson S, Jensen K, Lipton H L. A single base deletion in the 5′ noncoding region of Theiler’s virus attenuates neurovirulence. J Virol. 1992;66:1951–1958. doi: 10.1128/jvi.66.4.1951-1958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Racaniello V R. Poliovirus neurovirulence. Adv Virus Res. 1988;34:217–246. doi: 10.1016/s0065-3527(08)60519-9. [DOI] [PubMed] [Google Scholar]
- 41.Reed L J, Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 42.Reinarz A B G, Broome M G, Sagik B P. Age-dependent resistance of mice to Sindbis virus infection: viral replication as a function of host age. Infect Immun. 1979;3:268–273. doi: 10.1128/iai.3.2.268-273.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell D L, Dalrymple J M, Johnston R E. Sindbis virus mutations which coordinately affect glycoprotein processing, penetration, and virulence in mice. J Virol. 1989;53:1619–1629. doi: 10.1128/jvi.63.4.1619-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanway G, Hughes P J, Mountford R C, Reeve P, Minor P D, Schild G C, Almond J W. Comparison of the complete nucleotide sequences of the genomes of the neurovirulent poliovirus P3/Leon/37 and its attenuated Sabin vaccine derivative P3/Leon/12a1b. Proc Natl Acad Sci USA. 1984;81:1539–1543. doi: 10.1073/pnas.81.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, evolution. Microbiol Rev. 1994;58:492–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker P C, Griffin D E. The mechanism of altered Sindbis virus neurovirulence associated with a single amino-acid change in the E2 glycoprotein. J Virol. 1991;65:1551–1557. doi: 10.1128/jvi.65.3.1551-1557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tucker P C, Strauss E G, Kuhn R J, Strauss J H, Griffin D E. Viral determinants of age-dependent virulence of Sindbis virus for mice. J Virol. 1993;67:4605–4610. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyler K L, Gonzalez-Scarano F. Viral disease of the CNS: acute infections. In: Nathanson N, Ahmed R, Gonzalez-Scarano F, Griffin D E, Holmes K V, Murphy F A, Robinson H L, editors. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 837–853. [Google Scholar]
- 49.Tyler K L, Fields B N. Pathogenesis of viral infections. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 173–218. [Google Scholar]
- 50.Zhang L, Senkowski A, Shim B, Roos R P. Chimeric cDNA studies of Theiler’s murine encephalomyelitis virus neurovirulence. J Virol. 1993;67:4404–4408. doi: 10.1128/jvi.67.7.4404-4408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]